Research Article Open Access
Detection of Biological Environments for Endometrial Stromal and Mesenchymal Stem Cells Growth through a Quartz Crystal Microbalance Based Biosensor
Karekin D Esmeryan*
Georgi Nadjakov Institute of Solid State Physics, Bulgaria
- Corresponding Author:
- Esmeryan KD
Georgi Nadjakov Institute of Solid State Physics
72, Tzarigradsko Chaussee Blvd
1784 Sofia, Bulgaria
Tel: 9014170809
E-mail: karekin_esmerian@abv.bg
Received Date: May 22, 2015 Accepted Date: August 18, 2015 Published Date: August 21, 2015
Citation: Esmeryan KD (2015) Detection of Biological Environments for Endometrial Stromal and Mesenchymal Stem Cells Growth through a Quartz Crystal Microbalance Based Biosensor. Biosens J 4:120. doi:10.4172/2090-4967.1000120
Copyright: © 2015 Esmeryan KD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
The sensor response of 5 MHz gold electrode coated quartz crystal microbalances (QCMs) towards various environments for human endometrial stromal and mesenchymal stem cells growth is reported. The deposition of endometrial stromal cells onto sensor surface causes mass loading dependent resonance frequency downshift of 1.5 kHz and a corresponding increase in the dynamic resistance of 403 Ω. Furthermore, the QCMs demonstrate ability to detect negligible changes in the viscosity/density product of different biological environments. In addition, the sensors show reproducible sensor characteristics with maximum deviations from measurement to measurement within 50 Hz. The observed deviations are lower compared to the sensor signal, thereby confirming that the QCMs could be used as in situ detectors of various biological compounds or interactions, closely related to the in vitro fertilization analysis. These experiments open a possibility for integration of wide range in vitro investigations on lab-on-a-chip devices.
Keywords
Quartz crystal microbalance; Human endometrial stromal; In situ; In vitro; Lab-on-a-chip
Introduction
Nowadays, the women’s infertility introduces one of the biggest challenges in medicine, as the infertility rate has increased by 4% since 1980s, which is a serious drawback for the normal reproducibility of mankind [1]. The female infertility is a disease of the reproductive system defined by the failure to achieve a clinical pregnancy and can be caused by DNA damage, generic factors, hypothalamic-pituitary factors, environmental factors and/or the global stress [2-4]. Therefore, as an alternative solution, the interest in In vitro fertilization (IVF) has rapidly increased over the past decade [5-7]. The IVF is a process in which a female egg is fertilized by sperm outside the human body and involves both monitoring and stimulating the woman’s ovulation [6]. The fertilized egg (zygote) is cultured in a growth medium and is then implanted in the same or another woman’s uterus with an aim of establishing a successful pregnancy. During the In vitro fertilization, one of the most important steps in the establishment of pregnancy is the implantation [8]. There are many factors influencing the success rate of this process, which requires a subtle communication between the implanting embryo and the maternal endometrium [9]. In particular, the mesenchymal stem cells have a significant role in directing the development, growth, differentiation and functioning of the overlying epithelium [10]. The mesenchyme is embryonic connective tissue that is derived from the mesoderm and that interacts with the endoderm and ectoderm forming the three primary germ layers in the very early embryo. Such interactions are critical for the regulation of proliferation and differentiation of adult tissues as well [11]. On the other hand, the endometrial stromal cells, which are connective tissue cells derived from the inner mucous membrane of the woman’s uterus, provide a regulatory role for growth of the overlying epithelia. Moreover, they support the functional part of the uterus, called parenchyma, to which the human embryo is embedded [10]. Therefore, the interactions that occur between mesenchyme, embryonic stroma and epithelium have been investigated and analyzed in detail [10,12-15]. The most commonly used methods for cell characterization during growth; proliferation and differentiation are flow cytometry, binding and displacement studies, gel electrophoresis and immunophenotyping, as well as autoradiography [16,17]. These techniques are powerful and efficient (separation of DNA fragments ranging from 50 base pair to a few megabases), but suffer from several limitations. For instance, the electrophoretic approach is limited due to the passing current through the gel, causing heating and subsequent melting of the gel [18]. As a result, the buffering solution, which is used to reduce the pH changes due to the electric field, decreases its buffering capacity and the genetic material may not migrate consistently with each other. Moreover the results obtained with gel electrophoresis cannot be reliable if not previously validated [19]. In addition, the autoradiography has major disadvantages related to its sluggishness to yielding results, especially during quantitative studies, and lack of specificity [20]. Furthermore, most of these methods use bulky and expensive aparatus, which causes technical difficulties.
A strategy to overcome these limitations may come up of acoustoelectronics; in particular, quartz crystal microbalance (QCM) based biosensors [21-25]. Recently the QCMs have been used for detection of antimicrobial peptides [24], breast cancer [25], as immunosensor [23] and In vitro selection of various peptides [21]. The operation principle is related to the mass loading [26] or liquid’s viscosity-density [27] dependent resonance frequency shifts. The deposition of material in solid, liquid or gas phase on the sensor surface causes frequency variations proportional to the mass or viscosity/ density product of the substance. The main advantages of the QCM are its high sensitivity and resolution, fast response-recovery time, high dynamic range, low power consumption and relatively small size (from several mm to a few cm quartz crystals) [28]. These specific features allow In situ detection of biological interactions such as antigenantibody or receptor-ligand, as well as ppb concentrations of various compounds in biological suspensions.
The primary objective of this paper is to demonstrate the ability of the quartz crystal microbalance for In situ differentiation of biological environments for human endometrial stromal and mesenchymal stem cells growth. This could bring substantial improvements in In vitro fertilization analysis, by making them compatible to lab-on-a-chip devices. In section 2, the physical model describing the QCM’s operation in liquid environments is presented and discussed. Furthermore, the calibration process of the sensors and the experimental setup for biological investigations are described. In section 3, the resonance frequency and dynamic resistance calibration curves are presented. Finally, the QCM’s sensor response towards various biological environments for endometrial stromal and mesenchymal stem cells growth is examined and discussed.
Experimental
Operation principle of the QCM in liquids – physical background
The resonance behavior of the quartz crystal microbalance in liquids has been described theoretically by Kanazawa and Gordon [27]. The model is based on the coupling of the elastic shear waves in the crystal to the viscous shear waves in the liquid. The differential equation describing the propagation of shear waves in AT-cut resonators is the Helmholtz equation, yielding as solution undamped sinusoidal shearhorizontal wave traveling in a direction perpendicular to the crystal surface i.e. in YX plane:
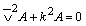 (1)
(1)
Where v-2 is the Laplacian; A is the amplitude of the wave; k is the wave number. In the liquid, the differential equation describing the shear wave is the diffusion equation, having as solution damped sinusoidal shear wave propagating in the Z direction away from the crystal:
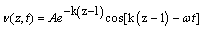 (2)
(2)
In eq. (2) v is the wave velocity, A is the amplitude, k is a propagation constant and ωt is the phase of the wave. The propagation constant can be described in terms of the viscosity η and density ρ of the liquid:
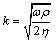 (3)
(3)
Here ρQ and μQ are the density and the shear modulus of the quartz, having values of 2650 kg/m3 and 2.95 × 1010 N/m2.
Calibration of the QCM
The sensor response of the QCM in liquids is expressed as proportional to the square root of viscosity/density product of the liquid according to eq. (4). As the QCM based biosensors are intended to operate in a liquid environment, in order to achieve accurate results, the behavior of their resonance frequency and dynamic resistance should follow the theoretical predictions. In this work, three 5 MHz gold electrode coated QCMs were used as biosensors, with frequency and resistance changes being monitored using spectrum analyzer SPM-14. The sensor characteristics were measured at room temperature in air and aqueous glycerol solutions in the concentration range of 0-90% wt.
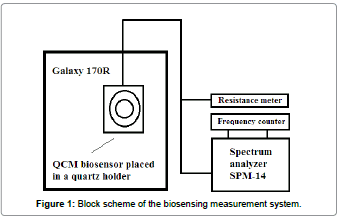
The biosensing measurement setup
The biological experiments were carried out in laboratory conditions using specially designed measurement system, illustrated on Figure 1. It consists of an incubator Galaxy 170R Eppendorf, a 5 MHz QCM mounted in a quartz crystal holder and connected to a spectrum analyzer SPM-14. A frequency counter HM 8123 was used for monitoring of the resonance frequency shifts. The deviations in dynamic resistance of the sensor were recorded through a resistance meter. Initially, the sensor device was placed in the incubator, which was set up to maintain constant temperature and humidity (36.7°C; ~100% RH), as well as constant CO2 and O2 concentrations of 6% and 13.6%, respectively. Afterwards, the QCM’s upper surface was immersed, one at the time, in various biological suspensions and subsequent changes in its resonance frequency and dynamic resistance were observed, proportionately to the viscosity/density product of each suspension. All experimental data were processed and recorded on a personal computer.
The chosen values of temperature, humidity, CO2 and O2 were found to provide favourable conditions for endometrial stromal and mesenchymal stem cells growth.
Results and Discussions
Investigation of the QCM’s sensor response in aqueous glycerol solutions
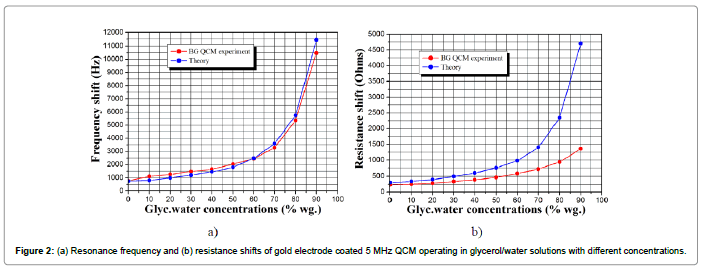
Figure 2 represents the resonance frequency and resistance shifts of three 5 MHz gold electrode coated QCMs versus glycerol/water solutions in the concentration range of 0-90% wt.
The blue lines represent the theoretical values calculated by the equation of Kanazawa and Gordon, while the red lines indicate the values obtained by the experiment. As seen, the frequency shifts follow the theoretical predictions in the entire range of concentrations. On the other hand, the resistance variations show substantial differences between theory and experiment, especially for highly viscous liquids (above 30% wt. glycerol concentrations).
However, the used QCMs are polished with a class of smoothness of 14. This is much higher class compared to the quartz crystals fabricated by Stanford Research System, for instance. Therefore, even upon immersion in highly viscous liquids, our QCMs retain lower level of energy losses; hence, lower resistance shifts. Each device was tested in three independent measurement cycles and the results showed reproducibility with a highest deviation within 50 Hz (10 ppm).
Differentiation of various biological environments using 5 MHz QCM
After calibrating the QCMs, their biosensing properties were tested toward various environments for endometrial stromal and mesenchymal stem cells growth. The results are summarized in Table 1 and Figure 3, respectively.
| QCM status | Δfr (Hz) | ΔR (Ω) |
| QCM 1 in air | 0 | 0 |
| QCM 1 with cells | 1500 | 403 |
| QCM 1 immersed in biological environment with cells | 1314 | 336 |
| QCM 2 in air | 0 | 0 |
| QCM 2 immersed in biological environment | 908 | 182 |
Table 1: Electrical characteristics of two 5 MHz QCMs, prior to and after immersion in various environments for endometrial stromal cells growth.
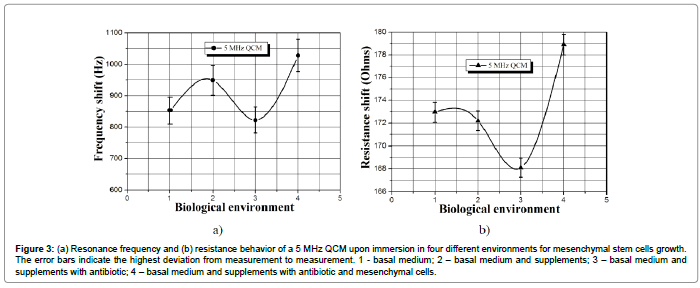
Figure 3: (a) Resonance frequency and (b) resistance behavior of a 5 MHz QCM upon immersion in four different environments for mesenchymal stem cells growth. The error bars indicate the highest deviation from measurement to measurement. 1 - basal medium; 2 – basal medium and supplements; 3 – basal medium and supplements with antibiotic; 4 – basal medium and supplements with antibiotic and mesenchymal cells.
The experimental data in Table 1 reveal that the deposition of endometrial stromal cells onto QCM’s surface leads to a proportional resonance frequency downshift of 1.5 kHz and a corresponding increase in the dynamic resistance of 403 Ω. Moreover, upon immersion of the sensor surface in biological suspensions with stromal cells, its response is influenced not only from the viscosity/density product of the liquid, but also from the mass loading effect caused by the cells [26]. The latter induces additional frequency and resistance deviations within 406 Hz and 154 Ω, respectively (see the 3rd and 5th row in Table 1). As shown in Figure 3, the situation with mesenchymal stem cells is similar and the QCM detects the differences between the biological environments.
It is clear that the addition of various components, such as antibiotic, stem cells or supplements, has strong impact onto sensor signal. Furthermore, the amplitude of error bars is lower, compared to the sensor signal, which implies that the latter is a physical phenomenon and not an artifact due to random fluctuations. This assumption was verified by performing analysis of variance (ANOVA) for a single factor with a null hypothesis that the data on Figure 3 are due to a random response. The statistical data treatment shows that the p-value for the resonance frequency is 0.027, which is less than 0.05 (significant level). Therefore, the model is significant and the null hypothesis for random response should be rejected. In other words, the frequency response of the QCM is due to changes in the viscosity/density product of the biological environment, rather than a random effect. In contrary, the p-value for the dynamic resistance is 0.51, which is much higher compared to the significant level. However, as depicted of Figure 2b, the resistance variations show substantial discrepancy between theory and experiment. It was mentioned in the previous chapter that such difference is most likely due to the smoothness of the QCM surface or due to electrical mismatch between the upper and lower electrode of the sensor.
Nevertheless, the experimental data on Figure 3 clearly indicate that the QCM could be used as in situ detector of various biological compounds or interactions, closely related to the in vitro fertilization. In turn, this opens a possibility for integration of wide range in vitro investigations on lab-on-a-chip devices.
Conclusions
This study presented systematic experimental investigations on the biosensing properties of 5 MHz gold electrode coated QCMs. The analysis showed that the resonance frequency and dynamic resistance of the sensors are strongly influenced by the presence of cells or by differences in the viscosity/density product of the liquid environments. In addition, the sensor devices demonstrated reproducibility and the highest deviation from measurement to measurement was within 50 Hz (10 ppm). This deviation is lower compared to the sensor signal, which is evidence that the QCM based biosensors could be used as in situ detectors of various biological compounds or interactions related to the in vitro fertilization.
Acknowledgements
The author wish to gratefully acknowledge hospital for female care “Nadejda”, in Sofia Bulgaria, for the provision of the biological equipment and substances. KE also thanks to Dr. Carlos Castano from the Nanocharacterization Center at Virginia Commonwealth University, USA, for his help with the ANOVA test and fruitful discussions.
References
- Maheshwari A, Hamilton M, Bhattacharya S (2008) Effect of female age on the diagnostic categories of infertility. Hum Reprod 23: 538-542.
- Smith EM, Hammonds-Ehlers M, Clark MK, Kirchner HL, Fuortes L (1997)Occupational exposures and risk of female infertility. J Occup Environ Med 39: 138-147.
- Zenzes MT (2000) Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update 6: 122-131.
- Newton CR, Sherrard W, Glavac I (1999) The fertility problem inventory: measuring perceived infertility-related stress. Fertil Steril 72: 54-62.
- Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, et al. (2007) A mild treatment strategy for in-vitro fertilization: a randomized non-inferiority trial. Lancet 369: 743-749.
- Allersma T, Farquhar C, Cantineau AE (2013) Natural cycle in vitro fertilization (IVF) for subfertile couples. Cochrane Database Syst Rev 8: CD010550.
- Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, et al. (2014) Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update 20: 808-821.
- Fluhr H, Krenzer S, Stein GM, Stork B, Deperschmidt M, et al. (2007) Interferon-γ and tumor necrosis factor-α sensitize primarily resistant human endometrial stromal cells to Fas-mediated apoptosis. J Cell Sci 120: 4126-4133.
- Herrler A, Von Rango U, Beier HM (2003) Embryo-material signaling: how the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online 6: 244-256.
- Arnold JT, Kaufman DG, Seppala M, Lessey BA (2001) Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 16: 836-845.
- Bigsby RM, Cunha GR (1986) Estrogen stimulation of deoxyribonucleic acid synthesis in uterine epithelial cells which lack estrogen receptors. Endocrinology 119: 390-396.
- Schwenke M, Kn√?¬ßfler M, Velicky P, Weimar CHE, Kruse M, et al.(2013) Control of human endometrial stromal cell motility by PDGF-BB, HB-EGF and Trophoblast-Secreted factors. PLoS ONE 8: e54336.
- Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, et al. (2002) Effect of gonadotropins on human endometrial stromal cell proliferation in vitro. Arch Gynecol Obstet 266: 223-228.
- Schuldiner M, Yanuka O, Eldor JI, Melton DA, Benvenisty N (2000) Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Nat Acad Sci USA 97: 11307-11312.
- Navaeian KE, Jalali M, Fathi Z (2015) Treating infertility in IVF clinics: Therapeutic potential of stem cells. J Genes Cells 1: 22-28.
- Singer GA, Strowitzki T, Rettiq I, Kimmiq R (1998) Flow cytometric detection and binding studies of human endometrial stromal cell epidermal growth factor receptor in monolayer culture: influence of progesterone. Mol Hum Reprod 4: 577-583.
- Shaer A, Azarpira N, Aghdaie MH, Esfandiari E (2014)Isolation and characterization of human mesenchymal stromal cells derived from placental deciduas basalis; Umbilical cord Wharton’s jelly and Amniotic membrane. Pak J Med Sci 30: 1022-1026.
- https://en.wikipedia.org/wiki/Gel_electrophoresis
- Chery CC, Moens L, Cornelis R, Vanhaecke F (2006) Capabilities and limitations of gel electrophoresis for elemental speciation: A laboratory’s experience. Pure Appl Chem, 78: 91-103.
- Stumpf WE (1981) Autoradiography: Advances in methods and application. JHistochem Cytochem 29: 107-108.
- Furusawa H, Murakawa A, Fukusho S, Okahata Y (2003) In vitro selection of N-Peptide-Binding RNA on a Quartz Crystal Microbalance to Study a Sequence-Specific Interaction between the peptide and Loop RNA. Chem Biochem 4: 217-220.
- Saint-Guirons J, Ingemarsson B (2012) Using a quartz crystal microbalance for the study of metastasis markers on intact cells. Methods Mol Biol 878: 175-183.
- Akter S, Rhee CK, Rahman AMd (2015) A highly sensitive quartz crystal microbalance immunosensor based on magnetic bead-supported bienzymes catalyzed mass enhancement Strategy. Biosens Bioelectron 66: 539-546.
- Wang KF, Nagarajan R, Camesano TA (2015) Differentiating antimicrobial peptides interacting with lipid bilayer: Molecular signatures derived from quartz crystal microbalance with dissipation monitoring. Biophys Chem 196: 53-67.
- Arif S, Qudsia S, Urooj S, Chaudry N, Arshad A (2015) Blueprint of quartz crystal microbalance biosensor for early detection of breast cancer through salivary autoantibodies against ATP6AP1. Biosens Bioelectron 65: 62-70.
- Sauerbrey GZ (1959) Verwendung von schwingquarzen zur wagung dunner schechten and zur mikrowagung. Physics 155: 206-222.
- Kanazawa KK, Gordon JG (1985) Frequency of a quartz microbalance in contact with liquid. Anal Chem 57: 1770-1771.
- Ballantine DS, Martin SJ, Wohltjen H, White RM, Zellers ET (1997) Chemical and Biological sensors. In: Acoustic wave sensors: Theory, Design, and Physico-Chemical Applications. Academic Press, USA
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 18306
- [From(publication date):
specialissue-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 17284
- PDF downloads : 1022