Research Article Open Access
Detection and Quantification of Pro-Inflammatory Cytokine in Sera and Urine of Sudanese Patients Infected with Schistosoma Haematobium
Hammad A1, Manahil Nuri2, Abdelbagi Alfadil3*, AMusa HA4, Osman MA5, Bashir A6, Nawal Eltayeb Omer7 and Yasir Hassan81Department of Parasitology National Ribat University, Sudan
2Department of Community medicine Alneelain University, Sudan
3Department of Immunology, National Ribat University, Sudan
4Department of Microbiology, National Ribat University, Sudan
5Department of Microbiology, University of Bacht Elruda, Sudan
6Department of Microbiology, Khartoum University, Sudan
7Department of Pathology, Khartoum College of Medical Sciences, Sudan
8Department of Biochemistry, Khartoum University, Sudan
- *Corresponding Author:
- Abdelbagi Alfadil
Department of Immunology
National Ribat University, Khartoum, Sudan
Tel: +249 912232021
E-mail: elfadilsss@yahoo.com
Received date: September 14, 2015; Accepted date: October 19, 2015; Published date: October 28, 2015
Citation: Hammad A, Nuri M, Alfadil A, Amusa HA, Osman MA, et al. (2015) Detection and Quantification of Pro–Inflammatory Cytokine in Sera and Urine of Sudanese Patients Infected with Schistosoma Haematobium. J Cytokine Biol 1:101. doi: 10.4172/jcb.1000101
Copyright: © 2015 Hammad A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Cytokine Biology
Abstract
Human infections with Schistosomiasis and other helminths induce strange immune responses which are characterised by the production of Th1 and Th2 cytokines. One hundred and thirty sera and urine were collected from patients with urinary schistosomiasis in two villages south Elduiem (Sudan). The disease were confirmed by finding Schistosoma haematobium eggs in urine using syringe filtration techniques. 70 individuals who were Schistosoma haematobium negative included in the study as controls 42 from endemic area and 28 from nonendemic area were tested IFNγ and IL 2 were found in urine and sera. There are a positive association between the production of cytokine and intensity of the infection. They are low intensity in control of endemic area which can be used for early detection of the disease in endemic area.
Keywords
ELISA, Shistocoma heamatobium, IL2, Pro-inflammatory cytokine.
Introduction
Schistosomiasis is one of the wide spread of all parasitic infection of man. The (WHO 2006) estimated that Schistosomiasis and soil transmitted helminths represent more than 40% of the global disease burden caused by all tropical diseases excluding malaria. Urinary Schistosomiasis caused by Schistosoma haematobium is endemic in 53 countries in the Middle East and most of the African continent Chitsulo, et al. especially in the rural areas where only the surface water bodies are sources of water supply [1,2]. Schistosoma haematobium infects over 112 million individuals and results in 150,000 deaths annually in sub-Saharan Africa [3]. In a study from Kenya, individuals with chronic infections and hepatosplenic disease appear to have predominantly Th1 responses to Schistosoma antigens and more severe pathology [4]. T Milner et al. in his study from Schistosoma-endemic area in Zimbabwe showed that egg positive people had significantly higher levels of specific antibodies, IL-2, IFN-γ and IL-23 [5]. In contrast, egg-negative individuals had significantly higher circulating IL-10, IL-4, IL-13, and IL-21 that were detected with high frequency in all participants.
Aim of the Study
Since World Health Organization (WHO) recommends that infection levels are determined prior to designing and implementing control programmes, as the treatment regimens depend on the population infection prevalence and the sensitivity of the parasitological infection diagnostic method is less reliable when infection levels are low. In this study we aimed to compare between parasitological methods and serological ones in diagnosing infected individuals in an endemic area.
Ethical Consideration
Ethical and institutional approval for the study was obtained from the medical research council of the National Ribat University. Permission for initiation of the study in the area was obtained from the Health Services Director (Eldwam locality). Objectives and methods were clearly explained to the community. Oral consent was obtained from the participants and parents/guardian before sampling.
Materials and Methods
Study design
This is a cross-sectional and case control study conducted to study Pro-inflammatory cytokines in sera and urine of Sudanese patients infected with Schistosoma haematobium.
Study area
This study was conducted in Eldwam locality which is approximately 120 km south to Khartoum. It is a well-known Schistosoma haematobium endemic zone in White Nile state in Sudan The principle source of water is White Nile river and there are large farms which grow sugar cane for sugar industry, also grow maize, wheat and vegetables. Fishing is carried in the White Nile. Temporary pools are created by the over flow of the White Nile during the rainy season. This locality was chosen because there is no other helminthic infections and low Schistsoma mansoni prevalence [6-8].
Study population
A total of 130 Sudanese patients from Eldwam locality whom were tested positive for Schistosoma haematobium eggs in urine were recruited in this study. 42 persons from the same locality whom were tested negative for Schistosoma haematobium eggs in urine and 28 healthy controls from a non-endemic area. Samples were collected during January 2014.
Sampling and Procedures
Urine and Stool collection and examination
A single terminal urine samples (20-50 ml) was collected in 50 ml containers from each individual of the study population. The samples were obtained between 10:00 am and 14:00 pm. Few drops of saponin solution were added to the samples with visible hematuria to enhance clarity in microscopy [9]. The specimens were appropriately labelled with identification numbers and placed in cold box with ice packs. They were processed 1-2 hrs after collection in the field. 10 ml was filtered through a 25 mm nucleopore filter (12 μm pore size) [10]. The filter was examined microscopically for Schistosoma haematobium eggs. The intensity was reported as number of egg/10 ml urine. The degree of intensity was categorized as light infection (≤ 50 ova/10 ml of urine) and as heavy infection ( >50 ova/10 ml urine) according to the World Health Organization [11]. To rule out Schistosoma mansoni eggs and other intestinal helminths, stool specimens were collected from all individual who have Schistosoma haematobium eggs in their urine, and processed following the Kato katz procedure [8]. The urine samples of the participants were aliquoted in cryotubes and stored at -20.
Blood for cytokines assay
Five millilitres of venous blood was collected in plain container from each Schistosoma haematobium infected volunteer and allowed to clot at room temperature. The sera were separated using centrifugation at 3000 rpm for 10 minutes. Then aliquoted in cryotubes and frozen at -20ºC. Their peripheral blood from all participant was examined for plasmodium using ICT for malaria with two species device (P.F and P.v) (SD Bio standard Diagnostics PVT-LTD India), to exclude malaria infection. All the participants were offered antihelminthic treatment with the recommended dose of praziquantel, 40 mg/kg body weight after collection of blood samples. Malaria cases were treated according to the treatment regime prescribed by the Ministry of health in Sudan.
A second blood and urine sample was collected from all the participant four weeks after treatment and processed as before. All the samples were transported frozen in cold box to the laboratory of Parasitology Department in the College of Medical laboratory Sciences National Ribat University, and stored at -80ºC. The samples were deforested for the first time for prior to testing.
Control samples
A group of 42 individuals were chosen from the area of the study on the basis that they had negative urine samples for Schistosoma haematobium ova and had no past history of Schistosoma. Infection in addition they were neither ill nor under any type of therapy at the time of sample collection. This represent controls from the endemic area. Another group of 28 apparently healthy individuals, living in nonendemic area were included in the study as controls from a nonendemic area.
Cytokines measurement
Enzyme - linked immunosorbent assay (ELISA) kits e Bioscience were used to determine the levels of two cytokines (IFNγ and IL-2) in serum and urine samples of Schistosoma haematobium infected individuals and controls from the endemic and non-endemic areas. All samples were run in single and the concentrations were calculated using standard curves.
Assay procedures
The assay procedure is the same for two cytokines both in sera urine (Manufacture instruction). Ninety six polystyrene micro titer plate (corning costar 9018) were coated with recommended concentration of capture antibody 100 μl/well in coating buffer. The plate was sealed and incubated overnight at 4ºC. Wells were aspirated (emptied) and washed five times with 250 μl/well washing buffer (one minute a time) was allowed for soaking during each wash step. Plate was blotted on absorbent paper. 200 μl of 1x assay diluents were added/well and the plate was incubated at room temperature for one hour (blocking). Aspiration and wash was done as before. Standards were diluted with 1x assay diluents. Two fold serial dilution of the top standards were performed to make the standard curve, and 100 μl/well of standard were added to appropriate wells, also 100 μl/well of samples were added to appropriate wells. The plate was sealed and incubated overnight at 4ºC. Aspiration/wash was done as before. 100 μl/well of detection antibody diluted in 1 x assay diluents was added to each well, and the plate was sealed and incubated at room temperature for one hour. Aspiration and washing was done as before. Subsequently 100 μl of Avidin-horseradish peroxidise diluted in assay diluents was added to the wells and sealed and incubated at room temperature for 30 minutes. Aspiration/wash was done as before (soaked in wash buffer for 1-2 minutes prior to aspiration, repeated for a total of 7 washes. 100 μl/well of substrate solution was added to each well and then incubated at room temperature for 15 minute. The reaction were stopped by adding 50 μl of stop solution. The absorbance was measured with a microplate reader at 450 nm.
Statistical analysis
The data were analyzed using Statistical Package for Social Sciences (SPSS version, 17). Thus parametric methods were used to evaluate results. The study population were categorized as infected (positive egg) or uninfected (endemic and non-endemic controls). Significance of difference, between mean values were assessed by student’s t-test. Pvalues less than 0.05 were regarded as significant. The comparison between Schistosoma egg positive and egg negative were analyzed by chi-square test. The relation between cytokine levels and infection intensity, as well as between cytokines in pair were conducted using a one-tailed non parametric spearman correlation procedure [12].
Results
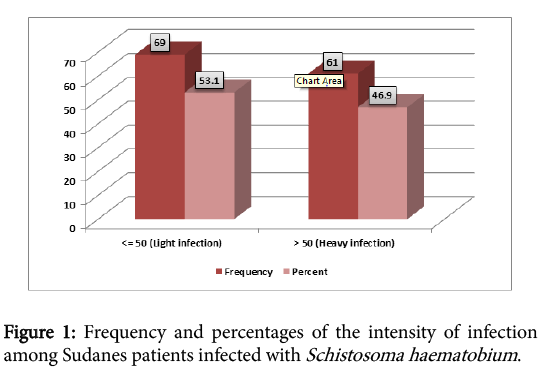
A total of 130 infected patients with Schistosoma haematobium were included in the study, 81 (62%) males and 49 (38%) females. The highest rate of infection were found among young people aged less 13 years (Table 1), Mean infection intensity for the study population was 61.92 eggs /10 ml urine, standard deviation 57.66. Figure 1 showed the intensity of the infection (53.1% light infection and 46.9% heavy infection), according to the WHO classification.
| Age groups | Frequencyofinfection | Percent(%) |
|---|---|---|
| less 13 | 96 | 73.85 |
| 13-23 | 32 | 24.61 |
| >23 | 2 | 1.54 |
| Total | 130 | 100 |
Table 1: Frequency of infection in different age groups of Sudanese patients infected with Schistosoma haematobium.
Cytokine Profile
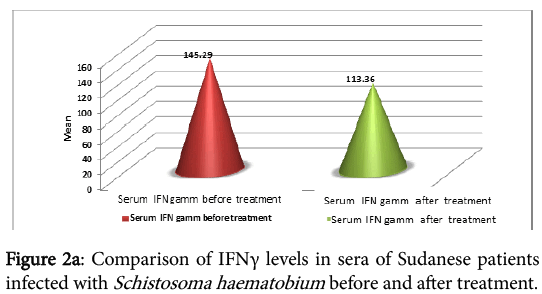
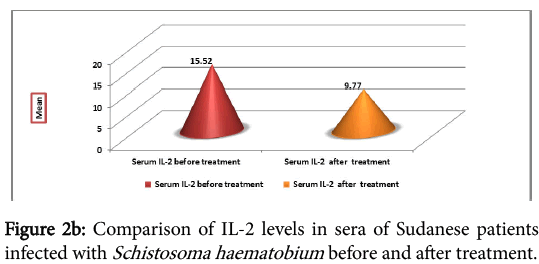
The cytokine profile measurement in sera of the infected individual, before and one month after treatment, showed high levels of IFNγ before treatment, but the difference was not significant (P=0.215) (Figure 2a). IL-2 was found to be high before treatment than after treatment. The difference before and after treatment was highly significant (P=0.007) (Figure 2b).
Table 2 summaries the cytokine profile in urine before treatment and one month after treatment. There were high levels of different cytokines before treatment than after treatment. The differences were statistically significant for IFNγ (P=0.000), IL-2 (P=0.000). The levels of IFNγ and IL-2 detected in serum and urine showed the same pattern, high before treatment and low after treatment.
| Cytokine | Mean | N | Standard deviation (SD) | P-value | |
|---|---|---|---|---|---|
| Pair 1 | IFNγ in urine before treatment | 86.17 | 106 | 159.26 | 0.000 |
| IFNγinurineafter treatment | 21.778 | 106 | 56.22 | ||
| Pair 2 | IL-2 in urinebefore treatment | 38.76 | 104 | 76.59 | 0.000 |
| IL-2inurineafter treatment | 7.04 | 104 | 21.89 |
Table 2: Cytokine profile in the urine of Sudanese patients infected with Schistosoma haematobium before and after treatment.
Table 3 summarize the serum level of cytokines detected in different groups (infected individual before and after treatment, controls from endemic and non-endemic area). IFNγ level in infected samples before and after treatment was 140.15 ± 232.63 and 112 ± 215.75 respectively, in controls from endemic area was 70.21 ± 96.66 and in controls from non-endemic area was 8.13 ± 10.31. There were differences in the levels of the IFNγ detected after treatment. IL-2 in infected samples was 15.42 ± 30.45 before treatment and 9.77 ± 18.52 after treatment. In control from endemic and non-endemic area IL-2 levels were 7.64 ± 17.76 and 3.12 ± 0.59 respectively. The highest levels of the tested cytokines detected in the three groups is IFNγ.
| Before treatment | After treatment | |||
|---|---|---|---|---|
| Samples group | Mean | Std | Mean | Std |
| Infected | 140.15 | 232.63 | 112.52 | 215.75 |
| Control from endemic | 70.21 | 97.66 | NA | NA |
| Control from non-endemic | 8.13 | 10.31 | NA | NA |
| Infected | 15.42 | 30.45 | 9.77 | 18.52 |
| Control from endemic | 7.64 | 17.76 | NA | NA |
| Control from non-endemic | 3.12 | 0.59 | NA | NA |
Table 3: Mean and standard deviation of the levels of cytokines in the sera of infected individuals before and after treatment and in the sera of controls groups.
Table 4 summarize the cytokine level in the different study groups are summarized as follows: comparison of the level of IFNγ in infected samples with the control samples from endemic and non-endemic area was found to be significant (P=0.045 and 0.005) respectively. When IFNγ level compared in endemic controls with the non-endemic one it was not significant (P=0.237).The comparison of IL-2 in infected samples with endemic controls was not significant (P=0.095) and the infected samples with non-endemic controls was significant (P=0.049) while the comparison of the two controls was not significant (P=0.520).
| Dependent Variable | Sample Groups | Sample Groups | Sig. |
|---|---|---|---|
| IFN γ in serum before treatment | Infected | Control from endemic | 0.045* |
| Control from non-endemic | 0.005* | ||
| Control from endemic | Infected | 0.045* | |
| Control from non-endemic | 0.237 | ||
| Control from non-endemic | Infected | 0.005* | |
| Control from endemic | 0.237 | ||
| IL-2 in serum before treatment | Infected | Control from endemic | 0.095 |
| Control from non-endemic | 0.049* | ||
| Control from endemic | Infected | 0.095 | |
| Control from non-endemic | 0.52 | ||
| Control from non-endemic | Infected | 0.049 | |
| Control from endemic | 0.52 |
Table 4: Comparisons of cytokines (IFNγ and IL-2) in the sera of different sample groups.*The mean difference is significant at the 0.05 level.
Table 5 shows sex cytokine profiles in patients before treatment. Females produce high levels of IFNγ and IL-2, than the males and there were significant differences (P=0.0085, P=0.0077 respectively).
| Cytokines | Sex | Mean ±STD | T-test | P-value |
|---|---|---|---|---|
| INFγ | Male (n=81) | 115.98 ±16.14 | 76.03 | 0.0085** |
| Female (n=49) | 178.82 ±314.87 | |||
| IL-2 | Male (n=81) | 14.01 ±29.72 | 24.91 | 0.0077** |
| Female (n=49) | 17.76 ±31.78 |
Table 5: Comparison of cytokine profile in sera of Sudanese males and females infected with S.haematobium before treatment.
The cytokine profiles in the different age groups before treatment are shown in Table 6. The levels of both cytokines (IFNγ and IL-2) appear to be influenced by age. High levels were detected in those >23 year of age. The differences in cytokine levels in different age groups were significant in case of IFNγ. But IL-2, shows no significant differences in age groups (<13) vs. (13-23) (P=0.0628).
| Cytokine | Age | Mean ± SD | Comparison | T-test | P-value |
|---|---|---|---|---|---|
| INFγ | <13 | 155.28 ±254.28 | (<13) vs.(13-23) | 38.41 | 0.0083** |
| 13-23 | 67.23 ±88.81 | (<13) vs. (>23) | 56.29 | 0.0059** | |
| >23 | 505.258 ±184.34 | (13-23) vs. (>23) | 73.16 | 0.0017** | |
| IL-2 | <13 | 15.44 ±32.29 | (<13) vs. (13-23) | 3.28 | 0.0628NS |
| 13-23 | 13.83 ±24.67 | (<13) vs. (>23) | 14.56 | 0.0124* | |
| >23 | 39.45 ±1.84 | (13-23) vs. (>23) | 12.79 | 0.0235* |
Table 6: Age cytokine profile in sera of infected groups before treatment.
The relation between the intensity of the infection and cytokine production appear in (table 7). Both cytokines (IFNγ and IL-2) were found to be high in heavy infected individuals and the differences were statistically significant (P=0.00069, P=0.0129, respectively).
| Cytokines | Groups | Mean ± SD | T-test | P-value |
|---|---|---|---|---|
| INFγ | Lightinfected group (n=69) | 97.71±126.69 | 82.19 | 0.00069* |
| Heavyinfected group (n=61) | 187.13±304.98 | |||
| IL-2 | Lightinfected group (n=69) | 13.93±28.98 | 11.64 | 0.0129* |
| Heavyinfected group (n=61) | 17.12±32.19 | |||
| Heavyinfected group (n=61) | 33.08±44.26 |
Table 7: Relation between intensity of infection and cytokine levels in the sera of Sudanese patients infected with S.haematobium
The level of cytokines in urine in relation to the intensity of infection were found to be as follows. IFNγ shows high levels in both heavy and light infection with significant differences (P=0.00069). IL-2 levels were relatively low in both high and low infection (Table 8).
| Cytokines | Groups | Mean ± SD | T-test | P-value |
|---|---|---|---|---|
| INFγ | Light infected group (n=69) | 111.20 ±193.13 | 58.29 | 0.00092** |
| Heavyinfected group (n=61) | 62.59 ±123.67 | |||
| IL-2 | Lightinfected group (n=69) | 40.22 ±84.95 | 17.41 | 0.0082* |
| Heavyinfected group (n=61) | 34.10 ±75.81 |
Table 8: Relation between intensity of infection and cytokine levels in the urine of Sudanese patients infected with S.haematobium.
Discussion
The relationship between age, intensity of infection and gender has been observed in many epidemiological situations, endemic and epidemic [6,13].
This study aimed to determine the relation between urinary and serum cytokine level in Sudanese individual infected with Schistosoma haematobium , which were measured before and after treatment. The cytokines studied were IFNγ, and IL-2 (markers of Th1 responses). All participants had a detectable levels of baths cytokines with different concentration. The most dominant cytokine was found to be IFNγ, that raised early in the infection with Schistosoma. Although it has been proposed that elevated levels of IFNγ may be associated with susceptibility to Schistosoma infection, however findings in this study suggest that IFNγ may be involved in early stages of the immune response to Schistosoma infection and this is in agreement with finding reported by Mutapi et al. and Mduluza et al. [14,15]. The IFNγ and IL-2 cytokines which observed in the study may indicate a regulatory role of Th1 cytokine in early responses to infection and this finding concur with Arnaud V et al. [16]. Also the study showed there was a decrease in IFNγ and IL-2 after treatment and this is on line with the Mutapi F et al. finding [14]. The Th1 cytokine IFNγ was found to exist during infection but the cytokine persisted at lower levels in treated individual and this may be use to modulating effect of IL-10. Th1 responses have been linked to the so called endemic normal [17,18]. When uninfected healthy individuals living in an area, where Schistosoma is transmitted, produce high levels of parasite specific IFNγ and low levels of IL-10.This coincide with our finding of these cytokine among endemic control individuals. This study also showed high levels of cytokines in females than in males. Similar observation were seen in immunoglobulin’s in other studies. This could be due to hormonal influence. We also observed in this study an association between age and cytokines levels. Furthermore this study showed a positive correlation between age and cytokine as evidenced by the increase of IFNγ with age and the decrease of IL-2 with aging. This finding is comparable with report of Milner et al. regarding IFNγ, but not IL-2. The cytokine levels in urine showed similar results as observed in serum, high before treatment and the levels were low after treatment. Some cytokines were detected with high concentration in urine than in sera which may be due to local production in the bladder. These finding concur with those reported by Gurgoze et al. who studied cytokines in serum and urine of children infected with urinary bacteria [19]. Very recently a similar finding was reported by Kariuki et al. who studied urinary cytokine and related it to urinary tract pathology in children [7].
Conclusion
Depending on high production of IFNγ and IL-2 and the low production of IL-4 and IL-5, the immunological classification of urinary Schistosomiasis in the study area could be considered in the acute stage of the disease. Also the result showed that IL-10 responses develop early compared to IL-5 and IL-4 responses and may be down modulating immune pathological responses that occur during the early phase of infection. IL-4 and IL-5 were working in reverse to IL-10 when studying these in sera and urine before and after treatment and we deduce that it was due to the modulating effect of IL-10.The study also showed that it is possible to use urine to study different cytokines in urinary Schistosomiasis which may indicate that there is a possibility of local production of cytokines in bladder.
Acknowledgements
We are grateful to the people of Hilat Salim and Saeed who agreed to participate in the study. We also acknowledge Abu Hania for his invaluable assistance in the field work. Financial support: This investigation received financial support from the Ministry of Higher Education and Scientific Research, special program for research and from the Faculty of Graduate Studies and Scientific Research National Ribat University.
References
- Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Trop 77: 41-51.
- MabataT, MichachOvji V. M Oguoma (2009) The prevalence of urinary schistosomiasis in Ogbadibo local Government area of Benue State, Nigeria. The journa l of infed Dis 98: 1528-8366.
- Rollinson D (2009) A wake up call for urinary schistosomiasis: reconciling research effort with public health importance. Parasitology 136: 1593-1610.
- Butterworth AE (1998) Immunological aspects of human schistosomiasis. Br Med Bull 54: 357-368.
- T Milner L Reilly, N Nausch, N MidlziMduluza, R Mazels and Mutapi (2010). Circulating cytokine levels and antibody responses to human Schistosomahaematobium: IL-5 and IL-10 levels depend upon age and infection status. Parasite Immunology 32: 710-721.
- Hagan P, Ndhlovu PD, Dunne DW (1998) Schistosome immunology: more questions than answers. Parasitol Today 14: 407-412.
- Kariuki H Njaanake, Simonsen PE, Vennervald BJ, Mukoko DA, Reimert CM, et al. (2014) Urinary cytokines in Schistosomahaematobium-infected schoolchildren from Tana Delta District of Kenya. BMC Infect Dis 14: 501.
- Katz N, Coelho PM, Pellegrino J (1970) Evaluation of Kato's quantitative method through the recovery of Schistosomamansoni eggs added to human feces. J Parasitol 56: 1032-1033.
- Cheesbrough M (1998) District laboratory Practice in Tropical Countries. Part 1, Cambridge University Press, London.
- Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK (1976) Field studies of a rapid, accurate means of quantifying Schistosomahaematobium eggs in urine samples. Bull World Health Organ 54: 159-162.
- Bergquist R, Johansen MV, Utzinger J (2009) Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol 25: 151-156.
- Sokal RR, Rohlf J (1995) Biometry: The principles and practice of statistics in biological research. Freeman and company.
- van Dam GJ, Stelma FF, Gryseels B, Falcão Ferreira ST, Talla I, et al. (1996) Antibody response patterns against Schistosomamansoni in a recently exposed community in Senegal. J Infect Dis 173: 1232-1241.
- Mutapi F, Winborn G, Midzi N, Taylor M, Mduluza T, et al. (2007) Cytokine responses to Schistosomahaematobium in a Zimbabwean population: contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect Dis 7: 139.
- Mduluza T, Ndhlovu PD, Midzi N, et al. (2003) Contrasting cellular responses in Schistosomahaematobium infected and exposed individuals from areas of high and low transmission in Zimbabwe. ImmunolLett 88: 249-256.
- Arnaud V, Li J, Wang Y, Fu X, Mengzhi S, et al. (2008) Regulatory role of interleukin-10 and interferon-gamma in severe hepatic central and peripheral fibrosis in humans infected with Schistosomajaponicum. J Infect Dis 198: 418-426.
- Gazzinelli G, Viana IR, Bahia-Oliveira LM, Silveira AM, Queiroz CC, et al. (1992) Immunological profiles of patients from endemic areas infected with Schistosomamansoni. MemInstOswaldo Cruz 87: 139-142.
- Viana IR, Sher A, Carvalho OS, Massara CL, Eloi-Santos SM, et al. (1994) Interferon-gamma production by peripheral blood mononuclear cells from residents of an area endemic for Schistosomamansoni. Trans R Soc Trop Med Hyg 88: 466-470.
- Gürgöze MK, Akarsu S, Yilmaz E, Gödekmerdan A, Akça Z, et al. (2005) Proinflammatory cytokines and procalcitonin in children with acute pyelonephritis. PediatrNephrol 20: 1445-1448.
- Montresor A, Crompton, David WT, Hall A, Bundy DAP, et al. (1998) Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: World Health Organization.
- [No authors listed] (2006) Schistosomiasis and soil-transmitted helminth infections--preliminary estimates of the number of children treated with albendazole or mebendazole. WklyEpidemiol Rec 81: 145-163.
Relevant Topics
- Anti-inflammatory cytokines
- Cytokine
- Cytokine array
- Cytokine function
- Cytokine inflammation
- Cytokine inhibitors
- Cytokine production
- Cytokine release syndrome
- Cytokine signaling
- Cytokine storm
- Cytokine therapy
- Cytokines function
- Inflammatory cytokines
- Intracellular cytokine staining
- Macrophage cytokines
- Pro-inflammatory cytokine
- Proinflammatory cytokines
- Role of cytokines
Recommended Journals
Article Tools
Article Usage
- Total views: 10950
- [From(publication date):
May-2016 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 10058
- PDF downloads : 892