Review Article Open Access
Design of Solar Lime Kiln
Rajaram Swaminathan* and Shikongo Tomas NadhipiteDepartment of Mechanical and Marine Engineering, Namibia University of Science and Technology, Namibia
- *Corresponding Author:
- Rajaram Swaminathan
Department of Mechanical and Marine Engineering
Namibia University of Science and Technology, Namibia
Tel: +264612072167
Fax: +264612079167
E-mail: rswaminathan@nust.na
Received date: September 15, 2017; Accepted date: September 18, 2017; Published date: September 25, 2017
Citation: Swaminathan R, Nadhipite ST (2017) Design of Solar Lime Kiln. Innov Ener Res S1:001. doi: 10.4172/2576-1463.S1-001
Copyright: © 2017 Swaminathan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Innovative Energy & Research
Abstract
Calcium oxide (CaO), commonly known as quicklime is a key ingredient of cement. It is produced by thermal dissociation of limestone (CaCO3) in a calcination reaction. The process of converting limestone to quicklime involves heating limestone to a temperature of 1200-1400K. This thermal dissociation reaction produces carbon dioxide (CO2). The heat required for the process is habitually supplied by the combustion of coal or, oil in the lime kiln. The combustion of these fuels produces additional CO2 emission which represents around 40% of the cement plant CO2 emissions. This emission can be reduced by switching to fuels, like natural gas, biomass or waste-derived fuels. The use of concentrated solar energy as the source of process heat is an energy efficient alternative.
This paper presents the design of a mini, scalable solar lime kiln which was designed from first principles. The kiln is designed to calcine small-grained (1-5 mm diameter) limestone particles. A solar dish collector is proposed to supply the required heat energy at the desired temperature. The heat is focussed on a heating element located centrally in a tilted rotary kiln driven by chain drive. The carbon dioxide emitted from the calcination reaction is released to prevent re-carbonation of limestone. The heating element can easily be replaced with another heat source such as electrical heating. The design procedure and design simulation are detailed in the paper.
Keywords
Solar lime kiln; CO2 emission; Fuels; Natural gas
Introduction
Production of quick lime (CaO) is a high-temperature process. It involves endothermic chemical reaction to thermally dissociate calcium carbonate into calcium oxide, with carbon dioxide as by-product.
Cement production is described by the chemical equation below:
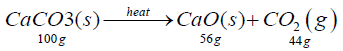
The thermal dissociation of limestone takes place at a calcination temperature of 1173K using thermal energy of 3010 kJ/kg of limestone produced [1]. On a mass basis, one kg of quicklime is produced from burning 1.786 kg of limestone, releasing 0.786 kg of carbon dioxide.
Normally, the heat for calcination is supplied by the combustion of carbon-based fuels. These conventional fuels have relatively high energy density and provide high-grade heat, but as a drawback, produce carbon dioxide both from the calcination reaction and from the combustion process. By replacing fossil fuels with concentrated solar energy as the primary energy source to carry out calcination reaction, the carbon dioxide from combustion is completely eliminated, thus reducing the carbon dioxide from cement industries which are the predominant producers of quick lime. The solar process also ensures production of very pure quicklime, which is not contaminated by combustion by-products.
Solar Concentrator
A parabolic solar collector transforms solar radiant energy into heat energy. In a concentrated form, this energy can be used to reach very high temperatures.
Solar thermal in lime manufacturing
The solar chemical reactors in lime processing plants are of two basic types:
Indirect receiver/reactors: Use an intermediate heat transfer fluid such as molten salt or solid separator wall between the receiver and reactor. The fluid or wall is directly heated by solar energy and energy transferred to the receiver.
Direct absorption receiver/reactors: They absorb sunlight directly on the reactor feed. The proposed design is of indirect type.
Kilns
Kilns are used for production of quicklime on a large scale and fall into two basic categories, namely the intermittent or continuous feed kilns. The choice for the process is based on the quality of product required and or the availability of fuel [1].
Lime kilns can be classified into three general groups; the vertical kiln, rotary kiln and the miscellaneous kiln. In this work, a rotary kiln was designed.
Rotary kiln
Rotary kilns are an advanced thermal processing tool used for processing solid materials at extremely high temperatures in order to cause chemical reaction or physical change. They are commonly used to carry out processes such as calcination, thermal desorption, organic combustion and heat setting.
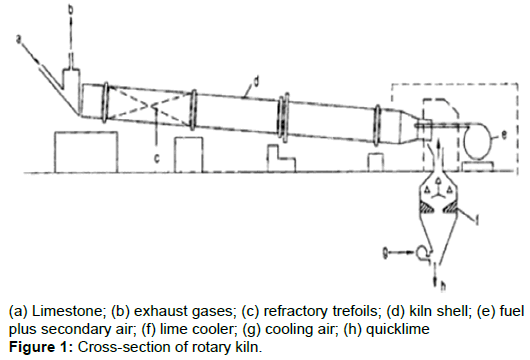
Although rotary kilns were originally developed for use in cement industries, due to their flexibility, they are now adopted for many industrial applications [2]. A cross-section view of a rotary kiln is shown in Figure 1 below [3].
A typical rotary kiln is made up of a rotating cylinder, sized specifically to meet the retention time and temperature requirements of the material to be processed [4]. The kiln is usually placed at an incline to allow gravity assist the materials to move through the rotating cylinder.
Although the rotary kilns are custom made around the material to be processed, some components are basic for rotary kiln [2], which are; drive assembly, riding rings, thrust rollers, trunnion wheels, discharge breeching, product discharge, exhaust gas system, refractory, heat source, raw material feed, air seal and kiln shell.
System concept
The configuration of the solar limekiln will be based on the working principles of a heat exchanger.
The heat exchanger allows the heat to be exchanged between two materials.
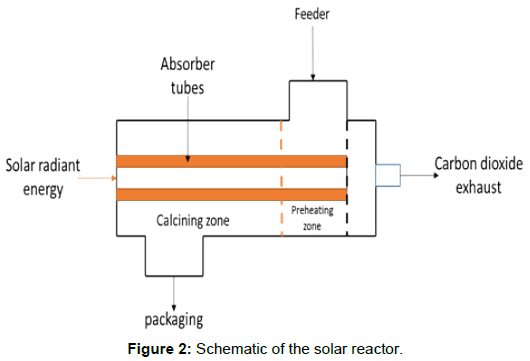
Raw materials enter the combustion chamber through the feeder. The combustion chamber rotates about the heating element (absorber tubes) to ensure evenly distribution of heat through the limestone particles. Solar energy enters the combustion chamber through the circular solar receiver. Energy from the sun is not directly transferred from the sun to the limestone particle. Figure 2 is the schematic layout of the design, which will lead to the final design of the kiln.
System design
For system design, the parameters considered are given in Table 1. The tilted rotary kiln is mounted on base supports and driven by chain drive.
| Parameter (Nomenclature) | Value (Unit) |
|---|---|
| Outer diameter (D) | 560 (mm) |
| Length of kiln (L) | 560 (mm) |
| Output (OP) | 1 (t/day) |
Table 1: System design parameters.
The kiln consists of 560 mm long cylindrical steel drum of 0.56 m internal diameter. The receiver is a Re-crystalized Silicon carbide cylinder, lined with absorber tubes of 300 mm long and 30 mm dimeter. The inner layer is made out of silicon porcelain material, with a maximum operating temperature of 1400 degrees.
The middle layer is the PUR foam that provides insulation to the reaction environment.
Parabolic Solar Dish
The collector’s parabolic geometry is fundamental to guarantee optimum thermal efficiency of the parabolic dish. A mathematical analysis was performed to find values that satisfy the design criteria based on the receiver diameter, concentration ratio and the receiver diameter. Table 1 presents the design parameters used.
High temperatures are achieved by having high concentration ratios
(C); which is the ratio between the area of the collector 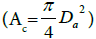 and
the area of the receiver
and
the area of the receiver 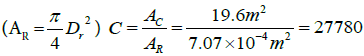
Table 2 gives information needed to calculate the thermal efficiency of the collector.
| Parameter (Nomenclature) | Value (unit) |
|---|---|
| Aperture diameter (Da) | 5 (m) |
| Focal point (F) | 1000 (mm) |
| Receptor diameter (Dr) | 30 mm |
Table 2: Design parameters.
The optical thermal efficiency (h0) of the receiver is given by: 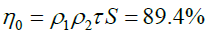
Temperature at the receiver can thus be estimated using the parameters in Tables 3 and 4.
| Parameter (Nomenclature) | Value (unit) |
|---|---|
| Receptor absorbance (ρ1 ) | 0.96 |
| Transmittance (τ) | 0.98 |
| Reflectivity of the receiver (ρ2 ) | 0.95 |
| Shape factor (S) | 1 |
Table 3: Parameters to calculate thermal efficiency.
| Parameter (Nomenclature) | Value (unit) |
|---|---|
| Solar direct radiation in Namibia (I1) | 6370 (W/m2) |
| ambient temperature (Tamb) | 298K |
| approximate temperature of the sun (Tsun) | 6000K |
| emissivity of the receiver (e) | 0.9 |
| maximum efficiency of solar collectors (n) | 0.4 |
| the thermal efficiency of the receiver (h0) | 91.20% |
| concentration ratio (C) | 27780 |
Table 4: Parameters to calculate temperature at the receiver.
The temperature at the receiver (Treceiver) is given by;
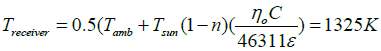
The achieved temperature is more than the required calcination temperature.
Kiln
The output (OP(t/day)) is linked to the inner diameter by the following formula [5-9]:
OP=0.135D2
It is desired to at least produce 1 ton of quicklime per day, hence the diameter of the kiln for the desired output is:
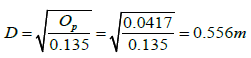
The selected kiln inner diameter is 0.56 m. The length is also kept equal to the diameter (0.56 m).
Operating the kiln between 9 am and 16 h 00, the calcination rate will be 0.039 kg of quicklime per second. An amount of 117.39 kJ/s is therefore needed for calcining at this rate.
Energy (Q) absorbed by the receiver (absorber tube) is given by: 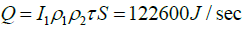
The designed system is able to meet the desired demand.
Results and Discussion
Understanding the heat transfer in the kiln is very crucial to help understand the heat losses to the environment. With no content inside, the heat transfer from the receiver to the kiln shell is strictly by convection. From the inner layer to the PUR foam and the steel is by conduction. From the outer layer to the outside environment is by convection.
The following assumptions will be applied during the heat transfer analysis,
• The thermal conditions at the boundaries does not change with time, hence heat transfer is steady.
• Heat transfer is one-dimensional since there is thermal symmetry at the midpoint.
• Thermal conductivity is constant.
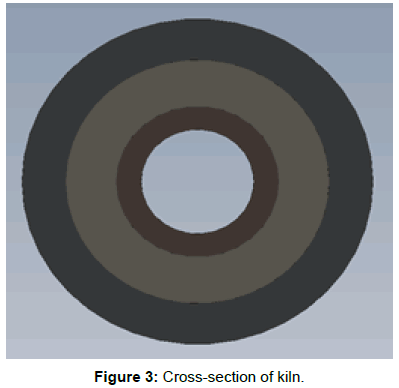
• The outer shell is at room temperature and the inner layer is at calcination temperature (Figure 3).
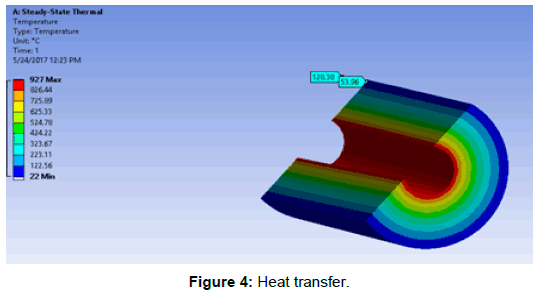
ANSYS was used to investigate the heat transfer in the kiln shell. The figure below was directly obtained from the simulation environment (Figure 4).
The results shows that the designed kiln possess very high thermal capacity.
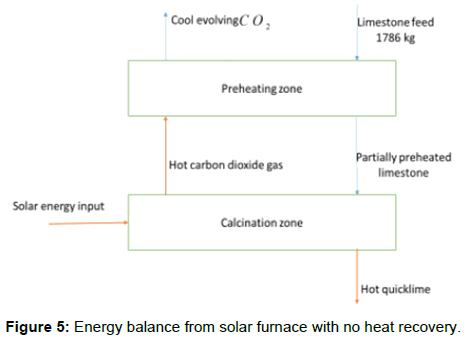
Heat and mass balance
Heat and mass balance for the designed system will be analyzed based on Figure 5 below. Since the solar kiln designed is desirably small, the limestone particles are not preheated by air. The evolving carbon dioxide gas transfer some heat to the new reactants. However, the total heat in the products is completely lost from the reaction chamber.
With no heat recovery in the system, the energy consumption is highest since the stored energy in the hot products is not recovered. This implies that all the available energy is consumed to raise the temperature from room temperature to the calcination temperature and to carry out calcination itself. An amount of 824 MJ per ton of quicklime is required to preheat the limestone in order to achieve calcination. With a heat recovery option, energy from the products can be utilized to pre-heat the incoming limestone particles thus minimizing energy consumption.
Carbon dioxide reduction
For each kilogram of quicklime produced, about 0.786 kg of carbon dioxide is produced. Burning fuel also releases carbon dioxide into the atmosphere. In the case of coal, the carbon dioxide emission is about 295 kg CO2 per ton of quicklime. Other types like natural gas releases 206 kg per ton of quicklime.
This implies that the carbon dioxide is reduced by approximately 27 percent when replacing fossil fuel with concentrated solar energy.
Conclusion
The system designed operate at an average peak solar flux concentration of 6.37 kW/m2. The receiver is made to withstand more than 1200K and the energy flow is controlled by a shutter in front of the receiver to regulate the temperature increase. The concentrated solar enters the circular aperture of the receiver. The receiver radiates heat into the reaction chamber, where particles of calcium carbonate is contained.
The carbon dioxide emitted from the calcination reaction is released to prevent re-carbonation of limestone. Since solar is not a reliable source of energy, heating element can easily be replaced with another heat source such as electrical heating and or coal power. The method of heating will not directly affect the performance of the lime kiln or the quality of lime produced. The main objective is to minimize the amount of carbon dioxide released during the production of cement.
References
- Oates J (1998) Lime and Limestone: Chemistry and technology, Production and Uses. 36: 36-2766.
- Feeco I (2014) The rotary kiln Handbook.
- Meier A. Design and Experimental Investigation of a Horizontal Rotary Reactor for the Solar Thermal Production of Lime. 29: 811-821.
- Bonaldi E (2002) Carbon dioxide Mitigation in the Lime Industry: Replacing Fossil Fuels with Concentrated Solar Energy, international lime Association Congress.
- Anton M , Enrico B, Gian MC, Wojciech L, Daniel W (2004) Solar Chemical Reactor Technology for the industrial solar production of Lime. 80: 1355-1362.
- Boynton R (1980) Chemistry and technology of lime and limestone.
- Oates J (2001) Private communication: Calcimatic kilns, Buxton: Derbyshire.
- Craig RA (2010) Investigating the use of concentrated solar energy to thermaly decompose limestone.
- Martin G (1932) Chemical Engineering and Thermodynamics applied to cement rotary kiln. 51: 405-406.
Relevant Topics
- Advanced Photovoiltoic Cells
- Advanced Photovoiltoic Panels
- Advanced Photovoiltoic Systems
- Advanced Photovoiltoic Technologies
- Alternative Energy
- Biomass Energy
- Coal Energy
- Energy Management
- Environmental Policy
- Green Energy
- Hydro Electric Energy
- Hydrogen Energy
- Hydropower Energy
- Photovoltoics
- Renewable Energy and Research
- Renewable Geothermal Energy
Recommended Journals
Article Tools
Article Usage
- Total views: 7364
- [From(publication date):
specialissue-2017 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 6265
- PDF downloads : 1099