Research Article Open Access
Design and Evaluation of Three Immuno-based Assays for Rapid Detection of Human Norovirus Virus-like Particles
Jessica Jenkins Broglie1, Matthew D Moore2, Lee-Ann Jaykus2 and Liju Yang1*1Biomanufacturing Research Institute and Technology Enterprise (BRITE), Department of Pharmaceutical Sciences, North Carolina Central University, USA
2Department of Food, Bioprocessing and Nutrition Sciences, North Carolina State University, USA
- *Corresponding Author:
- Liju Yang
Biomanufacturing Research Institute and Technology Enterprise (BRITE)
Department of Pharmaceutical Sciences
North Carolina Central University
Durham, NC 27707, USA
Tel: +1-919-530-6704
E-mail: lyang@nccu.edu
Received date: October 20, 2014; Accepted date: November 13, 2014; Published date: November 17, 2014
Citation: Broglie JJ, Moore MD, Jaykus L, Yang L (2014) Design and Evaluation of Three Immuno-based Assays for Rapid Detection of Human Norovirus Virus-like Particles. J Anal Bioanal Tech 5:220 doi: 10.4172/2155-9872.1000220
Copyright: © 2014 Broglie JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
This study designed and evaluated three versatile immuno-based assays for the rapid detection of GI.1 norovirus virus-like particles at low (0-3.0 μg/mL) levels: 1) enzymatic absorbance-based ELISAs, 2) a fluorescentbased immunoassay, and 3) a “signal-down” capture ELISA. Variables including controllable variations in assay format (indirect or sandwich), assay time, binding sequence, and reporter molecule (fluorophore or enzyme) were thoroughly investigated and optimized in all three assays. Selectivity tests in the three-hour absorbance-based ELISA using VLPs representing two GI and two GII strains indicated the assays were selective to GI strains over GII strains. The three-hour enzymatic absorbance-based assay turned out to be a robust and rapid method capable of detecting GI.I VLPs in the range of 0.037 to 0.555 μg/mL, and the three-hour fluorescent immunoassay was capable of detecting VLPs in a high concentration range of 0.5-2.0 μg/mL under optimized conditions. The “signal-down” capture ELISA was conceptually demonstrated for the detection of VLPs at concentrations in excess of 1.0 μg/ mL, but did not appear to be suitable for quantifying VLPs under its current conditions. The methods reported here provide proof-of-concept that various ELISA-type approaches could be further developed to provide robust norovirus detection assays having various detection ranges, limits, and linearity.
Keywords
ELISA; Norovirus; Rapid detection methods; GI.1 viruslike particle; Viral detection methods; Absorbance; Fluorescence
Introduction
With at least 154 documented strains [1] and the ongoing emergence of new variants to overcome herd immunity [2,3], Human Norovirus (HuNoV) is the most common cause of nonbacterial, acute gastroenteritis outbreaks worldwide [1,4,5], accounting for more than 21 million illnesses and hospitalizations, and at least 570 deaths in the United States each year (Centers for Disease Control and Prevention, 2013). NoVs are single-stranded RNA, non-enveloped viruses in the Calicivirdae family. They are classified into six genogroups (GI to GIV) and further subclassified into genotypes based on their capsid sequence [1]. NoV GI.1 was the first isolated genotype and has been considered as the prototype virus of the genus, although NoV GII.4 is currently the most frequently detected genotype in humans [6]. This genetic diversity, combined with a low infectious dose of 18 particles or less [7], a myriad of foodborne and waterborne transmission routes, a lack of general population immunity [5,8], and an ability to survive for a few hours to several weeks without loss of infectivity on multiple environmental surfaces [9,10], leads to frequent epidemics of NoV in a variety of settings, including schools, military units [11] and installations [12,13], prisons [14], restaurants, airplanes [15,16], cruise ships, and hospitals. In addition, human-to-human viral transfer is common – symptomatic, asymptomatic, and healthy individuals are all capable of spreading HuNoV [17], greatly increasing the likelihood of widespread infection, especially among high-risk groups like young children, the elderly, and immunocompromised individuals [18,19]. However, currently, the lack of rapid and sensitive assays for use in clinical and point-of-care settings has impeded the progress in the control and mitigation of HuNoV infection [5] for meeting the growing need to predict and prevent norovirus outbreaks.
Current approaches for detecting norovirus in clinical and environmental samples utilize a combination of electron microscopy techniques [20-24], molecular detection assays [7,10,17,25-27] and immunological methods [25,28-33]. Each approach strives for maximizing sensitivity and specificity while minimizing the time for detection [34]. Electron microscopy enables direct visualization of viral particles, but the technique is considered ineffective as a diagnosis tool, as it lacks efficacy in detecting viral loads in complex matrices [35]. Molecular detection methods, namely polymerase chain reaction (PCR) and its real time (RT) variants, require skilled operators and specialized equipment [20]. Their low detection limits are ideal for screening the low viral loads common to contaminated foods and environmental samples [35], but the actual assay sensitivity and specificity largely depend on the efficacy of the concentration, purification, and nucleic acid extraction steps as well as primer selection and amplification conditions [8]. In most cases, RT-PCR assays are limited to presenceabsence testing, as residual matrix inhibitors often prevent accurate quantification of viral load [8]. Rapid detection methods like enzymelinked immunosorbant assays (ELISAs) are economical and facile alternatives to the more expensive and technically complex molecular detection assays and microscopy techniques [5,20,34]. ELISAs can analyze a myriad of pathological samples using multiple screening modes and reporter molecules, giving the assay broad versatility, but their need for high viral loads [5,27,30] limits the assays’ application mostly in clinical settings. Despite their relatively lower sensitivity when compared to PCR-based methods [5,20], ELISA-based assays have been used to detect norovirus in human stool samples [5,25,36- 43], human sera [29], and food samples [26,31,32,44,45].
Current commercial NoV detection kits are in principle analogous to ELISAs, usually coupling lateral flow immunochromatography with colorimetry. For example, the RIDA® QUICK Norovirus test (R-Biopharm AG) uses a mixture of anti-norovirus monoclonal antibodies to test for GI and GII noroviruses in human stool. Color variations at the test and control lines on the test strip confirm the presence or absence of the viruses, with high (96%) accuracy. The ImmunoCard STAT!® Norovirus (Meridian Bioscience, Inc.) and the SDBIOLINE Norovirus (Standard Diagnostics, Inc.) assays apply the same principle and use color changes at test and control lines to detect GI and GII HuNoV in human feces. ALL.DIAG’s NOROTOP+® test can simultaneously detect antigens in both genogroups with high (>90%) sensitivity and specificity. Although these kits can provide a rapid (15-30 min) preliminary test of presence or absence of HuNov, none of them can quantify viral load in suspect foods and environmental samples.
Using norovirus GI.1 (Norwalk) virus-like particles (VLPs) as a model viral system, the objective of this study was to develop and evaluate several ELISA-based assays for rapid detection of varying concentrations of GI.1 VLPs (0.037-3.7 μg/mL). HuNoV VLPs are replication-incompetent, macromolecular protein assemblies with capsid structures and antigenic properties resembling those of innate norovirus particles [46-48]. They have been used in many research laboratory studies as a model of HuNoV for developing detection methods or evaluating inactivation methods for HuNoV. In this study, we first designed a conventional three-day ELISA derived from a previously published protocol used for recognition of norovirus and VLPs using a peptide displaying bacterial phage [28]. In the designed ELISA method here, in place of the bacterial phage, monoclonal antibody to GI.I VLP (mAb 3901) was used for target recognition, which was a mechanistically different recognition process than the phage display. The final signal detection was realized by using a horseradish peroxidase (HRP)-conjugated secondary anti-mouse IgG antibody and its subsequent enzymatic reaction, leading to a product detectable by absorbance. Through the optimization of binding and blocking time, this ELISA was then modified so that it could be completed in three hours, effectively reducing the assay time without compromising assay sensitivity. To further simplify the three-hour assay, a fluorophore was used as an alternative reporter molecule, where an Alexa Fluor® 488-conjugated secondary anti-mouse IgG antibody was used in place of the HRP-conjugated secondary antibody. Lastly, a “signal-down” capture ELISA was designed, in which the mAb 3901 was used as a capture antibody and the HRP-conjugated secondary anti-mouse IgG antibody as the detection antibody, and the binding of VLP to mAb prevented the access of detection antibody to the mAb, resulting in a reduced absorbance signal. This study describes the assay designs, their optimization, and compared the performances of all three assay configurations.
Materials and Methods
VLPs, antibodies and chemicals
Stock solutions of GI.1 VLPs and monoclonal anti-GI.1 VLP antibody (mAb 3901) at 3.7 and 2.2 mg/mL, respectively, were obtained from Doctor Robert Atmar’s laboratory at the Baylor College of Medicine (Houston, TX). Stock solutions of GI.6 (2.8 mg/mL), GII.2 (4.3 mg/mL), and GII.4 (1.3 mg/mL) VLPs were also acquired from the Atmar laboratory and used for assay specificity tests. Goat anti-mouse IgG (H+L) antibody labeled with horseradish peroxidase (HRP) (0.5 mg/mL) was purchased from Abcam (Cambridge, MA). Goat anti-mouse IgG (H+L) antibody labeled with green-fluorescent Alexa Fluor® 488 dye (2.0 mg/mL) was purchased from Invitrogen (Life Technologies, Grand Island, NY). Phosphate buffered saline (PBS), pH 7.4, was prepared in-house from a 1X (0.01 M) PBS recipe (Cold Spring Harbor Protocols) using NaCl, KCl, Na2HPO4, and KH2PO4, all purchased from Fisher Scientific. TweenTM 20 and SuperBlock T20 (PBS) Blocking Buffer were purchased from Fisher Scientific and Thermo Scientific, respectively.
The conventional three-day ELISA
The initial three-day ELISA was derived from an ELISA for VLP detection originally developed at the Baylor College of Medicine [28], which coupled overnight VLP immobilization and colorimetric detection using a peptide-displaying M13 phage and HRP-labeled M13 phage antibody and an enzyme-linked reaction for absorbance measurement. In this three-day ELISA, we adopted the overnight immobilization of VLPs, but replaced the peptide-displaying bacterial phage with the anti-GI.1 VLP monoclonal antibody 3901 (mAb 3901), and prolonged the blocking time to overnight. Figure 1A illustrates the three-day ELISA developed in this study, in which sequential overnight immobilization of VLPs and overnight blocking were applied, and followed by a cascade reaction using mAb 3901 and HRP-labeled antimouse IgG antibody for developing absorbance measurements. The assay was performed in medium-binding 96-well polystyrene plates (CostarTM 3591; Corning Incorporated, Corning, NY) by directly coating the wells with 50 μL aliquots of GI.1 VLPs at concentrations ranging from 0 to 1 μg/mL (0.037, 0.185, 0.37, 0.555, 0.74, and 0.925 μg/mL) or from 0 to 3.7 μg/mL (0.037, 0.204, 0.47, 2.04, and 3.7 μg/mL) overnight at 4°C. Aliquots of 50 μL of 0.01 M PBS were used as blanks. Following the overnight coating, each well was washed with 100 μL of 0.01 M PBS twice and filled with 100 μL of SuperBlock T20 (PBS) Blocking Buffer (Thermo Scientific). After the overnight blocking at 4°C, each well was rinsed with 100 μL of 0.01 M PBS containing 0.05% (v/v) TweenTM 20 (PBS-T) twice, and then filled with 50 μL of 0.0002 mg/mL mAb 3901 anti-GI.1 VLP antibody solution and incubated for 60 min at 37°C. The wells were washed with 100 μL of PBS-T twice and loaded with 50 μL aliquots of HRP-labeled goat anti-mouse IgG antibody (0.0001 or 0.0002 mg/mL). Following another 60 min incubation at 37°C, each well was washed with 100 μL of PBS-T twice and further reacted with 100 μL of TMB (3,3’,5,5’-tetramethylbenzidine) Peroxidase Substrate Microwell Substrate System (KPL, Gaithersburg, MD), a soluble chromogenic substrate for the HRP enzyme. After a 10-min reaction at room temperature, 50 μL of Stop Solution (KPL, Gaithersburg, MD) was added to each well to stop the enzymatic reaction. The plate was immediately read at 450 nm using a SpectraMax® M5 microplate reader (Molecular Devices, Sunnyvale, CA) equipped with the SoftMax Pro 5.4 software package.
The three-hour ELISA
More rapid assays are an ongoing goal of immunological and molecular-based methods for detecting foodborne pathogens [34] because timely identification of the etiological agent can help mitigate both the financial impact and size of an outbreak [49]. The three-hour ELISA used the same principle as the three-day assay with the coating and blocking steps both modified to 1 h at room temperature (Figure 1A). Also, to improve VLP adhesion to the well bottom during the VLP binding step, the plate was agitated for 5 min using a Fisherbrand fixedspeed nutating shaker every 30 min. All concentrations and volumes of GI.1 VLP, mAb 3901 anti-GI.1 VLP antibody, and HRP-conjugated goat anti-mouse IgG antibody remained identical to the three-day method. The performance of the assay was compared to the three-day ELISA.
Figure 1: Schematic illustrating the binding sequence of virus-like particles (VLPs), mAb 3901 anti-GI.1 VLP antibody, and HRP-conjugated or Alexa Fluor® 488 goat anti-mouse antibody in the (A) three-day and three-hour ELISA, (B) three-hour immunoassay with fluorescence detection, and (C) “signal-down” capture ELISA methods.
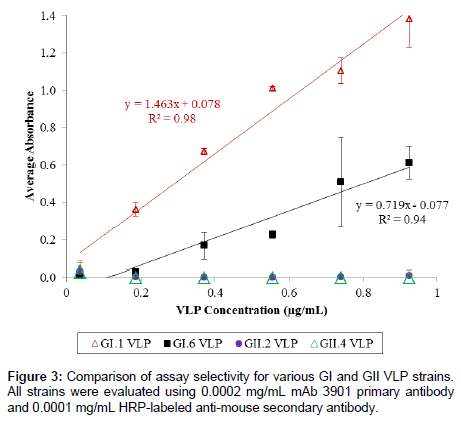
To test the selectivity of the ELISA assay, the absorbance signals of three other VLPs (representing GI.6, GII.2, and GII.4) were examined at six concentrations (0.037, 0.185, 0.370, 0.555, 0.740, and 0.925 μg/ mL) and compared to that of the GI.1 VLPs. The selectivity test was performed under the same conditions for all GI and GII strains, which used 0.0002 mg/mL mAb 3901 and 0.0001 mg/mL HRP-labeled secondary anti-mouse IgG antibody, and all other test conditions remained identical to the three-hour method for GI.1 VLP detection.
The three-hour immunoassay with fluorescence detection
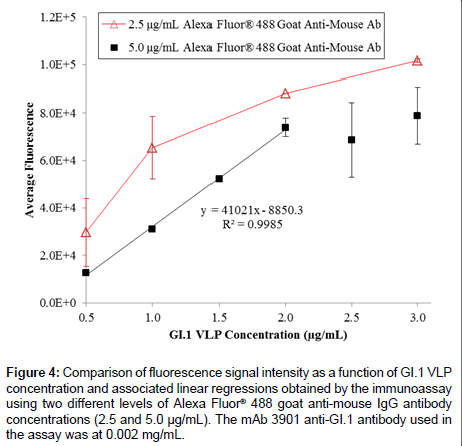
The fluorescence detection method was derived from the three-hour ELISA method by replacing the HRP-labeled anti-mouse secondary antibody with a fluorophore-labeled (Alexa Fluor® 488) anti-mouse IgG antibody, so that fluorescence intensity was immediately quantified following the addition of secondary antibody to the test wells. Figure 1B illustrates the detection principle. The assay was performed in CostarTM 3603 96-well black clear-bottom plates (Corning Incorporated, Corning, NY) by direct coating of GI.1 VLPs at concentrations ranging from 0 to 3 μg/mL (0, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 μg/mL). Opaque walls prevented crosstalk between samples in neighboring wells. Various concentrations (0, 0.002, 0.004, and 0.008 mg/mL) of mAb 3901 anti-GI.1 VLP antibody and Alexa Fluor® 488 goat anti-mouse IgG antibody at 2.0 or 5.0 μg/mL were tested to optimize the assay. Following a 1-hour incubation with the fluorophore-labeled secondary antibody, each well was rinsed with 100 μL of PBS-T solution twice, filled with 50 μL of 0.01M PBS, and read on a PHERAstar microplate reader (BMG LABTECH, Cary, NC) equipped with a 485/520 filter and the PHERAstar analytical software package.
The “signal-down” capture ELISA
Figure 1C illustrates the “signal-down” capture ELISA, in which overnight immobilization of mAb 3901 anti-GI.1 VLP antibody in the wells was followed by sequential capture of GI.1 VLPs and the reaction with HRP-labeled anti-mouse IgG antibody for developing enzymelinked absorbance measurement. The assay was performed by initially coating the wells of a sterile CostarTM 3591 96-well plate with 0.0002 mg/mL mAb 3901 anti-GI.1 VLP antibody solution overnight at 4°C. Following the coating, each well was rinsed with 100 μL of PBS-T twice and then filled with 50 μL of G1.1 VLPs ranging from 0 to 3.7 μg/mL (0.037, 0.204, 0.37, 2.04, and 3.7 μg/mL). The plate was incubated for 120 min at room temperature, with periodic rocking after 30 min using a 5/25 min (shaking/resting) cycle and a Fisherbrand fixed-speed nutating shaker to facilitate VLP adhesion to the immobilized primary antibody. Each well was rinsed with 100 μL of PBS-T twice to remove unbound VLPs and then filled with 50 μL of 0.0001 mg/mL HRPconjugated goat anti-mouse IgG antibody solution. Following a 60 min incubation at 37°C, all wells were washed with 100 μL of PBS-T twice and reacted with 100 μL of TMB solution for 10 min. Stop Solution (50 μL aliquots) was added to each well to quench the peroxidase reaction, and the plate was read immediately at 450 nm using the SpectraMax® M5 microplate reader.
Results and Discussion
The conventional three-day and three-hour ELISAs
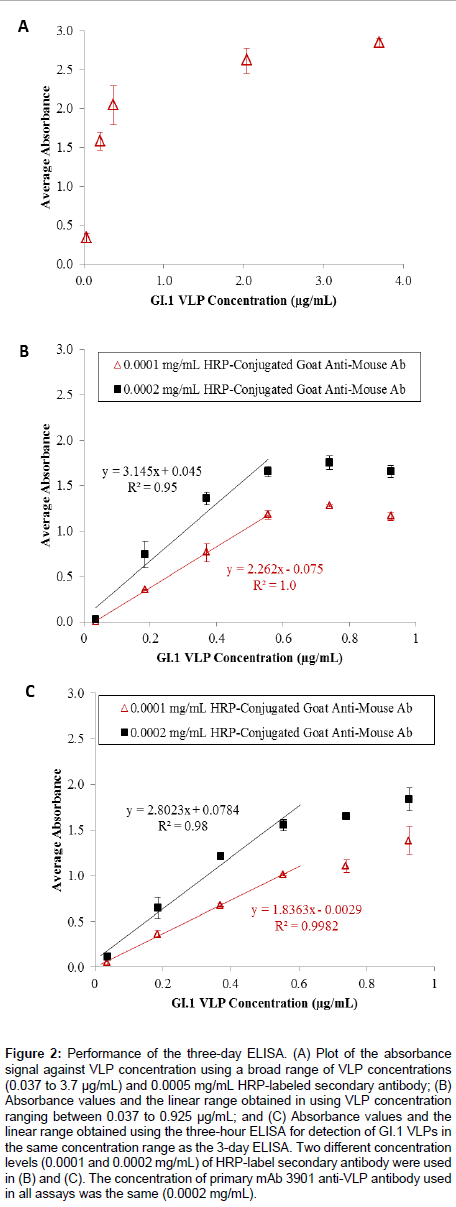
Figure 2A shows the results of the three-day ELISA for detecting GI.1 VLPs in a broad concentration range (from 0.037 to 3.7 μg/ mL). The absorbance signal continuously increased with increasing VLP concentration up to a concentration >1.0 μg/mL, after which the signal became saturated. This trend led to a closer examination of the absorbance signal in relation to VLP concentration in the low concentration range (<1.0 μg/mL). Figure 2B shows the results for detection of VLPs in a narrower concentration range (from 0.037 to 0.925 μg/mL) using two different concentrations of HRP-labeled antimouse IgG antibody (0.0001 and 0.0002 mg/mL). For both antibody levels, the absorbance signal continuously increased with increasing VLP concentration until it exceeded 0.555 μg/mL. Although the 0.0002 mg/mL secondary antibody concentration generated a greater signal for each evaluated VLP concentration, the overall linear range between the absorbance signal and the VLP concentration for both cases was the same (0.037 to 0.555 μg/mL), and the use of 0.0001 mg/mL secondary antibody solutions generated a better correlation between the absorbance signal and the VLP concentration (R2 = 1.0 vs. R2 = 0.95). The linear regression equations were y = 2.262 × - 0.075, and y = 3.145 x + 0.045, using the 0.0001 and 0.0002 mg/mL secondary antibody, respectively. The limits of detection (LOD) for the two assay conditions were calculated using LOD = x blank± 3σ , which were 0.153 μg/mL and 0.015 μg/mL, respectively. Though the linear ranges for both cases were sufficient for quantifying VLPs, the use of 0.0001 mg/mL secondary antibody solution appeared to be a more costefficient and material-saving assay.
Figure 2: Performance of the three-day ELISA. (A) Plot of the absorbance signal against VLP concentration using a broad range of VLP concentrations (0.037 to 3.7 μg/mL) and 0.0005 mg/mL HRP-labeled secondary antibody; (B) Absorbance values and the linear range obtained in using VLP concentration ranging between 0.037 to 0.925 μg/mL; and (C) Absorbance values and the linear range obtained using the three-hour ELISA for detection of GI.1 VLPs in the same concentration range as the 3-day ELISA. Two different concentration levels (0.0001 and 0.0002 mg/mL) of HRP-label secondary antibody were used in (B) and (C). The concentration of primary mAb 3901 anti-VLP antibody used in all assays was the same (0.0002 mg/mL).
Next, the investigation focused on reduction of the ELISA assay time to detection. By reducing the VLP immobilization time to 1 h at room temperature and the blocking time to 1 h at room temperature, the total assay time could be reduced to 3 h. Figure 2C shows the results of the three-hour ELISA for the detection of VLPs in the concentration range of 0.037 to 0.925 μg/mL, using 0.0001 and 0.0002 mg/mL secondary antibody levels. Similar to the three-day ELISA, the absorbance signal continuously increased with increasing VLP concentration for both secondary antibody concentrations, and the linear relationship between the absorbance signal and the VLP concentration was found in the range of 0.037 to 0.555 μg/mL The linear regression equations were y = 1.836 x - 0.0029 with R2 = 0.98, and y = 2.802 x + 0.0784 with R2 = 0.998, for 0.0001 and 0.0002 mg/mL secondary antibody levels, respectively. The detection limits were calculated to be 0.164 μg/mL when 0.0001 μg/mL secondary antibody was used, and 0.046 μg/mL when 0.0002 μg/mL secondary antibody was used. The results indicated both detection conditions allowed sufficient linearity and linear range for quantifying VLPs.
Comparison between the three-day and three-hour ELISAs showed that the magnitude of the absorbance signal for any given VLP concentration was slightly lower in the latter assay, but the overall linearity, the linear range, and the detection limits were not affected by the reduction of VLP coating time and blocking time. The three-hour ELISA assay effectively shortened the assay time, while still yielding comparable detection limits compared to the 3-day assay. The shorter assay was a robust and rapid method for detecting GI.1 VLPs in the low concentration range of 0.037 to 0.555 μg/mL. Other researchers have developed ELISAs for VLP detection using variable antigen ranges. Esseili and coworkers reported an assay for GII.4 VLP that can detect 0.05-2.50 μg (50 μL aliquots of 1, 5, 10, 20, and 50 μg/mL VLPs) [31], while Ghandi et al. [32] created an ELISA assay for GI.1 VLP that used 100 μg (100 μL aliquots of 1.0 μg/mL total protein) [32]. The GI.1 VLPbased ELISA developed at the Baylor College of Medicine – the basis for the assays developed in this study – used 0.63, 1.25, 2.5, and 5 μg in the initial antigen binding step [28]. Our results demonstrated that the three-hour ELISA is a sound alternative to the more time-intensive three-day method by reducing the antigen-binding and blocking steps without loss of assay sensitivity and detection range, and it is comparable to other ELISA methods reported in literature.
We further examined the selectivity of the assay using VLPs corresponding to three other HuNoV strains, including GI.6, GII.2, and GII.4. Figure 3 shows the results of the selectivity test for the 3-h ELISA. Although GI.6 VLPs could be detected at concentrations higher than 0.185 μg/mL and showed a linear relationship between absorbance signal and concentration, signal intensity for the GI.6 VLP was much lower (>50% less) than that of GI.1 VLPs. Both GII VLPs displayed no absorbance signals across the evaluated concentration range, suggesting the ELISA method was predominately selective for GI strains over GII strains. This was not an unexpected result considering that the selectivity of the ELISA method relied essentially on the specificity of mAb 3901, and in fact, a previous study has reported that mAb 3901 recognized a single epitope that was found in the majority of GI HuNoV but not GII strains [50].
The three-hour immunoassay with fluorescent detection
On the basis of the 3-h ELISA, we further investigated whether the use of a fluorophore as the reporter molecule on the secondary antibody could improve the assay performance. Figure 4 shows the results of GI.1 VLP detection in the range of 0.5 to 3.0 μg/mL using the three-hour immunoassay with fluorescent detection, using 0.002 mg/ mL mAb 3901, and two different concentrations Alexa Fluor® 488 goat anti-mouse antibody (2.5 and 5.0 μg/mL). The 2.5 μg/mL secondary antibody level unexpectedly generated a higher fluorescence signal at each VLP concentration than that using 5.0 μg/mL secondary antibody. This might be due to the higher molecule concentration in the 5.0 μg/ mL solution that actually inhibited mass transport of the secondary antibody to the surface-immobilized primary antibody, which has been observed in a previous study [51]. Although at both secondary antibody levels the fluorescence intensity increased with the increasing VLP concentration, only the 5.0 μg/mL secondary antibody level generated a sufficiently linear relationship across the tested VLP concentrations (ranging from 0.5 to 2.0 μg/mL); it also had a detection limit of 0.313 μg/mL. However, the fluorescent signal appeared to be saturated at high VLP concentrations (>2.0 μg/mL), which could be due to the selfquench phenomenon that commonly occurs in fluorescence detection at high concentrations of analyte.
Figure 4: Comparison of fluorescence signal intensity as a function of GI.1 VLP concentration and associated linear regressions obtained by the immunoassay using two different levels of Alexa Fluor® 488 goat anti-mouse IgG antibody concentrations (2.5 and 5.0 μg/mL). The mAb 3901 anti-GI.1 antibody used in the assay was at 0.002 mg/mL.
The “signal-down” capture ELISA
The “signal-down” capture ELISA detected VLPs by trapping them between the capture and detection antibodies, i.e. the mAb 3901 anti- VLP and the HRP-labeled anti-mouse IgG antibodies. In the absence of VLPs, the detection antibody freely binds to the capture antibody, yielding a high absorbance signal via the TMB-HRP enzymatic reaction. The presence of VLPs occupied some of the binding sites of the capture antibodies, reducing the binding of the detection antibody thus lowering the absorbance signal, which was expected to be correlated to the amount of captured VLPs. This design simplified the assay by reducing the number of requisite antigen-antibody and antibody-antibody interactions.
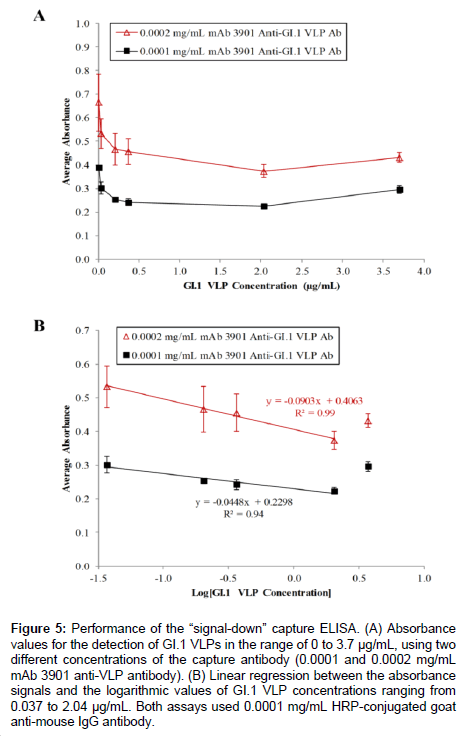
Figure 5A shows the results of the capture ELISA for the detection of GI.1 VLPs in the concentration range of 0 to 3.7 μg/mL using two concentration levels of capture antibody. At 0.0002 mg/mL mAb 3901 anti-GI.1 VLP antibody, the absorbance value steadily decreased from 0.664 ± 0.121 at 0 mg/mL GI.1 VLPs to 0.374 ± 0.028 at 2.04 μg/mL G1.1 VLPs (Figure 5A), confirming the assay worked as expected. The consecutive percentage changes in absorbance for VLPs at 0.037, 0.204, and 0.37 μg/mL were 19.7%, 12.6%, and 2.1%, respectively. The use of a lower concentration of capture antibody (0.0001 mg/mL mAb 3901 anti-GI.1 VLP) was expected to lower the baseline signal (in the absence of VLPs), thus to generate a greater percent reduction in absorbance signal at each evaluated VLP concentration. The results showed that, while the absorbance signal decreased as VLP concentration changed from 0 to 2.04 μg/mL, further validating the “signal-down” capture ELISA as a method for detecting VLPs, the consecutive percent changes in absorbance for VLPs at 0.037, 0.204, and 0.37 μg/mL were 22.8%, 15.9%, and 4.3%, respectively. As expected, these percentages were slightly greater compared to the results obtained using 0.0002 mg/ mL capture antibody. In both cases, the minor increase in absorbance value from 2.04 μg/mL GI.1 VLPs to 3.7 μg/mL G1.1 VLPs was possibly due to oversaturation of VLP binding during the capture step, leading to poor overall binding. The tests also showed no difference in the resulting signals when the blocking step was performed after capture antibody immobilization or after VLP capture.
Further analysis of the results demonstrated the linear relationship between the reduction in absorbance and the logarithmic value of VLP concentration in the range of 0.037 μg/mL to 2.04 μg/mL. As expected, the highest VLP concentration of 3.7 μg/mL fell outside the linear range for both primary antibody concentrations (Figure 5B), making reliable quantification implausible at high VLP concentrations due to oversaturation during the binding step. Regardless, the results yielded two linear regression equations in the detection of VLPs in the range of 0.037 to 2.04 μg/mL: y= -0.0903 x + 0.4063 with R2 = 0.99 and the detection limit of 14.4 μg/mL VLPs, when 0.0002 mg/mL capture antibody was used, and y = -0.0448 x + 0.2298 with R2 = 0.94 and the detection limit of 0.00058 μg/mL at 0.0001 mg/mL capture antibody level.
Figure 5: Performance of the “signal-down” capture ELISA. (A) Absorbance values for the detection of GI.1 VLPs in the range of 0 to 3.7 μg/mL, using two different concentrations of the capture antibody (0.0001 and 0.0002 mg/mL mAb 3901 anti-VLP antibody). (B) Linear regression between the absorbance signals and the logarithmic values of GI.1 VLP concentrations ranging from 0.037 to 2.04 μg/mL. Both assays used 0.0001 mg/mL HRP-conjugated goat anti-mouse IgG antibody.
Okame et al. [33] developed a cross-reactive sandwich ELISA for detection of multiple GI and GII strains with a similar lower detection limit (0.0024 μg/mL) [33], but their assay required five antibodies (four primaries and one labeled secondary). Conversely, the capture ELISA presented in this study needed only one primary (for capture) and one labeled secondary antibody (for detection), drastically reducing assay time and reagent need. On the whole, the “signal down” assay showed large standard deviations; a narrow range of absorbance change across the range of VLP concentrations tested (at 0.0001 mg/mL secondary antibody level); and poorer detection limits relative to the other assays. At this point, it would be a poor choice for quantitative detection of VLPs and should be considered as a “proof-of-concept” that may merit additional optimization.
Comparison of detection ranges and sensitivities
The results indicated that all assays were capable of detecting GI.1 VLPs, but each assay had different detection ranges and sensitivities (Table 1). Of all the ELISA-derived methods developed in this study, the 3-h ELISA appeared to be the best choice for rapid detection of GI VLPs in the low concentration range of 0.037 to 0.555 μg/mL, effectively providing more rapid detection without losing analytical sensitivity when compared to the 3-day assay. The use of the fluorescent reporter molecule yielded a sufficient linear relationship between the fluorescence signal and the VLP concentration in the range of 0.5 to 2.0 μg/mL, at the optimized test condition (0.002 mg/mL mAb3901 and 5.0 μg/mL). Velappan and coworkers showed that fluorescent and enzymatic immunosorbant assays were equally effective as plate screening methods [52]. In our study, although the 3-h traditional enzymatic ELISA showed greater analytical sensitivity, the fluorescent assay was able to detect a broader VLP concentration range. It may be that using a combination of the enzymatic and fluorescent-based immunoassays might result in combined low assay detection limits and wider detection concentration ranges. The “signal-down” capture ELISA was able to detect VLP but further optimization is necessary. For example, manipulating the orientation of the immobilized capture antibody might improve assay performance.
| Method | R2 Value | Linear Range (μg/mL) | Detection Limita (μg/mL) |
|---|---|---|---|
| Three-Day (3-d) ELISA | 0.95-1.0 | 0.037-0.555 | 0.153 (L1)b; 0.015 (L2)c |
| Three-Hour (3-h) ELISA | 0.98-0.998 | 0.037-0.555 | 0.164 (L1); 0.046 (L2) |
| Three-Hour (3-h) Fluorescent Immunoassay | 0.9985 | 0.5-2.0 | 0.313 (L2) |
| “Signal-Down” Capture ELISA | 0.94-0.99d | 0.037-2.040 | 0.00058 (L1); 14.4 (L2) |
aDetection limit was calculated using LOD = XBlank +3 σ with 99% confidence level.
bL1 denotes the lower primary (3-d, 3-h ELISA) or secondary (3-h immunoassay) antibody employed in the assay.
cL2 denotes the higher primary (3-d, 3-h ELISA) or secondary (3-immunoassay) antibody employed in the assay.
dR2 values were the coefficient between the absorbance signal and the logarithmic value of VLP concentration.
Table 1: Correlation Values, Ranges, and Limits.
The usefulness of an assay is significantly determined by its specificity and sensitivity for the target antigen [4]. The specificities of all the methods tested in this study were mechanistically dependent on the specificity of the primary antibody, which in this case was specific to GI but not GII HuNoV strains. It can be inferred that the specificity of the fluorescent assay and the “signal-down” capture ELISA would be similar to the 3-h ELISA, although studies are underway to confirm this. Of course, additional optimization could improve each method, and further validation is needed by examination of fecal extracts derived from HuNoV infected individuals and direct comparisons to other established methods.
Conclusion
In this study, three versatile immuno-based assays for rapid detection of GI.1 norovirus virus-like particles in the concentration range of 0-3.0 μg/mL were designed and evaluated. Controllable variations in assay format (indirect or sandwich), assay time, binding sequence, and reporter molecule (fluorophore or enzyme) were thoroughly investigated and optimized in all three assays. The study demonstrated that adjusting both the time and temperature of the antigen binding and blocking steps in enzymatic-based ELISA gave rise to rapid, reliable assays that could detect the same concentration range of VLPs (0.037 to 0.555 μg/mL) as a more time-intensive alternative. Use of a fluorophore instead of an enzyme labeled secondary antibody allowed the development of a robust method for quickly detecting a broad range of higher VLP concentrations (0.5 to 2.0 μg/mL). While in need of additional optimization, a “signal-down” approach was also demonstrated. This developmental work adds to the knowledge needed to develop rapid and sensitive assays for HuNoV detection in clinical and point-of-care settings [5]. Eventually, such assays may also be relevant to detection of HuNoV in contaminated food and water. Such methods may ultimately contribute to the reduction of the HuNoV disease burden.
Acknowledgements
This study was supported by Agriculture and Food Research Initiative Competitive Grant no. 2011-68003-30395 from the USDA National Institute of Food and Agriculture (NIFA). The authors are grateful to Doctor Robert Atmar at the Baylor College of Medicine for generously providing the VLPs and anti-GI.1 VLP antibody for this study, to Doctor Soo Hwan (Caleb) Suh and Mrs. Audrey Adcock for their assistance with ELISA optimization, and to Brittany Mertens, and Drs. Blanca Escudero-Abarca and Orlin Velev for critical review of this study.
References
- Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, et al. (2006) Norovirus classification and proposed strain nomenclature. Virology 346: 312-323.
- Cannon JL, Lindesmith LC, Donaldson EF, Saxe L, Baric RS, et al. (2009) Herd immunity to GII.4 noroviruses is supported by outbreak patient sera. J Virol 83: 5363-5374.
- Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, et al. (2008) Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med 5: e31.
- Patel MM, Hall AJ, Vinjé J, Parashar UD (2009) Noroviruses: a comprehensive review. J Clin Virol 44: 1-8.
- Hall AJ, Vinjé J, Lopman B, Park GW, Yen C, et al. (2011) Updated norovirus outbreak management and disease prevention guidelines. MMWR 60(RR03): 1-15.
- Glass RI, Parashar UD, Estes MK (2009) Norovirus gastroenteritis. N Engl J Med 361: 1776-1785.
- Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, et al. (2008) Norwalk virus: how infectious is it? J Med Virol 80: 1468-1476.
- Jaykus L, D’Souza D, Moe C (2013) Foodborne Viral Pathogens. In: Doyle MP, Buchanan RK (ed) Food Microbiology: Fundamentals and Frontiers, 4th edition, American Society for Microbiology Press, 619-649.
- Liu B, Chien Y-W, Papafragkuo E, Hsiao H-M, Jaykus L, et al. (2009) Persistence of human noroviruses on food preparation surfaces and human hands. Food Environ Virol 1: 141-147.
- D'Souza DH, Sair A, Williams K, Papafragkou E, Jean J, et al. (2006) Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol 108: 84-91.
- Thornton SA, Sherman SS, Farkas T, Zhong W, Torres P, et al. (2005) Gastroenteritis in US Marines during Operation Iraqi Freedom. Clin Infect Dis 40: 519-525.
- Wadl M, Scherer K, Nielsen S, Diedrich S, Ellerbroek L, et al. (2010) Food-borne norovirus-outbreak at a military base, Germany, 2009. BMC Infect Dis 10: 30.
- Yap J, Qadir A, Liu I, Loh J, Tan BH, et al. (2012) Outbreak of acute norovirus gastroenteritis in a military facility in Singapore: a public health perspective. Singapore Med J 53: 249-254.
- Public Health England (2014) Summary of surveillance of norovirus and rotavirus. PHE Monthly National Report 10 July: 1-8.
- Kirking HL, Cortes J, Burrer S, Hall AJ, Cohen NJ, et al. (2010) Likely transmission of norovirus on an airplane, October 2008. Clin Infect Dis 50: 1216-1221.
- Kornylo K, Kim DK, Widdowson MA, Turabelidze G, Averhoff FM (2009) Risk of norovirus transmission during air travel. J Travel Med 16: 349-351.
- Liu P, Escudero B, Jaykus LA, Montes J, Goulter RM, et al. (2013) Laboratory evidence of norwalk virus contamination on the hands of infected individuals. Appl Environ Microbiol 79: 7875-7881.
- Koo HL, DuPont HL (2009) Noroviruses as a potential cause of protracted and lethal disease in immunocompromised patients. Clin Infect Dis 49: 1069-1071.
- Green KY (2014) Norovirus infection in immunocompromised hosts. Clin Microbiol Infect 20: 717-723.
- Rabenau HF, Stürmer M, Buxbaum S, Walczok A, Preiser W, et al. (2003) Laboratory diagnosis of norovirus: which method is the best? Intervirology 46: 232-238.
- Escudero-Abarca BI, Rawsthorne H, Goulter RM, Suh SH, Jaykus LA (2014) Molecular methods used to estimate thermal inactivation of a prototype human norovirus: more heat resistant than previously believed? Food Microbiol 41: 91-95.
- Straub TM, Höner zu Bentrup K, Orosz-Coghlan P, Dohnalkova A, Mayer BK, et al. (2007) In vitro cell culture infectivity assay for human noroviruses. Emerg Infect Dis 13: 396-403.
- Cheetham S, Souza M, Meulia T, Grimes S, Han MG, et al. (2006) Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J Virol 80: 10372-10381.
- Otto PH, Clarke IN, Lambden PR, Salim O, Reetz J, et al. (2011) Infection of calves with bovine norovirus GIII.1 strain Jena virus: an experimental model to study the pathogenesis of norovirus infection. J Virol 85: 12013-12021.
- Kele B, Lengyel G, Deak J (2011) Comparison of an ELISA and two reverse transcription polymerase chain reaction methods for norovirus detection. Diagn Microbiol Infect Dis 70: 475-478.
- El-Senousy WM, Costafreda MI, Pintó RM, Bosch A (2013) Method validation for norovirus detection in naturally contaminated irrigation water and fresh produce. Int J Food Microbiol 167: 74-79.
- Gilpatrick SG, Schwab KJ, Estes MK, Atmar RL (2000) Development of an immunomagnetic capture reverse transcription-PCR assay for the detection of Norwalk virus. J Virol Methods 90: 69-78.
- Rogers JD, Ajami NJ, Fryszczyn BG, Estes MK, Atmar RL, et al. (2013) Identification and characterization of a peptide affinity reagent for detection of noroviruses in clinical samples. J Clin Microbiol 51: 1803-1808.
- Brinker JP, Blacklow NR, Estes MK, Moe CL, Schwab KJ, et al. (1998) Detection of Norwalk virus and other genogroup 1 human caliciviruses by a monoclonal antibody, recombinant-antigen-based immunoglobulin M capture enzyme immunoassay. J Clin Microbiol 36: 1064-1069.
- Hirneisen KA, Kniel KE (2012) Comparison of ELISA attachment and infectivity assays for murine norovirus. J Virol Methods 186: 14-20.
- Esseili MA, Wang Q, Saif LJ (2012) Binding of human GII.4 norovirus virus-like particles to carbohydrates of romaine lettuce leaf cell wall materials. Appl Environ Microbiol 78: 786-794.
- Gandhi KM, Mandrell RE, Tian P (2010) Binding of virus-like particles of Norwalk virus to romaine lettuce veins. Appl Environ Microbiol 76: 7997-8003.
- Okame M, Shiota T, Hansman G, Takagi M, Yagyu F, et al. (2007) Anti-norovirus polyclonal antibody and its potential for development of an antigen-ELISA. J Med Virol 79: 1180-1186.
- Dwivedi HP, Jaykus LA (2011) Detection of pathogens in foods: the current state-of-the-art and future directions. Crit Rev Microbiol 37: 40-63.
- Knight A, Li D, Uyttendaele M, Jaykus LA (2013) A critical review of methods for detecting human noroviruses and predicting their infectivity. Crit Rev Microbiol 39: 295-309.
- Okitsu-Negishi S, Okame M, Shimizu Y, Phan TG, Tomaru T, et al. (2006) Detection of norovirus antigens from recombinant virus-like particles and stool samples by a commercial norovirus enzyme-linked immunosorbent assay kit. J Clin Microbiol 44: 3784-3786.
- Burton-MacLeod JA, Kane EM, Beard RS, Hadley LA, Glass RI, et al. (2004) Evaluation and comparison of two commercial enzyme-linked immunosorbent assay kits for detection of antigenically diverse human noroviruses in stool samples. J Clin Microbiol 42: 2587-2595.
- de Bruin E, Duizer E, Vennema H, Koopmans MP (2006) Diagnosis of Norovirus outbreaks by commercial ELISA or RT-PCR. J Virol Methods 137: 259-264.
- Dimitriadis A, Marshall JA (2005) Evaluation of a commercial enzyme immunoassay for detection of norovirus in outbreak specimens. Eur J Clin Microbiol Infect Dis 24: 615-618.
- Richards AF, Lopman B, Gunn A, Curry A, Ellis D, et al. (2003) Evaluation of a commercial ELISA for detecting Norwalk-like virus antigen in faeces. J Clin Virol 26: 109-115.
- Morillo SG, Luchs A, Cilli A, Ribeiro CD, Calux SJ, et al. (2011) Norovirus 3rd Generation kit: an improvement for rapid diagnosis of sporadic gastroenteritis cases and valuable for outbreak detection. J Virol Methods 173: 13-16.
- Wilhelmi de Cal I, Revilla A, del Alamo JM, Román E, Moreno S, et al. (2007) Evaluation of two commercial enzyme immunoassays for the detection of norovirus in faecal samples from hospitalised children with sporadic acute gastroenteritis. Clin Microbiol Infect 13: 341-343.
- Costantini V, Grenz L, Fritzinger A, Lewis D, Biggs C, et al. (2010) Diagnostic accuracy and analytical sensitivity of IDEIA Norovirus assay for routine screening of human norovirus. J Clin Microbiol 48: 2770-2778.
- Tian P, Bates AH, Jensen HM, Mandrell RE (2006) Norovirus binds to blood group A-like antigens in oyster gastrointestinal cells. Lett Appl Microbiol 43: 645-651.
- Tian P, Engelbrektson AL, Jiang X, Zhong WM, Mandrell RE (2007) Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: a possible mechanism of bioaccumulation. J Food Prot 70: 2140-2147.
- Pumpens P, Grens E (2002) Artificial genes for chimeric virus-like particles. In Khudyakov YE, Fields HA (ed) Artificial DNA: Methods and Applications, CRC Press, 249-327.
- Pattenden LK, Middelberg AP, Niebert M, Lipin DI (2005) Towards the preparative and large-scale precision manufacture of virus-like particles. Trends Biotechnol 23: 523-529.
- Herbst-Kralovetz M, Mason HS, Chen Q (2010) Norwalk virus-like particles as vaccines. Expert Rev Vaccines 9: 299-307.
- Lopman BA, Reacher MH, Vipond IB, Hill D, Perry C, et al. (2004) Epidemiology and cost of nosocomial gastroenteritis, Avon, England, 2002-2003. Emerg Infect Dis 10: 1827-1834.
- Hale AD, Tanaka TN, Kitamoto N, Ciarlet M, Jiang X, et al. (2000) Identification of an epitope common to genogroup 1 "norwalk-like viruses". J Clin Microbiol 38: 1656-1660.
- Karlsson R, Fägerstam L, Nilshans H, Persson B (1993) Analysis of active antibody concentration. Separation of affinity and concentration parameters. J Immunol Methods 166: 75-84.
- Velappan N, Clements J, Kiss C, Valero-Aracama R, Pavlik P, et al. (2008) Fluorescence linked immunosorbant assays using microtiter plates. J Immunol Methods 336: 135-141.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15938
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11316
- PDF downloads : 4622