Design and Evaluation of Naproxen Proliposomal Gels
Received: 01-Nov-2021 / Accepted Date: 22-Nov-2021 / Published Date: 29-Nov-2021
Abstract
The study was aimed to develop a proliposomal formulation for anti-inflammatory drug Naproxen. Proliposomes with various concentrations of mannitol, phospholipid and cholesterol were prepared using thin film hydration technique (vacuum rotatory evaporator). The optimization of proliposomal formulation was achieved based on Average size, Entrapment efficiency and drug content. The proliposomal formulation was incorporated in gel (carbopol) and characterized for their rheology and in-vitro drug release studies. The results of formulations revealed that maximum entrapment efficiency was dependent on phospholipid and cholesterol concentration. Rheological studies revealed that the proliposome formulation containing 2% w/w carbopol is stable for topical drug delivery. The in-vitro studies revealed that proliposomal gel formulation exhibits increased skin permeation showing sustain release when compared to that of pure drug.
Keywords
Proliposomes; Naproxen; Film hydration; Antiinflamatory; Rhelogocal studies
Introduction
In the past few decades, considerable attention has been focused on the development of new drug delivery system (NDDS). The NDDS should ideally fulfill two prerequisites. Firstly, it should deliver the drug at a rate directed by the needs of the body, over the period of treatment. Secondly, it should channel the active entity to the site of action. Conventional dosage forms including prolonged release dosage forms are unable to meet none of these. At present, no available drug delivery system behaves ideally, but sincere attempts have been made to achieve them through various novel approaches in drug delivery [1].
Novel drug delivery system aims at providing some control, whether this is of temporal or spatial nature or both, of drug release in the body. In recent years, vesicles have become the vehicle of choice in drug delivery. Lipid vesicles were found to be of value in immunology, membrane biology, diagnostic techniques, and most recently, genetic engineering [2-4]. Vesicles can play a major role in modeling biological membranes, and in the transport and targeting of active agents. Encapsulation of a drug in vesicular structures can be predicted to prolong the existence of the drug in systemic circulation and perhaps, reduces the toxicity if selective uptake can be achieved [5]. The phagocytic uptake of the systemic delivery of the drug loaded vesicular delivery system provides an efficient method for delivery of drug directly to the site of infection, leading to reduction of drug toxicity with no adverse effects.
Naproxen is a non-steroidal anti-inflammatory drug with strong analgesic and lesser action on inflammation and pyrexia (fever). Naproxen half-life is 2-5 hours [6]. Naproxen is well absorbed after oral administration peak plasma concentration is usually achieved within 2-3 hours after its oral administration. It is highly (99%) bound to plasma protein and hence it need to be administered 2-3 times a day. Hence, there is every need for formulating a sustained release form for Naproxen to improve its therapeutic efficacy and patient compliance.
Materials and Methods
Naproxen was obtained as a gift sample from Divis Laboratories, Hyderabad. Phosphotidyl Choline, Mannitol, Cholestrol, Mannitol, Carbopol 934 were purchased from SD Fine chemicals, Mumabai. All other chemicals and reagents used were analytical grade.
Preparation of naproxen loaded proliposomes
The proliposomes containing naproxen was prepared by film deposition on carrier method using vacuum rotary evaporator. The naproxen proliposomes was done by preparing the different formulations by varying the concentration of phosphatidyl choline and cholesterol. Required quantity of mannitol was placed in 100ml round bottom flask which was held at 60-70 temperature and the flask rotated at 80-90 rpm for 30 min under vacuum. After complete drying the temperature of water bath was lowered to 20-30℃. Naproxen, Phosphotidyl Choline and Cholesterol were dissolved in mixture of organic solvents (chloroform: methanol, 6:4, v/v) and 5ml of aliquot of organic solution was slowly introduced into the flask via the solvent inlet tube. After complete drying second aliquot (5ml) was introduced. After complete dying, the vacuum was released and proliposomes were placed in a desiccator overnight and then sieved with 100 mesh. The collected powder was transferred into a glass bottle and stored at the freeze temperature (Table 1) [7].
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Naproxen(mg) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Phosphotidyl Choline (mg) | 100 | 100 | 150 | 150 | 50 | 150 | 100 | 50 | 50 |
| Cholesterol | 150 | 100 | 50 | 100 | 100 | 150 | 50 | 50 | 150 |
| Mannitol (g) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Chloroform(ml) | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Methanol(ml) | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
Table 1: Formulation Design.
Preparation of carbopol gel base
2gm of carbopol 934 was weighed and dispersed in distilled water. Then, propylene glycol was added and the mixture was neutralised by drop wise addition of 1% triethanolamine. Mixing was continued until the transparent gel was obtained and allowed to swell for 24 hours. Similarly 2% and 3% carbopol gels were prepared.
Preparation of proliposomal gels
Proliposomes containing naproxen(separated from the unentrapped drug) were mixed into the 2% carbopol gel by using mortar and pestle, the concentration of proliposomes in the gel being 1%. All optimized formulations were incorporated into different carbopol gels (1% and 3%).
Characterization of proliposomes
Vesicle size and count: Average size and size distribution proliposomes was determined using optical microscope. A drop of distilled water was added to proliposome granules on a glass slide without a cover slip, and the process of liposome formulation was observed using optical microscope with 100X magnification. Size of liposomal vesicles was measured at different locations on the slide. From the obtained results size distribution and average size of liposome vesicles was determined.
Surface morphology: The surface morphology of proliposomes and plain mannitol particles were examined by scanning electron microscopy (SEM) after coating with gold. After goldcoating of proliposome and plain mannitol particles, their surface morphology was viewed and photographed.
Drug content: Naproxen content in proliposomes was assayed by an UV-visible spectrophotometer. Proliposomes (100mg) were dissolved in 10ml methanol by shaking the mixture for 5 mins. One ml of the resultant solution was taken and diluted to 10ml with methanol. Then, aliquots were withdrawn and absorbance was recorded at 323 nm using UV-visible spectrophotometer (Lab India 3200).
Entrapment efficiency: Separation of unentrapped drug from the liposomal suspension was done by centrifugation method. The entrapment efficiency of proliposomes was determined after hydration with distilled water. 10ml of phosphate buffer (pH 7.4) was added to proliposomes granules and then subjected to sonicate for 10 mins using ultra sonicator (Citizen, India). The liposomal suspension was subjected to centrifugation on a cooling centrifuge (REMI TR-01) at 15000rpm for 30 mins for the separation of unentrapped drug. The clear supernatant (1ml) was taken and diluted to 10ml with buffer and absorbance was recorded at 323 nm using UV-visible spectrophotometer (Lab India 3200). Then calculate the percentage drug in the each formulation.
Entrapment Efficiency ={(Ct-Cf)÷Ct }×100
Ct – concentration of total drug
Cf – concentratin of free drug
Yield of proliposomes: After complete drying the proliposome powders were collected and weighed accurately. The yield of proliposomes was calculated using the formula [8].
Percentage Yield= Total weight of proliposomes/ Total weight of drug+Weight of added materials*100
Characterization of gel
Gel base was evaluated for following parameters for both plain gel and gel loaded with proliposomes.
Physical appearance: All prepared proliposomal gel formulations have been observed for their visual appearance, such as transparency, colour, texture, grittiness, greasiness, stickiness, smoothness, stiffness, tackiness and clarity was determined by using clarity chamber with black and white background.
pH of formulation: pH measurement of the gel was carried out of the formulation was measured by using a digital pH meter (Lab India SAB 5000), dipping the glass electrode completely into the gel system. The observed pH values were recorded for all formulations (F1-F9) in triplicates.
Rheological properties
The rheological properties of prepared gels were estimated using a Brookfield viscometer. Sample holder of the Brookfield viscometer was filled with the gel sample, and then spindle was inserted into sample holder. The spindle was rotated at 100 rpm. All the rheological studies were carried out at room temperature. A viscosity measurement was done in triplicate. Viscosity of 1, 2 and 3% carbopol gel was determined and selected the optimized formulation.
Drug Content
For determination of drug content, accurately weighed quantity (1gm) of gel equivalent to 10 mg of naproxen was dissolved in phosphate buffer (PH 7.4) and analyzed by UV-Vis Spectrophotometer at 323 nm and the drug content was calculated.
In vitro studies
Franz diffusion cell was used for the in vitro drug release studies. Semi permeable membrane was placed between donar and receptor chamber of diffusion cell. Receptor chamber was filled with freshly prepared 30ml 7.4 PH phosphate buffer. Proliposomal gel equivalent to 1gm was placed on semi permeable membrane. The franz diffusion cell was placed over magnetic stirrer with 500rpm and temperature was maintained at 37±10C. 5ml of samples were withdrawn periodically and replaced with fresh buffer. The withdrawn samples were periodically diluted and analysed for drug content using UV visible spectrophotometer (Lab India 3200) at 323 nm.
Results and Discussion
In a preformulation study the optimum concentrations of mannitol, phospholipid and cholesterol was determined to obtain stable liposomes devoid of aggregation, fusion and sedimentation. Naproxen proliposomes was prepared using thin film hydration technique and method was found to be well suited for the production of liposomes without aggregation. Amount of mannitol, phospholipid and cholesterol was found to be very critical in the preparation and stabilization of proliposomes.
The most important parameter, which needs to monitor during proliposome preparation for its best performance, is the vesicle size and size distribution of liposomes. Several reports, showed the effect of liposome size on the drug release as well as drug deposition in the skin.
A positive correlation was observed for both variables phospholipid and cholesterol in case of liposome vesicle size. Thus, with increase in the concentration of phospholipid and cholesterol vesicle size was found to be increased. Proliposomal sample was placed under digital microscope (Metzer, India) and hydrated with water. Then formation of vesicle was observed within the liposomal dispersion. Results of average vesicle size and distribution were calculated for count and distribution.
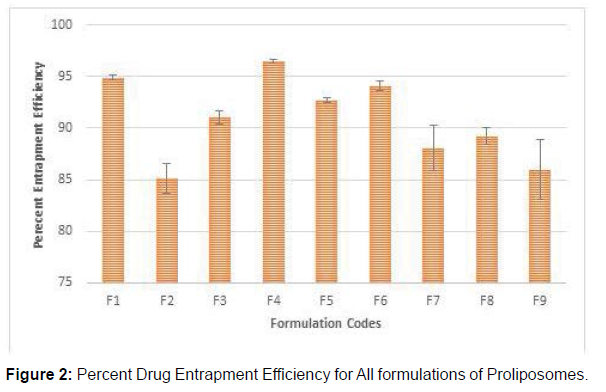
Entrapment efficiency is an important parameter in case of liposomes as it majorly effects the drug release and skin deposition. Results show that with increase in the concentration of phospholipid and cholesterol entrapment efficiency found to be increased. In the present study, the observed entrapment efficiency for all batches of Naproxen proliposome formulation was in the range of 85.12 to 96.5% were tabulated in table no.2. Among all Naproxen proliposomal formulations F1-F9 had maximum vesicle size and entrapment efficiency which were selected for the further study (Table 2) (Figures 1 and 2).
| S.No. | Formulation Code | Avg particle size for 100 Particles | Drug Content | Percentage yield | Entrapment Efficiency |
|---|---|---|---|---|---|
| 1 | F1 | 5.34 ± 0.023 | 95.03 ± 0.543 | 93.4 ± 0.324 | 94.9 ± 0.244 |
| 2 | F2 | 4.43 ± 0.123 | 86.4 ± 0.734 | 90.7 ± 0.534 | 85.12 ± 1.48 |
| 3 | F3 | 2.65 ± 0.076 | 93.7 ± 0.664 | 89.5 ± 0.654 | 91.02 ± 0.613 |
| 4 | F4 | 6.06 ± 0.012 | 96.8 ± 0.249 | 95.4 ± 0.123 | 96.5 ± 0.205 |
| 5 | F5 | 4.34 ± 0.231 | 94.7 ± 0.984 | 94.3 ± 0.221 | 92.7 ± 0.249 |
| 6 | F6 | 5.12 ± 0.167 | 94.8 ± 0.860 | 94.8 ± 0.212 | 94.1 ± 0.509 |
| 7 | F7 | 3.21 ± 0.221 | 92.4 ± 1.70 | 88.7 ± 0.321 | 88.1 ± 2.19 |
| 8 | F8 | 2.69 ± 0.148 | 90.6 ± 0.748 | 89.2 ± 0.817 | 89.2 ± 0.817 |
| 9 | F9 | 2.34 ± 0.321 | 87.5 ± 0.953 | 86.5 ± 0.265 | 86.02 ± 2.90 |
Table 2: Average Particle Size distribution of Proliposomes.
The Naproxen content in the proliposomes were observed in the range of 86.4% to 96.8% at various drug to phospholipid ratios. From the above results it is concluded that F4, F1, F5 and F6 formulations showed maximum drug content when compare to other formulations. The percent yield of formulations was found to be increase with increase in phospholipid concentration. The results of % yield of various formulations were found to be in the range of 86.5 ± 0.265 to 95.4 ± 0.221% as the drug to phospholipid ratio in proliposomes was changed.
Viscosity measurement
Rheological studies revealed that 2% carbopol gel showing better rheological properties when compared to 1% and 3% carbopol gels. So 2% carbopol was used for preparation of proliposomal gel. Viscosity of the gel was measured by Brookfield viscometer. Viscosity of proliposomal gel showed 1156cps at 100rpm.
pH measurement
The PH of the developed formulation was in accordance with human skin pH rendering them more acceptable. Therefore formulated Proliposomal gel was suitable for topical application. The pH values of prepared Proliposomal gels were within the limits of 5.5 to 5.8.
Drug content (%)
The prepared Naproxen proliposomal gel was subjected to drug content uniformity and it was found to 98.55 % which indicated the drug uniformly dispersed throughout the formulation.
In vitro drug release
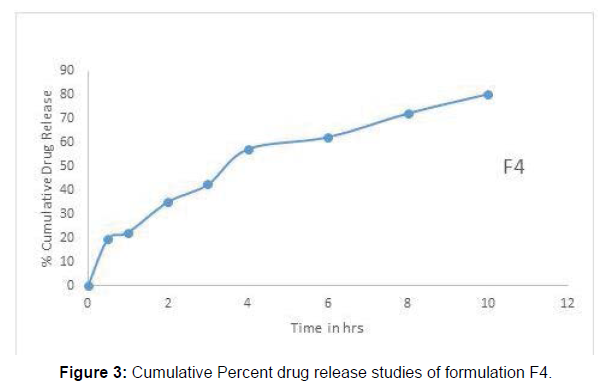
The result of In vitro release of naproxen from the gel formulation clearly shows that the gels have ability to retain the drug for prolonged periods. The % CDR of proliposomal gel formulation F4 was found to be 80.5 % as shown in Fig. and which follows Higuchi model. The ‘n’ values for all the formulation were found to be more than 0.5. This indicates that the release approximates nonFickian diffusion mechanism (Figure 3).
Summary and Conclusion
In this study, it was found that as the phospholipid concentration was increased, it resulted in corresponding increase in the entrapment efficiency of reconstituted liposomes. The ingredient for longer period of time, which shows the sustained release behaviour of formulations. Phospholipid-rich domains of vesicle might have helped to enhance the percent entrapment of lipophilic drug molecule like Naproxen in lipid bilayer, which indicate that entrapment of Naproxen in reconstituted liposome was found to be dependents mainly on the lipid concentration. Increase in lipid concentration in proliposome was also able to control the release of the active Naproxen.
In conclusion, a sustained delivery of Naproxen can be achieved by proliposomal drug delivery system. Phospholipids, being the major component of liposomal system, can easily get integrated with the skin lipids and maintain the desired hydration conditions to improve drug permeation. Fusion of lipid vesicles with skin contributed to the permeation enhancement effect. The phospholipid was found to have a significant influence on the lipid matrix of the stratum corneum, suggesting a disruption of the intercellular lipid lamellar structure and act as penetration enhancer. Hence as the phospholipid concentration was increased, it would increase the permeation of drug following application on the skin. The free flowing properties of the proliposomes granules will be beneficial in formulating the proliposomes as a solid dosage form. In-vitro studies concluded that enhanced skin permeation and retention of Naproxen was observed and was due to lipo-solubilized state of drugs within proliposomes which helped to produce the depot effect.
Acknowledgement
The author was thankful to the principal and management for providing chemicals and premises for the carrying out of the research work.
References
- Robinson JR, Lee VHL (1987) Controlled Drug Delivery: Fundamentals and Applications. Marcel Dekker, Inc 7.
- Ogihara-Umeda I, Sasaki T, Toyama H, Oda K, Senda M, et al. (1997) Rapid Diagnostic Imaging of Cancer using Radiolabeled Liposomes. Cancer Detect Prev 21:490.
- Park JW, Hong K, Kirpotin DB, Papahadjopoulos D, Benz CC (1997) Immunoliposomes for Cancer Treatment. Adv Pharmacol 40:399.
- Kao GY, Change LJ, Allen TM (1996) Use of Targeted Cationic Liposomes in Enhanced DNA Delivery to Cancer Cells. Cancer Gene Therapy 3:250-256.
- Todd JA, Modest EJ, Rossow PW, Tökés ZA (1982) Liposome Encapsulation Enhancement of Methotrexate Sensitivity in a Transport Resistant Human Leukemic Cell Line. Biochem Pharmacol 31:541-546.
- Deo MR, Sant VP, Parekh SR, Khopade AJ, Banakar UV (1997) Proliposome-Based Transdermal Delivery of Levonorgestrel. J Biomat appl 12:77-88.
- Gaikwad VL, Yadav VD, Dhavale RP, Choudhari PB, Jadhav S (2012) Effect of Carbopol 934 and 940 on Fluconazole Release from Topical Gel. Curr Pharma Res 2:487-493.
Citation: Suryam G, Desu PK, Kishore B (2021) Design and Evaluation of Naproxen Proliposomal Gels. Clin Pharmacol Biopharm, 10: 240.
Copyright: © 2021 Suryam G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1612
- [From(publication date): 0-2021 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 1140
- PDF downloads: 472