Depressive Symptoms Exacerbate the Effects of HSV-1 Infection on Cognitive Function in Middle Age
Received: 06-Jan-2018 / Accepted Date: 15-Jan-2018 / Published Date: 19-Jan-2018 DOI: 10.4172/2476-213X.1000122
Abstract
Objective: The purpose of this article is to examine how psychosocial distress and HSV-1 might interact to foster early cognitive vulnerability in otherwise healthy middle-aged adults. Several environmental risk factors, including mental stress and chronic viral infections, can increase cognitive vulnerability and lead to cognitive decline. Considering the anticipated dramatic increase in the number of older adults with dementia in the next 40 years and the current lack of dementia cures, it is imperative that we explore any modifiable risk factors for brain vulnerability that may facilitate the development of interventions to prevent or delay the onset of severe cognitive impairment.
Methods: A total of 113 participants, 63 female and 50 male, were recruited for this study. 15 cc of blood was obtained by venipuncture from consenting volunteers and screened with the ELISA tests to assess seropositivity for HSV-1 IgG antibodies. Cognitive vulnerability was operationalized as lower cognitive function across any of the following 3 domains: global, memory, executive function. The Beck Depression Inventory (BDI-II) was used to assess depressive symptoms.
Results: After controlling for age, gender, and education, there was a significant main effect for HSV-1, F(1,106)=7.908, p=0.01, but not for depressive symptoms, on global cognition. However, this main effect was qualified by a statistically significant interaction between the factors, F(1,106)=5.046, p=0.03. No significant main effects or interactions were found for memory or executive function.
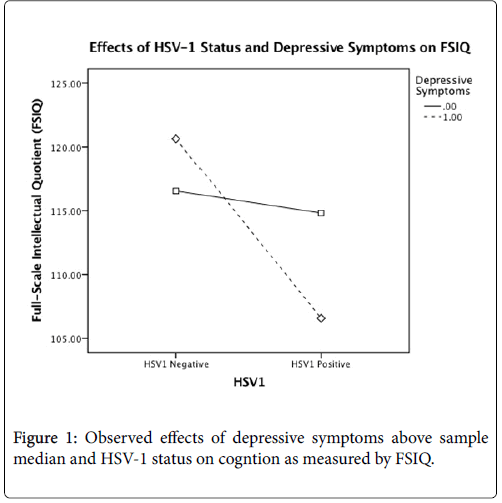
Conclusion: The results of this study show that even subclinical depressive symptoms can exacerbate the negative effect of HSV-1 infection on global cognitive function. We found that seropositive individuals reporting at least some depressive symptoms exhibited worse performance on a test of intelligence than seropositive individuals without depressive symptoms.
Keywords: Depression; Executive function; Memory; Cognitive impairment; Dementia; Middle-age adult; Global cognitive function
Introduction
Intact cognitive function is critical for one’s physical and mental health as well as overall quality of life in older age [1]. However, several environmental risk factors, including mental stress and chronic viral infections, can increase cognitive vulnerability and lead to cognitive decline. Considering the anticipated dramatic increase in the number of older adults with dementia in the next 40 years [2] and the current lack of dementia cures, it is imperative that we explore any modifiable risk factors for brain vulnerability that may facilitate the development of interventions to prevent or delay the onset of severe cognitive impairment.
Psychosocial distress, as measured by symptoms of depression, is highly prevalent among middle-aged adults who are often overburdened with caretaking responsibilities andcareer demands [3].Depression is known to contribute to cognitive vulnerability [4] across many domains including memory, psychomotor skills, executive function, and general intelligence [5]. Early studies posited that a lack of effortful processing leads to decreased attention, which affects all areas of cognition [6]. More recent theories have claimed that depression-related cognitive decline is related to dysfunctional neural networks, not the lack of motivation that is inherent to depression [7]. In fact, some believe that it lies on the same neuropathological continuum as mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) [8].
Another factor that can contribute to cognitive vulnerability in midlife is exposure to ubiquitous environmental pathogens such as Herpes Simplex Virus type 1 (HSV-1), also known as the cold sores virus. Approximately 60% of all adults carry the HSV-1 virus [9]. HSV-1 is usually acquired through contact with infected saliva and other bodily fluids. The virus lies dormant in the trigeminal ganglion and infected individuals normally experience no symptoms [10,11]. However, periods of latency can be interspersed with periods of reactivation, accompanied by fever, chills, and cold sores around the mouth [12]. In a worst-case scenario, the virus can lead to a variety of severe brain disorders such as encephalitis [13], Bell’s Palsy [14], and corneal blindness [15]. In addition, the presence of HSV-1 has been associated with the genesis of amyloid plaques, the hallmark symptom of AD [16,17]. Thus, researchers have become interested in the potential cognitive side-effects of HSV-1. While on its own, HSV-1 does not appear to have deleterious cognitive effects in persons without encephalitis [18], the virus appears to exacerbate cognitive vulnerabilities related to other chronic illnesses such as schizophrenia and bipolar disorder [19-21].
Since HSV-1 reactivations are often triggered by stress [22], this study examines how psychosocial distress and HSV-1 might interact to foster early cognitive vulnerability in otherwise healthy middle-aged adults.
Method
A total of 113 participants (63 female, 50 male) aged 40-60 (M=49.4, SD=6.3) were recruited from the community using online ads, newspaper ads and flyers. Participants were excluded if they had an overt chronic disease or major psychiatric disorder as reported on a medical history questionnaire. Other exclusion criteria included participants with hypertension (systolic blood pressure>140 mmHg or diastolic blood pressure>90 mmHg), diabetes (fasting blood glucose>7.0 mmol/L) dyslipoproteinemia (cholesterol>6.2 mo/L, triglycerides>4.5 mmol/L) or a baseline IQ<85. The self-reported ethnic distribution of this sample was 61.1% Caucasian, 21.2% Latino, 6.2% African American, 3.5% Asian, and 8.0% other.
Participants provided approximately 15 cc of blood, obtained through venipuncture of the antecubital vein. A portion of the blood was centrifuged and the serum was frozen for later HSV-1 testing. Enzyme-linked immunosorbent assay (ELISA) was used to assess seropositivity for HSV-1 IgG antibodies using the commercially available HSV-1 IgG ELISA kits (Calbiotech, Spring Valley, CA) per manufacturer instructions. All samples were analyzed in duplicate and the values averaged. Subjects were considered positive if optical density exceeded 0.9. Participants also underwent a neuropsychological assessment. Cognitive vulnerability was operationalized as lower cognitive function across any of the following 3 domains: global, memory, executive function. Global cognition was measured with the full-scale intellectual quotient (FSIQ) on the Wechsler Abbreviated Scale of Intelligence II (WASI-II). Memory was measured using a composite z score consisting of the averaged, sample-based z scores on the California Verbal Learning Test-II (CVLT-II) immediate recall, delayed recall, and recognition. Executive function was measured using a z composite score consisting of the averaged sample-based z scores on the Wechsler Adult Intelligence Scale-III (WAIS-III) digit span forward and backwards subtests, total completion time of trails A and trails B, and the Controlled Oral Word Association Test (COWAT). The scores for trails A and B were multiplied by -1 before inclusion in the composite, so that higher scores reflect better performance. The Beck Depression Inventory (BDI-II) was used to assess depressive symptoms. As the sample was comprised of community dwelling adults with relatively low levels of depressive symptoms (mean BDI<16, mild depression), BDI scores were dichotomized using a median split (m=7) to create two groups: some depressive symptoms and no depressive symptoms. Two-way analyses of variance (ANOVAs) were used to test the interaction between HSV-1 status (HSV-1+ versus HSV-1-) and depressive symptoms (some depressive symptoms versus no depressive symptoms) on cognitive function (i.e. global cognition, memory, and executive function) after controlling for gender, age, and educationAn alpha level of 0.05 was used as the criterion for statistical significance. Statistical analyses were conducted using SPSS 23.0 (IBM Corporation, Chicago, IL).
Results
The demographic data for participants are included in Tables 1 and 2. These data show an ethnically diverse, middle-age sample that is representative of the state of Texas where this study took place. 70 participants were seropositive for HSV-1 (61.9%), the remaining 43 (38.1%) were not. Average BDI-II score was 8.20 (SD=6.37). 62 participants reported BDI-II scores greater than the sample median of 7 (54.9%) and the remaining 51 reported scores below the mean (45.1%)
| Mean | SD | Range | |
|---|---|---|---|
| Age | 49.36 | 6.33 | 40-60 |
| Full-Scale Intellectual Quotient (FSIQ) | 113.46 | 15.53 | 76-145 |
| Years of Education | 16.42 | 2.78 | Oct-30 |
| Beck Depression Inventory-II (BDI-II) | 8.2 | 6.37 | 0-35 |
Table 1: Descriptive Statistics of Variables used in Analysis of Variance (ANOVA).
| N | % | |
|---|---|---|
| Ethnicity | ||
| Caucasian | 69 | 61.10% |
| Latino | 24 | 21.20% |
| African American | 7 | 6.20% |
| Asian | 4 | 3.50% |
| Other | 9 | 8.00% |
| HSV-1 Status | ||
| HSV-1 Negative | 43 | 38.10% |
| HSV-1 Positive | 70 | 61.90% |
| Gender | ||
| Male | 50 | 44.20% |
| Female | 63 | 55.80% |
| Depressive Symptoms (BDI-II) | ||
| BDI-II < 7 | 51 | 45.10% |
| BDI-II >7 | 62 | 54.90% |
Table 2: Frequency Statistics of Variables used in Analysis of Variance (ANOVA).
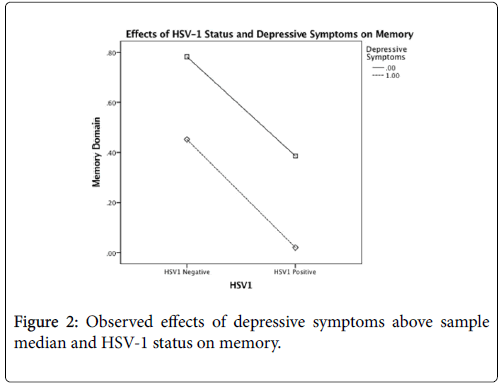
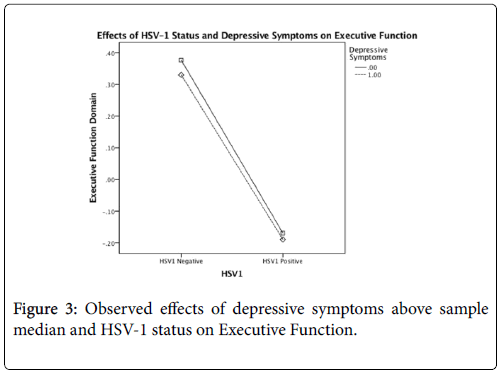
After controlling for age, gender, and education, there was a significant main effect for HSV-1, F(1,106)=7.908, p=0.01, but not for depressive symptoms, on global cognition. However, this main effect was qualified by a statistically significant interaction between the factors, F(1,106)=5.046, p=0.03 (Table 3). The interaction plot (Figure 1) suggests that HSV-1 seropositivity has a detrimental effect on FSIQ scores only for those who report at least some depressive symptoms. After controlling for age, gender, education, and FSIQ, no significant main effects or interactions were found for memory or executive function (all p>.05) (Tables 4 and 5) (Figures 2 and 3).
| df | F | MS | p | |
|---|---|---|---|---|
| Age | 1 | 0.38 | -74.1 | 0.54 |
| Gender | 1 | 0.26 | -51.3 | 0.61 |
| Education | 1 | 8.72* | -1696.4 | 0.00 |
| HSV-1 | 1 | 7.91* | -1537.9 | 0.01 |
| BDI | 1 | 0.57 | -111.71 | 0.45 |
| HSV-1*BDI | 1 | 5.046** | -981.45 | 0.03 |
Note: * p < 0.01, **p < 0.05
Table 3: Analysis of Variance (ANOVA) Between HSV-1 Status and Depressive Symptoms on Global Cognition.
| df | F | MS | p | |
|---|---|---|---|---|
| Age | 1 | 0.34 | -2.3 | 0.56 |
| Gender | 1 | 0.26 | -1.73 | 0.61 |
| Education | 1 | 0.45 | -3.05 | 0.5 |
| FSIQ | 1 | 2.66 | -17.84 | 0.1 |
| HSV-1 | 1 | 0.59 | -3.93 | 0.45 |
| BDI | 1 | 0.46 | -3.08 | 0.5 |
| HSV-1*BDI | 1 | 0 | -0.01 | 0.97 |
Note: * p < 0.01, **p < 0.05
Table 4: Analysis of Variance (ANOVA) Between HSV-1 Status and Depressive Symptoms on Memory.
| df | F | MS | p | |
|---|---|---|---|---|
| Age | 1 | 5.23* | -16.77 | 0.02 |
| Gender | 1 | 0.43 | -1.37 | 0.52 |
| Education | 1 | 1.71 | -5.47 | 0.2 |
| FSIQ | 1 | 0.32 | -1.02 | 0.57 |
| HSV-1 | 1 | 2 | -6.4 | 0.16 |
| BDI | 1 | 0.01 | -0.03 | 0.93 |
| HSV-1*BDI | 1 | 0 | 0 | 0.97 |
| Note: * p < 0.01, **p < 0.05 | ||||
Table 5: Analysis of Variance (ANOVA) Between HSV-1 Status and Depressive Symptoms on Executive Function.
Discussion
The results of this study show that even subclinical depressive symptoms can exacerbate the negative effect of HSV-1 infection on global cognitive function. We found that seropositive individuals reporting at least some depressive symptoms exhibited worse performance on a test of intelligence than seropositive individuals without depressive symptoms. However, we did not find any effects of HSV-1 or depression on memory or executive functioning.
These results are consistent with the current body of literature reporting that depression independently leads to cognitive decline 5, as well as the additive effects of biological and psychological risk factors on cognitive outcomes [23], specifically HSV-1 seropositivity and other psychological disorders [24]. It is possible that we did not find effects on memory and executive function beyond FSIQ because this study population was younger, healthier, and more educated, all factors which protect against mild cognitive impairment (MCI) [25].
One potential mechanism that could account for our results is the inflammatory response of the immune system. Under certain conditions, HSV-1 travels from the site of infection on the epithelium to the nerve endings where retrograde axonal transport carries the virus to the sensory ganglia [26]. In this way HSV-1 has direct access to the central nervous system. Subclinical levels of HSV-1 have been found in the central nervous system, but they are not enough to lead to viral encephalitis [27]. Nevertheless, this chronic infection consisting of repeating cycles of latency and reactivation is enough to activate an inflammatory immune response [28] and cause neuronal degradation [24]. This can lead to an overall decrease in cognitive reserve as measured by FSIQ. The negative impact of depression on cognition has been clearly demonstrated, however the relationship between depression and cognitive decline is multi-faceted. While cognitive decline associated with depression is thought to be caused by lack of motivation or as a response to decreased functionality itself [29], others posit that an underlying neuropathology might lead to depression and cognitive impairment [8]. No matter the mechanism, HSV-1 infection can result in lower cognitive reserve, which in turn exacerbates the effects of depression on cognition.
While drawing conclusions about the results of this study, however, we need to consider its limitations as well. As this was a cross-sectional study, it is difficult to determine the directionality of the detected effect. We cannot determine if lower FSIQ preceded HSV-1 infection, or if it is the result of it. One possibility, as stated in our proposed mechanism, is that HSV-1 creates cognitive vulnerability by decreasing cognitive reserve. However, another possibility is that people with lower FSIQ are prone to maladaptive behaviors that expose them to HSV-1. We also cannot rule out the effects of an unknown genetic or environmental factor. One potential environmental factor that was not considered in this study is socio-economic status (SES). Increased rates of depression [30], HSV-1 [31], and lower IQ [32] have been associated with lower SES. Because SES is closely related to stress, viral infection, and cognition, it will be important for future studies to determine how SES impacts the relationship between these variables. Future research should address these limitations through longitudinal studies incorporating SES variables to assess true causality.
A methodological limitation of this study is that the assay used to detect HSV seropositivity did not distinguish between HSV-1 and HSV-2. However, due to the differing prevalence rates between HSV-1 (70%) and HSV2 (17%) 9 we can be reasonably certain that most of our sample is positive for HSV-1. Another potential limitation of this study was the method used to determine depressive symptoms. BDI scores from this sample were dichotomized using a median split. Since participants with severe depression were excluded from this study, our sample included mostly participants reporting mild levels of depressive symptoms (BDI<16), making it impossible for us to utilize the recommended clinical cutoffs for depression [33]. Thus, we made a decision to employ a median split, creating two relatively healthy groups, one reporting some depressive symptoms, and another one reporting very few. While dichotomizing continuous variables results in some loss of information, dichotomization is favored in cases where the variable in question is skewed, and studies have shown that the use of a dichotomized variable generally leads to the same conclusion [34]. Further research is necessary to determine if the effects of clinical depression and chronic viral infection have comparable effects on cognition in other populations.
These findings are intriguing and raise a number of questions and directions for further exploration. One question is whether these effects are consistent over time and across different age groups. The ability to identify risk factors at an earlier age means steps for earlier intervention can be taken. If the combined effects are still significant in older age, then treating depression and viral infection may lead to more favorable cognitive outcomes, even in those who are already cognitively impaired. The potential for other types of viral infections and life stress to have the same effects on cognition are very likely, however more research is necessary to determine if these effects carry over to other mood disorders and viral infections. Further delineation of the mechanism would help lead to earlier intervention and better preventative care.
In conclusion, we found that, in middle-aged adults, the combination of depressive symptoms with HSV-1+ status is related to poorer global cognitive function. Because of the high prevalence of HSV-1 and depression in the population [9,35], many otherwise healthy individuals are at risk for cognitive decline. Currently there is no cure for cognitive dysfunction. The prevalence of dementia in the United States is estimated to be between 2.9 million [2] and 3.8 million [36]. The rate of dementia is projected to double every 20 years to 81.1 million by 2040 [2]. One of the most effective methods we have is to intervene before any damage occurs to prevent cognitive dysfunction. It is important to identify the risk factors preceding cognitive impairment to know how to target them with interventions. By targeting these risk factors early on, the chance of prolonging or preventing the onset of dementia is greatly improved.
References
- Gaugler JE, Yu F, Krichbaum K, Wyman JF (2009) Predictors of Nursing Home Admission for Persons with Dementia. Med Care 47: 191-198.
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, et al. (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366: 2112-2117.
- Hammer LB, Neal MB (2008) Working sandwiched-generation caregivers: Prevalence, characteristics, and outcomes. The Psychologist-Manager Journal 11: 93-112.
- McDermott LM, Ebmeier KP (2009) A meta-analysis of depression severity and cognitive function. J Affect Disord 119: 1-8.
- Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L (2010) Cognitive impairment in major depression. Eur J Pharmacol 626: 83-86.
- Hartlage S, Alloy LB, Vázquez C, Dykman B (1993) Automatic and effortful processing in depression. Psychol Bull 113: 247-278.
- Austin M-P, Mitchell P, Goodwin GM (2001) Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 178: 200-206.
- Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, et al. (2010) Late-Life Depression, Mild Cognitive Impairment, and Dementia: Possible Continuum? Am J Geriatr Psychiatry 18: 98-116.
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, et al. (2006) Trends in herpes simplex virus type 1 and type 2 seroprevalence in the united states. JAMA 296: 964-973.
- van Velzen M, Ouwendijk WJD, Selke S, Pas SD, van Loenen FB, et al. (2013) Longitudinal study on oral shedding of herpes simplex virus 1 and varicella-zoster virus in individuals infected with HIV: Oral HSV-1 and VZV Shedding in HIV Patients. J Med Virol 85: 1669-1677.
- Baringer JR, Swoveland P (1973) Recovery of Herpes-Simplex Virus from Human Trigeminal Ganglions. N Engl J Med 288: 648-650.
- Arduino PG, Porter SR (2008) Herpes Simplex Virus Type 1 infection: overview on relevant clinico-pathological features. J Oral Pathol Med 37: 107-121.
- Whitley RJ, Gnann JW (2002) Viral encephalitis: familiar infections and emerging pathogens. Lancet 359: 507-513.
- McCormick DP (2000). Herpes simplex virus as a cause of Bell’s palsy. Rev Med Virol 10: 285-289.
- Steiner I, Benninger F (2013) Update on Herpes Virus Infections of the Nervous System. Curr Neurol Neurosci Rep 13: 414.
- Bourgade K, Garneau H, Giroux G, Page AYL, Bocti C, et al. (2015) β-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 16: 85-98.
- Itzhaki RF (2014) Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Front Aging Neurosci 6: 202.
- Aiello AE, Haan MN, Blythe L, Moore K, Gonzalez JM, et al. (2006) The Influence of Latent Viral Infection on Rate of Cognitive Decline over 4 Years. J Am Geriatr Soc 54: 1046-1054.
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Cole S, et al. (2004) Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorder. Biol Psychiatry 55: 588-593.
- Schretlen DJ, Vannorsdall TD, Winicki JM, Mushtaq Y, Hikida T, et al. (2010) Neuroanatomic and cognitive abnormalities related to herpes simplex virus type 1 in schizophrenia. Schizophr Res 118: 224-231.
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB (2011) Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res 128: 61-65.
- Chida Y, Mao X (2009) Does psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav Immun. 23: 917-925.
- Borrell-Carrio F, Suchman AL, Epstein RM (2004) The Biopsychosocial Model 25 Years Later: Principles, Practice, and Scientific Inquiry. Ann Fam Med 2: 576-582.
- Prasad KM, Watson AM, Dickerson FB, Yolken RH, Nimgaonkar VL (2012) Exposure to Herpes Simplex Virus Type 1 and Cognitive Impairments in Individuals With Schizophrenia. Schizophrenia Bull 38: 1137-1148.
- Tervo S, Kivipelto M, Hänninen T, Vanhanen M, Hallikainen M, et al. (2004) Incidence and Risk Factors for Mild Cognitive Impairment: A Population-Based Three-Year Follow-Up Study of Cognitively Healthy Elderly Subjects. Dement Geriatr Cogn Disord 17: 196-203.
- Miller CS, Danaher RJ, Jacob RJ (1998) Molecular Aspects of Herpes Simplex Virus I Latency, Reactivation, and Recurrence. Crit Rev Oral Biol Med 9: 541-562.
- Becker Y (1995) HSV-1 brain infection by the olfactory nerve route and virus latency and reactivation may cause learning and behavioral deficiencies and violence in children and adults: A point of view. Virus Genes 10: 217-226.
- Li H, Zhang J, Kumar A, Zheng M, Atherton SS, et al. (2006) Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology 117: 167-176.
- Yaffe K, Blackwell T, Gore R, Sands L, Reus V, et al. (1999) Depressive symptoms and cognitive decline in nondemented elderly women: A prospective study. Arch Gen Psychiatry 56: 425-430.
- Gavin AR, Walton E, Chae DH, Alegria M, Jackson JS, et al. (2010) The associations between socio-economic status and major depressive disorder among Blacks, Latinos, Asians and non-Hispanic Whites: findings from the Collaborative Psychiatric Epidemiology Studies. Psychol Med 40: 51-61.
- Smith JS, Robinson NJ (2002) Age-Specific Prevalence of Infection with Herpes Simplex Virus Types 2 and 1: A Global Review. J Infect Dis 186: S3-S28.
- Strenze T (2007) Intelligence and socioeconomic success: A meta-analytic review of longitudinal research. Intelligence 35: 401-426.
- Dozois DJA, Dobson KS, Ahnberg JL (1998) A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment 10: 83-89.
- DeCoster J, Iselin A-MR, Gallucci M (2009) A conceptual and empirical examination of justifications for dichotomization. Psychol Methods. 14: 349-366
- Pratt LA, Brody DJ (2014) Depression in the U.S. household population, 2009-2012. NCHS Data Brief 172: 1-8.
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, et al. (2007) Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology 29: 125-132.
Citation: Cassill C, Steward K , Eagan D, Kaur S, Kruzliak P, et al. (2018) Depressive Symptoms Exacerbate the Effects of HSV-1 Infection on Cognitive Function in Middle Age. J Clin Infect Dis Pract 3: 122. DOI: 10.4172/2476-213X.1000122
Copyright: © 2018 Cassill T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 10853
- [From(publication date): 0-2018 - Dec 04, 2024]
- Breakdown by view type
- HTML page views: 10066
- PDF downloads: 787