Review Article Open Access
Dental Caries: A Microbiological Approach
Khushbu Yadav1* and Satyam Prakash21Krishna Medical Technical Research Center, Purwanchal University, Janakpurdham, Nepal
2Department of Biochemistry, Janaki Medical College Teaching Hospital, Tribhuvan University, Janakpurdham, Nepal
- *Corresponding Author:
- Khushbu Yadav
Medical Microbiologist and Lecturer, Krishna Medical Technical Research Center
Purwanchal University, Janakpurdham, Nepal
Tel: +977-9841603704
E-mail: meetkhushi20@gmail.com
Received date: March 10, 2017; Accepted date: April 04, 2017; Published date: April 10, 2017
Citation: Yadav K, Prakash S (2017) Dental Caries: A Microbiological Approach. J Clin Infect Dis Pract 2:118. doi: 10.4172/2476-213X.1000118
Copyright: © 2017 Yadav K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical Infectious Diseases & Practice
Abstract
Tooth decay, also known as dental caries is an epidemic, microbiological contagious disease of the teeth that ends in localized dissolution and damage of the calcified structure of the teeth. This disease occurs due to multiple factors such as interactions within the plaque community, host physiology, diet, fluoride, pH and the nature of the tooth enamel, and dominance of Streptococcus mutans . The time factor is significant for the commencement and development of caries in teeth. The main instigation and progress of dental caries involves acidogenic and aciduric Gram-positive bacteria such as Streptococcus, Lactobacillus and Actinomycetes colonizing the supragingival biofilm which impede with usual nutrition intake, verbal communication, self-worth and daily habitual behavior. Nutritional influences on craniofacial development, oral cancer and other oral infectious diseases are expensive to treat. In spite of development in science of oral diseases, dental caries extend to be a global health concern affecting human being of different age groups. With this concern, this review article highlights different microbiological perspectives of dental caries in broader sense and its update will help to upgrade the recent trends of microbiology in dental caries and also formulating various developmental programs towards oral hygiene.
Keywords
Biofilms; Dental caries; Glucosyltransferase; Microbial ecology; Streptococcus
Introduction
WHO declares that deprived oral health and its related diseases may have dreadful effect on common health as well as eminence of life. Dental caries is a common and major public health oral disease which hampers the attainment and protection of oral health in different age groups [1,2]. The prevalence pattern and severity of dental caries varies with age, sex, race, socio-demographic characteristics, economic status, geographical location, food practice and oral hygiene habits within the same country or region in various parts of the world [3].
During the last few decades, the incidence of microbial diseases has amplified drastically. Microorganisms are the super bug agent responsible for causing dental caries. Many facultatively and obligately anaerobic bacteria dominate the microbial community of dental caries [2]. But, the most important etiological agent of dental caries is Streptococcus mutans [3]. Tooth decay takes place when a vulnerable tooth surface is colonized with cariogenic microbes and dietary source of sucrose or refined sugar. Fermentation of carbohydrate leads to production of lactic acid by the action of bacteria which melts the hydroxyapatite crystal structure of the tooth which grounds caries [4,5].
The experience of pain, problem with eating, chewing, smiling and communication due to missing, discolored or spoiled teeth have a foremost impact on people’s everyday life [2]. Low self-esteem, adverse pregnancy outcomes, increased risk of myocardial infarction, cardiovascular disease, respiratory, erectile, diabetes complications, cavernous sinus thrombosis and Ludwig angina are associated with dental caries which can be crucial to human beings [6-8]. Moreover, oral diseases hamper activities at school, at occupation and at residence causing millions of school and employment hours to be vanished each year throughout the globe [3,9]. As a consequence, the treatment need is enhanced nowadays.
Globally, there is a great interest in the use of antimicrobial agents for prevention and treatment of dental diseases [6,10]. The emergence of antibiotic resistance has been identified due to inappropriate prescription and practice of antibiotic use [5,6]. In the recent years, dentists and dental professionals has shifted their interest from narrow spectrum antibiotic prescriptions to broad-spectrum aminopenicillins due to enhancement of bacterial isolates resistant to the former antibiotics in prescription practices [5,6,11]. Antibiotic resistance increases the morbidity associated with dental caries and contributes substantially to rising costs of care and the need for more expensive drugs [5,6,12,13]. This review paper focuses on the inference of definite bacteria in tooth decay, biofilm in the tooth cavity and drug resistance and preventive measures to reduce the susceptibility of dental caries.
Definition
The word caries derives from the Latin for rot or rotten. According to Bader, dental caries is a chronic contagious disease caused by a complete interaction of oral microorganism in dental plaque, diet and a broad array of host factors ranging from societal & environmental factors to genetic & biochemical/immunologic host responses [14].
Historical Evidence of Caries in Hominines and Early Humans
There has been long history of dental caries. Caries is a very old disease and it is not exclusive of the human species. Evidences of dental lesions compatible with caries have been observed in creatures as old as: Paleozoic fishes (570-250 million years), Mesozoic herbivores dinosaurs (245-265 million years), prehominines of the Eocene (60- 25 million years), and Miocenic (25-5 million years), Pliocenic (5- 1.6 million years), and Pleistocenic animals (1.6-0.01 million years) [15,16]. Caries has also been detected in bears and other wild animals and it is common in domestic animals [17].
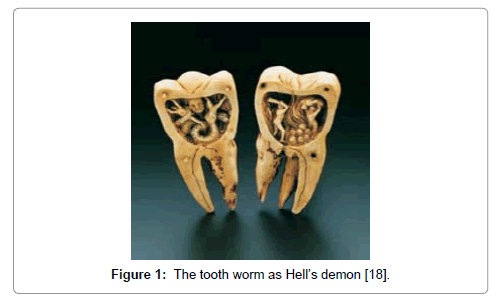
The rate of caries remained low through the Bronze and Iron ages. The increase of caries during the Neolithic period may be attributed to the increase of plant foods containing carbohydrates. The beginning of rice cultivation in South Asia also believed to have caused an increase in caries. The earliest theory was the “tooth worm theory” proposed by the ancient Chinese in 2500 BC, where it posited a tooth worm as the cause of this rottenness [18-21]. In 350 BC Aristotle observed figs and sweets caused tooth decay and by the 12th century, caries was described as the condition of having holes in the teeth or cavities. A Sumerian text from 5000 BC describes a “tooth worm” as the cause of caries (Figure 1).
Figure 1: The tooth worm as Hell’s demon [18].
Pre-Restorative Era (up to 1850 A.D.)
In this era, numerous observations were made about the causes of dental caries. But, the understanding of dental caries, its causes and treatment did not progress much until the 18th century. The first full text on dental diseases and their treatment was published in 1728 when Pierre Fauchard, a French surgeon, wrote “Le Chirurgien Dentiste.” Fauchard rejected the tooth worm theory of dental caries. Instead, he described enamel hypoplasia as “an erosion of the enamel” [22] and recommended that hypoplastic areas be smoothed using files. Fauchard suggested total excavation of carious cavities and filling them with lead, tin, or gold foil.
Until the eighteenth century, dental treatment was rather simple and was based on extraction of teeth and use of traditional remedies. With the beginning of the second industrial revolution in 1875, dentistry was on the edge of practicing an innovative revolt that paid attention on conserving teeth rather than extracting them (Figure 2).
Figure 2: Pierre Fauchard (Father of Modern Dentistry) [20].
The Restorative Era: 1850-Present
With the start of the second industrial revolution, significant economic and social changes took place in Europe and the United States. The dispersion of wealth and the creation of a class of middle- income working families in large cities created demands for restoring rather than extracting teeth. Late in the nineteenth century, dentists were faced with an increasing demand to conserve teeth from the ravages of dental caries. Amalgam was first used in Europe, but in 1855, Drs. W. M. Hunter and E. Townsend in the United States published a formula of amalgam that consisted of tin, silver, and mercury [22].
In 1883, a battery-powered electric dental engine was developed to ease the practice of dentistry with the introduction of faster dental engines and handpieces. These developments in technology, however, were not associated with advancement in knowledge of the diagnosis, etiology, and management of dental caries. This task was left to the pioneering works of Tomes, Webb, Black, and Miller, among others [23,24].
In the 1890s, W.D. Miller proposed chemoparasitic caries theory explained that bacteria colonized the oral cavity and produced acids that liquefy tooth structures in the presence of fermentable carbohydrates [25,26]. In 1924, Killian Clarke illustrates a spherical bacterium in chains isolated from carious lesions as Streptococcus mutans . In the 1950s, Keyes and Fitzgerald working with hamsters demonstrate that caries was infectious and caused by an acid-producing Streptococcus . In 1960s, accepted that the Streptococcus isolated from hamster caries was the same as S. mutans described by Clarke [27].
Epidemiology
Today, tooth decay is increasing in incidence in the elderly in the United States and elsewhere as aging populations retain more of their dentition, making more tooth surfaces available for the disease processes. United States Surgeon General’s report shows that 45% of children ages 5 to 17 have cariously affected teeth and the problem is particularly severe among children in specific populations [28].
Globally, 36% population has dental caries in their permanent teeth and 9% population has in baby teeth [29]. The occurrence of this disease is most frequent in Latin American countries, countries in the Middle East, South Asia, and least rampant in China [30]. Dental caries is the most widespread chronic childhood disease in the United States five times more common than asthma affecting 60-90% of school children and the vast majority of adults approximately 29% to 59% [31,32] above the age of 50 years’ experience caries [2,9,33]. Among children in the United States and Europe, 20% of the population endures 60% to 80% of having dental caries [34]. Bacterial vaginosis is more frequent in females with bacterial infection of caries [35]. Total spending on dental services in the United States will be over $65 billion this year, with arguably about half of this a direct or indirect result of dental caries [36].
Types
Primary caries
It can take place on different tooth surfaces. On an approximal surface, the lesion initiates and appears below the contact area between teeth. Caries on an occlusal surface is also a localised phenomenon in pit and fissure. On both occlusal and approximal surfaces, enamel caries is a three-dimensional subsurface demineralisation that spreads along the enamel prisms [37,38].
Secondary caries
It is a lesion located at the margin of a dental restoration which characterizes a caries lesion adjacent to the margin with signs of demineralisation (wall lesions) alongside the cavity wall which could be outcome of micro leakage. However, clinical and microbiological studies suggest that this leakage does not lead to active demineralisation below the restoration [37,38].
Microbial Ecology
The microbial ecology of caries includes the biology of oral bacteria within related habitats i.e.,
The habitat
The hard (enamel and tooth root) and soft (mucosal) tissue surfaces in the mouth provide a variety of microhabitats with distinctly different structural and environmental parameters [39]. In particular, the non- shedding surface of enamel allows the accumulation of a biofilm that provides a protected habitat with a variety of niches that support a wide range of bacterial genera and species [40].
Microorganisms associated with tooth decay
Two species of the ‘mutans streptococci’ viz. Streptococcus mutans and Streptococcus sobrinus are the principal agents of enamel caries [6,41-44]. Lactobacillus and Actinomyces are also associated with caries. Actinomyces odontolyticus colonizes infants before eruption of teeth [45]. Some root caries lesions are dominated by Actinomyces naeslundii, A. israelii and A. gerencseriae [46-50]. The other significant species involved in caries includes Streptococcus mitis, Bifidobacterium and Actinomyces , a group of ‘low pH’ aciduric isolates which have been isolated from white spot lesions in humans [6,46,51]. In contrast to bacteria that lower plaque pH including Veillonella and Actinomyces associated with caries.
Diversity among strains of species of oral bacteria
It is well accepted that phenotypic characteristics among strains of bacterial species can vary. Changes in the phenotypes in plaque could influence bacterial selection and survival [52,53] and the overall activities of the plaque community. In addition to generation of diversity through changes in their genomic DNA strains of species of oral bacteria colonizing a mouth may be influenced by ‘clonal replacement’ where new clones replace the existing clones in a habitat and contribute to strain diversity [53].
Adaptation by oral bacteria to environmental parameters
Phenotypic adaptation and stress response mechanisms such as tolerance of acid, starvation, oxygen, fluoride and expression of urease [44,46-49,52,54-57]. that are common among strains of a species can be regarded as support of bacteria to survive stresses common to their habitats due to ‘stress proteins’ [58-61].
The biofilm mode of growth
Dental plaque is a multifaceted biofilm community where bacterial populations exist as separated micro-colonies in physiologically diverse environments. Biofilm cells exhibit different characteristics from the same cells growing in suspended culture [62].
Etiological Agent of Tooth Decay [6,38,63-67]
Different microorganisms can exist in single or poly-microbial communities in caries. i.e.
• Gram positive cocci: - Streptococcus mutans, S. mitis, S. salivarus, S. sanguis, S. intermedius, S. vestibularis, Staphylococcus aureus, Atopobium spp, Peptostreptococcus spp, Enterococcus fecalis .
• Gram positive rods: - Actinomyces odontolyticus, A. naeslundii, A. viscosus, A. israelii, Lactobacillus fermentum, L. acidophillus, Bifiodobacterium dentium, Propionibacterium spp.
• Gram negative cocci: - Veillonella parvula, Nesseria spp.
• Gram negative rods: - Bacteriodes denticola, B. melaninogenicus, Fusobacterium necrophorum, F. mortiferum, Escherishia coli, Klebsiella pneumoniae, Enterobacter aerogens, Citrobacter freundi, Pseudomonas fluorescence, Haemophilus spp, Prevotella spp, Leptotrichia spp.
• Yeasts: - Candida albicans, C. tropicalis, C. glabrata .
Mutans streptococci (MS)
Mutans streptococci are the foremost cariogenic pathogens in tooth decay. They are highly acidogenic, producing short-chain acids which soften hard tissues of teeth. Three isozymes of glucosyltransferases catalyze and metabolize sucrose to synthesize insoluble extracellular polysaccharides, which increase their adherence to the tooth surface and persuade biofilm formation [6,68,69]. The most significant mutans streptococci isolated from tooth caries samples are S. mutans [6] and S. sobrinus. S. mutans is more cariogenic than S. sobrinus because of specific cell-surface proteins, which assist in its primary attachment to the tooth. But, such proteins are deficient in S. sobrinus (Table 1) [38].
| S.No. | Groups | Species |
|---|---|---|
| 1 | Streptococcus mutans group | S. mutans S. sorbinus S. ratti |
| 2 | Streptococcus salivarus group | S. salivarius S. infantarius S. vestibularis |
| 3 | Streptococcus mitis group | S. mitis S. oralis S. infantis S. cristatus S. perois |
| 4 | Streptococcus sanguinis group | S. sanguinis S. parasanguinis S. gordonii |
| 5 | Streptococcus anginosus group | S. anginosus S. intermedius S. constellatus |
Table 1: Five Major Groups of Viridans Group Streptococci [38,68].
Streptococcus mutans
Number of sugars and glycosides such as glucose, fructose, sucrose, lactose, galactose, mannose, cellobiose, glucosides, trehalose, maltose and group of sugar alcohols are metabolized by the bacterial action of S. mutans. S. mutans synthesizes intracellular glycogen like polysaccharides in the presence of extracellular glucose and sucrose [38,70]. An important factor in the colonization and establishment of S. mutans in the dental biofilm are mutacins (bacteriocins) produced by S. mutans (Table 1) [38,71].
Virulence Factors of S. mutans
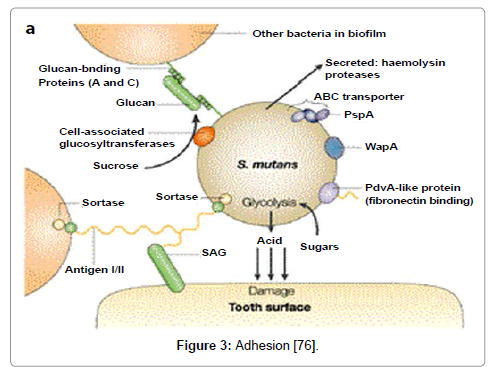
Adhesion
The adhesion of S. mutans within dental plaque can be mediated via sucrose-independent as well as sucrose-dependent mechanism. Sucrose-independent adhesion, salivary components within the acquired enamel pellicle may initiate the attachment process whereas in sucrose-dependent adhesion, sucrose can be principally accountable for developing colonization to the tooth surface (Figure 3).
Figure 3: Adhesion [76].
Sucrose-independent adhesion
Sucrose-independent adhesion of S. mutans is strongly predisposed by antigen I/II, an 185kDa surface protein which is found on most oral streptococci [72]. They has been designated by a variety of names including P1, SpaP, Sr, PAc, and antigen B. Proteins within the antigen I/II family share structural similarity based on amino acid domains, but display variable functionality with respect to binding salivary agglutinins, salivary pellicle components, and other plaque bacteria [73,74]. The alanine-rich and proline-rich domains are thought to be primarily responsible for the interaction between antigen I/II and salivary components [75-79].
Importance of Saliva
Viscous saliva is less effective than watery saliva in clearing effect of carbohydrates which provides physical protection. Chemical protection contains calcium, phosphate, fluoride, buffers, bicarbonate, phosphate, and small proteins that neutralize the acids after ingestion of fermentable carbohydrates. Antibacterial substances in saliva work against the bacteria [6]. If salivary production is reduced due to illness or medications or radiation therapy, the teeth are at increased risk for decay [80].
Sucrose-dependent adhesion
The major mechanism behind sucrose-dependent adhesion is the action of glucosyltransferases (GTFs) in the synthesis of both water soluble (dextran) and water-insoluble glucans (mutan). Apparently, sucrose-dependent adhesion can fasten binding by glucan coated S. mutans present inside the dental plaque [81,82] which synthesize by extracellular GTFs that bound the salivary pellicle.
The glucan binding domain (GBD) of the GTFs [83] consists of two non-enzymatic glucan binding proteins, GbpA, GbpD [84,85] and other GbpC acts as a cell surface glucan receptor which is only observed under certain stressful growth conditions [86]. Alternatively, the wall-associated protein WapA contributes to sucrose dependent adhesion indirectly. The interruption of other genetic loci also has been correlated with decreased sucrose-dependent adhesion [87].
Non-enzymatic glucan binding proteins
Non-enzymatic glucan-binding proteins (GBPs) are GbpA [88]; subsequently GbpB [89], GbpC [86], and GbpD [84]. Proteins capable of binding glucan were assumed to contribute to sucrose dependent adhesion and possibly to the cohesive nature of the dental plaque biofilm. The role of glucan is important in the caries process.
Carbohydrate Metabolism
Potential virulence factors are the proteins involved in the metabolism of sucrose, glucans or other carbohydrates which include a fructosyltransferase (Ftf), a fructanase (FruA), an extracellular dextranase (DexA), and proteins responsible for intracellular polysaccharide accumulation (Dlt1-4) [90].
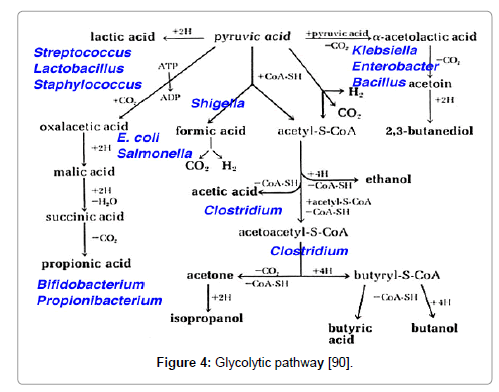
Acidogenicity
S. mutans contains glycolytic pathway which produce lactate, formate, acetate, and ethanol as fermentation products [91]. Strains deficient in lactate dehydrogenase (LDH) display reduced cariogenicity [92,93] and the dearth of lactate dehydrogenase (LDH) is lethal. The acidogenicity of S. mutans leads to ecological changes in the plaque flora that includes an elevation in the proportion of S. mutans and other acidogenic and acid-tolerant species (Figure 4).
Figure 4: Glycolytic pathway [90].
Acid-Tolerance
The acid tolerance response (ATR) constitutes together with the activity F1F0-ATPase proton pump and change in gene and protein expression. ATR has been revealed to defend the organisms from a sub-lethal pH challenge [94] and acid shock or growth at acidic pH has been allied with changes in the expression of over 30 proteins in vitro medium [95,96]. The acid tolerance of S. mutans is largely mediated by an F1F0-ATPase proton pump but also involves adaptation with an accompanying change in gene and protein expression. Evidence suggests that acid tolerance may be aided by the synthesis of water insoluble glucan and the formation of a biofilm.
Maintenance of Intracellular pH
S. mutans FATPase acitivity is enhanced with the fall of pH [97,98] maintaining pH gradient of approximately one pH unit. At the meantime, the fatty acid profile of the membrane shifts, decreasing permeability to protons, and excretion of acidic end products increases [99].
Biofilm Formation
Dental plaque is considered as a biofilm.There are many variables coupled with growth in a biofilm including adhesion, nutrient flow, and co-aggregation can persuade growth rate, gene expression and quorum sensing in ways that differ from life in a planktonic environment. gtfB and gtfC genes are directly involved in biofilm development with the variability of gene expression in response to the environment. Evidence advocates that these genes can be both independently transcribed and co-transcribed. Hudson and Curtiss, by using the chloramphenicol acetyltransferase (cat ) reporter gene demonstrated increased expression of the gtfB/C genes in response to sucrose or when bound to an artificial tooth pellicle [100].
Steps of biofilm formation
a. Association – Dental pellicle forms on the tooth & provides bacteria surface to attach.
b. Adhesion – Within hours, bacteria loosely binds to the pellicle.
c. Proliferation – Bacteria spreads throughout the mouth & begins to multiply.
d. Microcolonies – Microcolonies are formed, streptococci secrete protective layer (slime layer).
e. Biofilm formation – Microcolonies from complex groups with metabolic advantages.
f. Maturation – The biofilm develops a primitive circulatory system.
Progression of Dental Biofilm
Dental plaque forms through a sequential events resulting in a structurally and functionally organized species rich microbial community [6,101]. Once plaque forms, its species composition at a site is characterized by degree of stability among the component species. This stability is due to a balance imposed by numerous microbial interactions [102], such as conventional biochemical interactions where complex host glycoproteins catabolize to develop food chains and cell to cell signaling which leads to coordinated gene expression within the microbial community [103,104].
Streptococcus sobrinus
S. sobrinus has been implicated in caries development particularly in instances where caries development appears to be independent of S. mutans . S. sobrinus exhibits higher acid production and acid tolerance compared to S. mutans (Table 2) [29,38,97,105].
| S.No. | Bacterial isolates | Colony morphology | Gram’s reaction |
|---|---|---|---|
| 1. | S. mutans | Very small, spherical, white-grey,γ-haemolysis on BA | Gram positive cocci in chains |
| 2. | S. mitis | Raised center, very small, black white colony, α- haemolysis on BA | Gram positive cocci in chains |
| 3. | S. vestibularis | White, convex, glossy with entire edges colony, α- haemolysis on BA | Gram positive cocci |
| 4. | Enterobacter spp | Lactose fermenter colony on MA | Gram negative rods |
Table 2: Colony morphology and Gram’s staining reaction of bacteria isolated from dental caries patients [38].
Streptococcus mitis
Streptococcus mitis are commensal bacteria which belong to the viridans streptococci group usually arranged in short chains in the shape of cocci [106]. These Gram-positive bacteria are part of human oral flora, usually nonpathogenic but commonly cause bacterial endocarditis which colonizes hard surfaces in the oral cavity such as dental hard tissues as well as mucous membranes. The development of this microbial community is reliant on numerous factors including adherence, signalling, nutritional adaption, and host modulation. In addition, environmental conditions such as pH, temperature, oxygen availability, organic metabolites etc. may be involved in Streptococcus mitis colonization [107,108].
Streptococcus gordonii
S. gordonii is a non pathogenic commensal streptococci considered as integral members of the human oral florais part of the group viridians of Streptococci . Streptococcus gordonii initiates colonization through formation of a monospecies biofilm. The human tooth is covered by pellicle containing lipids and proteins, including salivary agglutinin glycoprotein. The receptors for salivary agglutin glycoprotein located on S. gordonii and other pioneer colonizers recognize and bind to the pellicle. S. gordonii cells bound to the surface of the tooth initiates a signal transduction pathway known as BrfAB which regulates adhesive activity [109].
Lactobacilli
Lactobacilli are dominant part of the flora, considered as pioneer microbes in the development of caries particularly in dentin [110]. It inhabits the deep cavities, and their number correlates with the quantity of carbohydrates [38,111]. The isolation of Lactobacilli is from deep caries lesions but rarely just earlier than the development of dental caries and in the early tooth decay.
Enterococci
Enterococci are gram positive, round or ovoid, facultative anaerobic bacteria. They are part of normal flora however they also colonize oral mucus membranes and skins especially in hospital settings [38,112].
Staphylococcus aureus
Most of the healthy individuals (30%) are the carrier of S. aureus , often carrying the bacterium on their skin, mucous membranes of anterior nares. Such carriers serve as the source of infection to themselves as well as to others [38,113].
Actinomycetes
Actinomycetes are not powerfully acidogenic or acid tolerant but are also carbohydrate users which are copious in the human mouth and persuade root surface caries in hamsters and gnotobiotic rats [38,114].
Etiological Theories of Dental Caries
Different theories have been specified for the cause of dental caries which are as follows:
Chemical theory
It is often known as acid theory. This theory proposes that the putrefaction of protein leads to the production of acid formation in oral cavity producing ammonia and subsequently get oxidized to nitric acid which destroys the teeth [115].
Parasitic theory
This theory is also known as septic theory. It suggests that decomposition of the enamel and dentin are caused by filamentous microorganisms (denticolae) in the enamel cuticle and in carious lesions leads to tooth decay [115].
Chemo-parasitic theory
In this theory, W. D, Miller highlights that the fermentable carbohydrates are degraded to form acids by the metabolism and secretion of enzymes of different microbes present in the oral cavity. These acids demineralize the enamel and the disintegrated enamel is subsequently mechanically removed by force of mastication [116].
Proteolytic theory
Gottileb in 1947 provoked that microbes enter the organic pathways of the enamel and start caries by proteolytic action in this theory. Subsequently, the inorganic salts are dissolved by acidogenic bacteria [116].
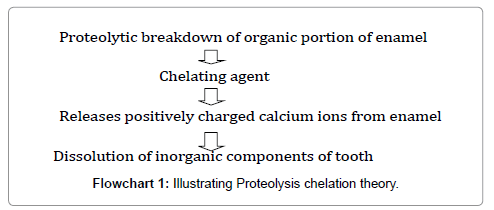
Proteolysis chelation theory
This theory reveals that tooth decay initiates from an earliest bacterial and enzymatic proteolytic action on the organic matter of enamel without preliminary demineralization. This causes the emancipation of a several complexing agents such as aminoacids, polyphosphates and organic acids which soften the crystalline hydroxyapatite [115] (Flowchart 1).
Caries Transmission
Cariogenic bacteria are not present in humans by birth. But, transmission of mutans streptococci is from caregivers, usually mothers, by mouth-to-mouth transmission via kissing or by sharing a spoon during feeding [117].
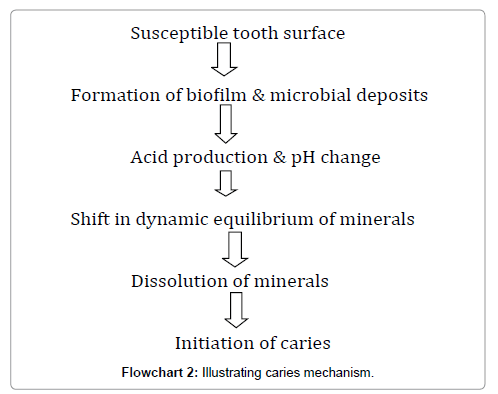
Caries mechanism
The bacterial flora and host defense systems are in the process of being established. Caries development is dependent on the following factors [118] (Flowchart 2):
Susceptible tooth and host
Implantation of S. mutans can occur only when teeth are present, because the teeth provide a non-shedding surface for colonisation of the micro-organisms. The amount of S. mutans depends on the number of erupted teeth present [119]. Sometime after eruption, newly exposed enamel surfaces undergo the final stages of post-eruptive maturation and hardening. This period immediately after eruption and prior to final maturation, is when the tooth is most susceptible to caries. The presence of structural developmental defects in enamel may increase the caries risk and may manifest as partial or total loss of enamel (hypoplasia). Irregular surfaces such as pits and grooves lead to plaque retention, increased S. mutans and decreased elimination of carbohydrates. Dentin, when exposed, provides little resistance to acid attack [120].
Fermentable carbohydrate diet
The formation of dental caries is associated with the carbohydrate component of the diet. Oral micro-organisms, especially S. mutans , utilize certain carbohydrates to form a sticky matrix that facilitate them to stick to the teeth. Organic acids are formed from the carbohydrates, which demineralize the teeth [121]. The frequent consumption of soluble carbohydrate as well as their prolonged contact with tooth surfaces is highly significant risk factors [122].
Microflora
The micro-organisms are responsible for dental caries. The early colonizers of these micro-organisms are mainly mutans streptococci, produce large amounts of acid, especially lactic acid, which are potent in causing tooth demineralisation [123]. Attachment of the S. mutans is now thought to be independent of sucrose and mediated by adhesions on the bacterial surfaces interacting directly with the salivary proteins, which form the pellicle on the tooth surface.
Time
Time is a significant factor in the progress of caries in relation to the frequency and amount of exposure of the liquid which will affect both the severity of the lesions and the number of teeth involved. The frequency of contact of the substrate has a major role in cariogenicity during a 24-h period [118].
Stages & Symptoms of dental caries
The different five stages of dental caries with symptoms are as follows (Figures 5 and 6):
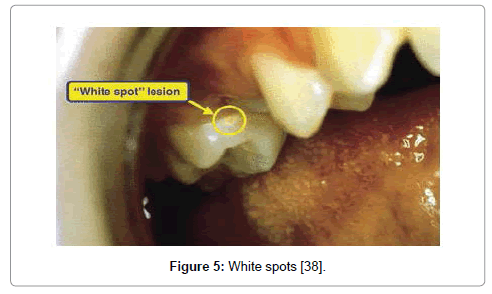
Figure 5: White spots [38].
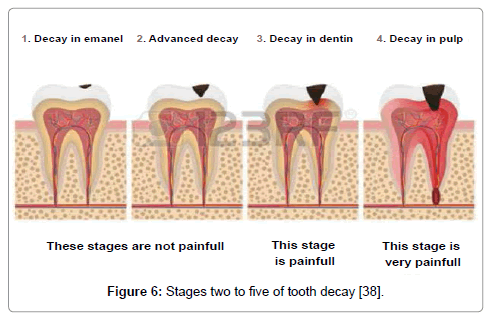
Figure 6: Stages two to five of tooth decay [38].
a. Stage One: white spots
The appearance of yellowish spots or chalky white areas on the surface of tooth due to the loss of calcium is the first stage of tooth decay which is still reversible with appropriate treatment. There are no subjective symptoms, including pain (Figure 5) [2].
b. Stage Two: enamel decay
The enamel of tooth begins flouting beneath the exterior layer with the outside intact during this stage. The surface of the tooth ruins when decay persists. Such type of damage is irreversible with no pain or sensitivity [123].
c. Stage Three: dentin decay
In this stage, the decay progresses beyond the enamel into the dentin with pain [124].
d. Stage Four: involvement of the pulp
The infection of the pulp of the tooth starts in the fourth stage due to the action of bacteria. Blood vessels and nerves in the pulp die due to pus formation [123].
e. Stage Five: abscess formation
This is the final stage of the infection which reaches to the root tip of the tooth causing severe pain. The bones surrounding the tooth also get infected and visible swelling on the cheeks, along the affected side is observed [124].
Problems Associated with Tooth Decay
The complications of tooth decay are toothache, pulpitis, tooth loss, tooth discoloration, oral cancer, endocarditis, cavernous sinus thrombosis and Ludwig angina can be fatal [6,38,125].
Dental Caries Risk Assessment
Risk factors are either “causal” or “associated” is one of the most important queries. Initially, the consumption of sugar, plaque, hygiene regimen and the host were considered as local caries process were the associated risk factors for caries. Burt in 2005 highlights that these factors should be comprehensive and stated that “Social determinants of health and population health are also associated with caries”. Major three types of factor related to tooth decay risk assessment have been defined:
Risk factor or caries-promoting factor
The exposure that plays an essential role in caries development is known as risk factor.
a. Sugar consumption
The growth rate of many oral bacteria increases and changes the composition of the microflora in a caries-promotion with sucrose- rich diet [126]. Acid-producing micro-organisms, such as the mutans streptococci in dental plaque, play a crucial role in the caries development [127].
b. Tooth location
Molars and premolars are more susceptible towards tooth decay as these teeth have lots of grooves, pits and crannies that can accumulate food particles. As a consequence, they’re difficult to maintain hygienic and clean. Plaque can fabricate and bacteria can increase between back teeth, producing the acid that demolishes tooth enamel.
Risk indicator
The exposure that co-exists with an increased probability of developing a disease is known as risk indicator [128].
a. Socio-economic factors
Low socio-economic status and immigrant background that ultimately influence oral hygiene standards and attitudes to tooth care in children and adolescents are social risk indicators [129,130].
b. Age
In the United States, cavities are more frequent in children and teenagers and older adults also are at elevated jeopardy. Teeth can wear down and gums may recede, making teeth more vulnerable to root decay by overtime. Older adults can possibly use supplementary medications that decrease saliva flow, escalating the risk of dental caries [131].
c. Dry mouth
Dry or dehydrated mouth is caused by a deficiency of saliva. Substances found in saliva also assist to oppose the acid produced by bacteria and can help in restore early caries. Certain medications, some medical conditions, radiation to head or neck, or certain chemotherapy drugs can augment risk of cavities by reducing saliva production [132].
d. Worn fillings or dental devices
Dental fillings can weaken, begin to break down or develop rough edges over the years which allow plaque to build up more simply and make it harder to remove. Dental devices can also stop fitting well, allowing decay to begin beneath them [133].
e. Eating disorder
Significant tooth erosion and cavities can also be due to anorexia and bulimia. Stomach acid from repeated vomiting (purging) cleanses over the teeth and instigates melting the enamel. Eating disorders can also hinder with saliva production [130].
f. Heartburn
Gastroesophageal reflux disease (GERD) or heart burn can cause stomach acid to flow into mouth (reflux), wearing away the enamel of teeth leads to significant tooth damage [131].
g. Genetic factors
The presence of bacteria and carbohydrates are necessary for caries to develop. Whether this actually happens depends upon the inherited or acquired resistance of the teeth [134].
h. Malnutrition
Horowitz (1998) stated that children who are malnourished pre-, peri- or post-natally and/or who are of low birth weight are likely to have hypomineralised or hypoplastic primary teeth. These teeth have a higher risk of becoming carious and are more susceptible to mutans streptococci colonization [134].
Risk Inhibitor
Risk inhibitor is an exposure that prevents with the probability of developing a disease.
a. Proper tooth brushing and use of fluoride toothpaste
The declination of caries can occur with the use of fluoride toothpaste with daily tooth brushing. Fluoride has significant effect in caries prevention which is mostly due the topical effect of different fluoride vehicles after tooth eruption [133].
b. Antibacterial therapy
Products such as chlorhexidine rinses are effective [135].
c. Fermentable carbohydrates
Reduce the amount and frequency of ingestion.
d. Salivary flow
Salivary flow can be increased by chewing sugarless gum, for example, those with a non-sugar sweetener such as xylitol [136].
Immunology of Caries with Relation to IgA
IgA deficiency is a comparatively widespread disease affecting 1:1000 individuals associated with tooth decay. Evidence suggests that IgA deficiency fell into two groups in terms of oral antibody: i.e. those with compensatory IgM antibodies against S. mutans in saliva and those without.
Increased caries activity has been reported in Panhypo-or agammaglobulinemia. Human parotid IgA antibodies against surface antigen I/II of S. mutans could block S. mutans adhesion to saliva-coated hydroxyapatite highlighting the mechanism of protection available to the host against certain cariogenic bacteria. Serum antibodies, intragingival antibodies, complement, and granulocytes are continuously extravagating from the periodontal crevice and into the oral environment. These components may bestow modest defense to the tooth in the cervical area, but they are not likely to be important in coronal portions of the teeth [137] (Table 3).
| Tests | S. mutans |
S. mitis | S. vestibularis | S. aureus | S. albus | K. pneumoniae | Pseudomonas spp. | P. Vulgaris | Enterobacter spp. |
|---|---|---|---|---|---|---|---|---|---|
| Catalase | - | - | - | + | + | + | + | + | + |
| Coagulase | ND | ND | ND | + | - | ND | ND | ND | |
| Oxidase | - | - | - | - | - | + | + | - | - |
| Motile | - | - | - | - | - | - | - | + | + |
| Indole | + | - | - | - | - | - | - | + |
|
| Citrate | - | - | - | + | + | + | + | + | + |
| Urease | - | - | + | + | - | + | + | + | - |
| Optochin | R | R | R | ND | ND | ND | ND | ND | ND |
| Bile solubility | R | R | R | ND | ND | ND | ND | ND | ND |
| Fructose | AG | A | A | A | A | A | NR | A | A |
| Lactose | A | A | A | A | AG | AG | NR | NR | A |
| Arabinose | NR | NR | NR | A | AG | NR | NR | A | NR |
| Sucrose | A | A | A | A | AG | NR | NR | AG | A |
| Maltose | A | NR | A | A | A | A | NR | NR | A |
| Glucose | A | A | A | A | A | A | A | A | A |
| Mannitol | ND | ND | ND | A | NR | ND | ND | ND | ND |
| Keywords: NR-No reaction; A-Acid production; ND-Not done; +ve=Positive; -ve=Negative; R- Resistant [38]. | |||||||||
Table 3: Identification of bacterial isolates from dental caries by biochemical tests.
Diagnosis
• Examination of all visible tooth surfaces using a good light source, dental mirror and explorer for a small chalky area or cavity [2,38].
• Early, uncavitated caries is often diagnosed by blowing air across the suspect surface [2,38,138].
• X-rays of tooth are used for less visible areas of teeth in particular caries between the teeth [2,38].
• Lasers without ionizing radiation also now used for detection of interproximal de cay [2,38,139].
• Dental fluorosis and developmental defects of the tooth including hypomineralization of the tooth and hypoplasia of the tooth are used for dental caries [2,38,139].
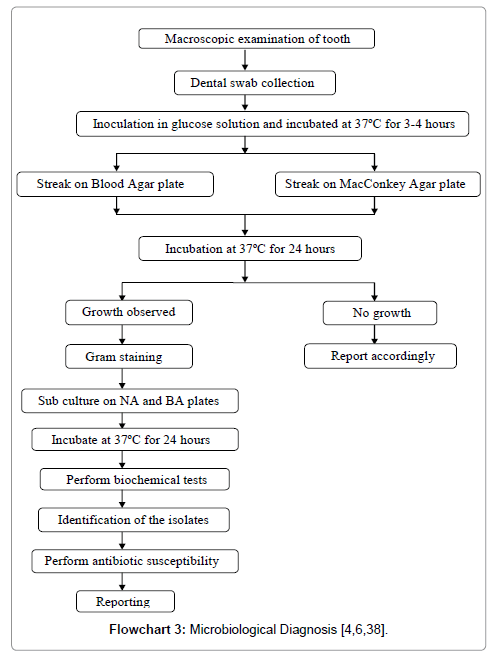
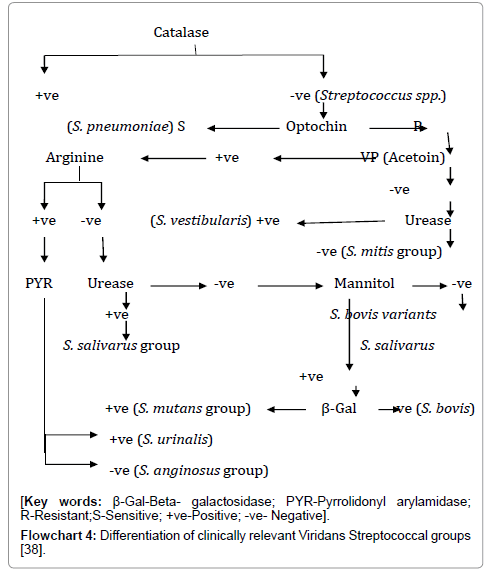
Microbiological Diagnosis (Table 3, Flowcharts 3 and 4)
Flowchart 4: Differentiation of clinically relevant Viridans Streptococcal groups [38].
Antibiotics commonly used in dental practice
Both anaerobic and aerobic Gram positives and Gram negatives bacteria are involved in most of the oral infections. A depiction of the most-prescribed antibiotics in dental practice is listed.
β-Lactam antibiotics
In dentistry, penicillins are the most broadly used antimicrobial agents among all β-lactams antibiotics [6,38]. The primary interest to dental practitioners is the narrow-spectrum penicillinase-sensitive agents as penicillin G and penicillin Vand the broad-spectrum aminopenicillins as ampicillin and amoxicillin. Penicillin V, phenoxymethylpenicillin is active against streptococci and most oral anaerobes which is orally administered [6,38,140]. Phenoxymethylpenicillin is effective against a majority of α-haemolytic streptococci and penicillinase-negative staphylococci.
Aerobic Gram-positive organisms, including Actinomyces , Eubacterium , and Peptostreptococcus species, are sensitive together with anaerobic Gram negative organisms, such as, Bacteroides, Prevotella, and Porphyromonas, Fusobacterium, and Veillonella species. The majority of Staphylococcus aureus strains have developed resistance to the drug. Acute purulent infections, post-extraction infections, and salivary gland infections is treated with Phenoxymethylpenicillin frequently prescribed by dental practitioners [6,38,141,142].
Sometime, ampicillin is used in the experimental treatment of dento-alveolar infections when the antibiotic sensitivity patterns of the causative organisms are unidentified [38,141]. It is also universal to combine some penicillin with β-lactam inhibitory substances such as clavulanic acid, sulbactam, or tazobactam which block the β-lactamase enzyme produced by the bacteria from functioning and amplify the capability of the β-lactam antibiotic to work [38,143].
Metronidazole
Microbial RNA synthesis on bacteria is inhibited by the action of metronidazole. This drug is active against almost all strict anaerobes including Bacteroides,Eubacterium, Fusobacterium , and Peptostreptococcus species. The drug is indicated in the treatment of acute necrotizing ulcerative gingivitis and for moderate to severe odontogenic infections, frequently in combination with penicillins [6,38,140,141].
Tetracyclines
Tetracyclines are broad-spectrum bacteriostatic drugs. It binds to the 30S ribosomal subunit of bacteria, and specifically inhibits the binding of aminoacyl-t-RNA synthetases to the ribosomal acceptor site, thus inhibiting protein synthesis [38,141]. Tetracycline, doxycycline, and minocycline are the well-known members of this family of antibiotics.
In dentistry, tetracyclines are used with a few accomplishments as adjunctive management in localized aggressive periodontitis [38,141]. Due to few side effects of tetracyclines, it is not recommended for children or pregnant women as it can discolor developing teeth and alter bone development [38,144].
Macrolides and lincosamides
Macrolides are bacteriostatic drugs having activity against streptococci, staphylococci, and some oral anaerobes [140]. It exhibits their action by interfering with bacterial protein synthesis by binding to the 50S ribosomal subunit to the donor site during the translocation step [143]. Erythromycin, clarithromycin, and azithromycin are members in this family.
In penicillin-allergic patients, erythromycin is used as a substitute of penicillins with an additional benefit of being active against β-lactamase producing strains [38,141]. Clindamycin, potent bactericidal antibiotic is a lincosamide having a wider host range than erythromycin. It is effective against both aerobic and anaerobic species of bacteria and exhibits its action by interfering with protein synthesis. In dentistry, clindamycin has its main indication in penicillin-allergic patients who require antibiotic prophylaxis prior to dental treatments [38,145].
Antibiotic Resistance
Bacterial resistance to antimicrobials can be clear from two perspectives either genotypically, where the bacteria carry certain resistance elements or phenotypically, where the bacteria can survive and grow above a certain level of antibiotics in the laboratory. Clinically, the bacteria are capable to multiply in humans in the presence of drug concentrations during therapy [6,146]. Bacterial resistance to antimicrobial agents can be either natural (inherent, intrinsic) or acquired.
a. Natural (inherent, intrinsic) resistance
In this category of resistance, all isolates of a certain bacterial species are not sensitive to the antimicrobial as there is lack of certain structures in bacteria which serve as the target molecules for the antimicrobial or the lack of metabolic processes essential for the activation of the antimicrobial [147].
Example, enterococci are intrinsically resistant to cephalosporins as there are no penicillin-binding proteins in enterococci that bind cephalosporins drugs with high affinity. Consequently, intrinsic resistance is due to lack of metabolic processes and is also observed amongst oral microbes [38,148]. For example, Actinomyces species, Streptococcus species, and A. actinomycetemcomitans lack nitroreductase enzyme which is necessary to convert metronidazole to its active metabolites. Thus, these species are not affected by the drug at normal therapeutic concentrations. Facultative anaerobic metabolism limits the uptake of aminoglycosides because of the absence of an electron-transport chain [38,149].
b. Acquired resistance
Acquired resistance in microorganisms is evolved due to genetic alteration which can be attained by two processes:
a) Chromosomal mutation: It occurs in the pre-existing bacterial genome.
b) Horizontal gene transfer: It is the most recurrent pathway for the propagation of antibiotic resistance genes. Most frequently, Horizontal gene transfer occurs between bacteria both within and outside species. A resistance gene may be inserted into transferable genetic elements (plasmids, transposons, and integrons) and may be linked within them to other resistance genes in horizontal gene transfer [146].
Mechanisms of Acquired Antibiotic Resistance
Specialized defense mechanisms are utilized by bacteria for their survival in an environment in which antimicrobials are designed to kill them. The principal ways utilized by bacteria to render antimicrobials ineffective are as follows:
a. Alteration of target site
The target molecules are structurally altered to prevent antibiotic binding. An example, the targets of fluoroquinolones is the alteration of ribosomal target sites in the DNA gyrase/topisomerase genes. Modification of the PBPs may occur through mutation in the chromosomal genes encoding the enzymes or through the acquisition of foreign homologous genes or fragments of genes from related species encoding new PBPs. This is more prevalent in Gram positive cocci but seen less frequently in Gram negative bacteria. Methicillin resistant S. aureus (MRSA) is known to produce an alternative PBP (2a) that bypasses the consequence of the antibiotic [150,151]. Resistance to β-lactam antibiotics might be caused by the production of low-affinity PBPs. This resistance mechanism is pervasive along with the oral viridans streptococci such as Streptococcus oralis, Streptococcus sanguis and Streptococcus mitis [152].
b. Modification of metabolic pathway
Antibiotics are expelled from cell entry. Porin channels are utilized by several antibiotics when entering Gram negative bacteria. Thus, the diminished expression of porins results in impermeability or decreased uptake that often leads to antibiotic resistance [27,145,153].
c. Reduced drug accumulation
Efflux pump is the mechanism where antibiotics are pumped out of the cell where bacteria can actively efflux the antimicrobial agent. The most important five families of the efflux system are: MFS: Major Facilitator Superfamily; RND: Resistance Nodulation-Division; SMR: Small Multidrug Resistance; ABC: ATP Binding Cassette; and MATE: Multidrug and Toxic Extrusion [27,145].
d. Drug inactivation or modification
Antibiotics are inactivated through enzymatic degradation. As for example, this mechanism is resistance against β-lactam antibiotics because of inactivation of β-lactamases. These enzymes present resistance to the most extensively used 24 antimicrobials in medical and dental practice, i.e. β-lactams [6,38,147].
Antimicrobial Resistance Pattern of Dental caries
The study carried by Olajokun et al. reported 45.6% S. mutans, 41.2% Lactobacillus spp and 13.2% S. aureus were isolated from carious lesion. This study also showed 43.2% mixed growth of S. mutans and Lactobacillus spp. 38.6% of S. mutans and S. aureus , 18.2% of Lactocillus spp and S. aureus. Pefloxacin, chloramphenicol, ceftriaxone and ciprofloxacin were the most effective drug (76.1% to 92.2%) against the caries [38,154].
The study conducted at Dental Unit of General Hospital Minna, Nigeria reported Staphylococcus aureus had the highest degree of occurrence with 31 isolates, followed by Streptococcus mutans with 23 isolates, while the least was Lactobacillus spp with 4 isolates. All the strains of Streptococcusmutans , and Staphylococcus aureus were sensitive to ofloxacin and nitrofurantoin and totally resistant to co-trimozazole and erythromycin. Only a small percentage of these strains were sensitive to chloramphenicol, ceftriaxone, gentamycin, tetracycline, cefuroxime and cefotaxime. Lactobacillus strains were totally resistant to all the antibiotics except ofloxacin [38,155].
A study conducted by Maripandi et al.reported 87 bacterial isolates were associated with caries from which 63 were facultative anaerobes and 24 were anaerobes. Among facultative anaerobes, 22.98% were Streptococcus mutans , 13.79% were S. salivarius, 14.97% were Candida spp and among anaerobes 12.64% were Prevotella spp and 5.74% were Fusobacterium spp. All the isolates of Streptococcus spp were resistant to penicillin and sensitive to tetracycline [156].
S. aureus was found to be the most prevalent organism in tooth decay followed by S. mutans and Lactobacillus species in a study conducted at Department of the Rajah Muthiah Dental College and Hospital Annamalai University. Lactobacillus species were resistant to gentamycin, tetracycline, cotrimoxazole and cholramphenicol except ofloxacin [157]. The bacterial isolates implicated in dental caries were Streptococcus mutans, Klebsiella pneumoniae , Staphylococcus albus, Proteus vulgaris and Pseudomonas aeruginosa. Streptococcus mutans (53.13%) andonly 6.25% of Proteus vulgaris and Pseudomonas aeruginosa were isolated from caries. All isolates were sensitive to ciprofloxacin, gentamycin and norfloxacin while highest level of resistance was shown to chloramphenicol and tetracycline in this study [38,158].
In a study conducted by Salman and Senthikumar, 55.38% were S. mutans and 7.69% were S. sobrinus . All S. mutans and S. sobrinus isolates were vulnerable to penicillin, ampicillin, cefotaxime, cephalothin, cefazolin, methicillin, erythromycin and chloramphenicol. Penicillin and ampicillin were the most effective antibiotics against S. mutans and S. sobrinus and no resistance was found in this study [159]. As per Dwivedi et al., the resistance percentages of bacterial isolates were found to be 48% of penicillin V, 66% of tetracycline, 90% of amoxicillin, 78% of cloxocillin, 60% of erythromycin, 26% of penicillin V/amoxicillin and 30% of amoxicillin/ erythromycin [160].
In a study conducted by Bancescu et al., 73 isolates were S. oralis , 6 isolates were S. mitis , 5 isolates were S. sanguis and one isolate was S. gordonii associated with caries. The isolates were 78.8% susceptible to penicillin, 93% were susceptible to ampicillin, 98% were susceptible to cefotaxime, and 90.6% were susceptible to erythromycin. All the isolates were susceptible to clindamycin and chloramphenicol but 41.2% were resistant to tetracycline [161].
In a study conducted by Williamet al.on dental caries, the antibiotic resistance pattern of S. mutans and S. sobrinus towards penicillin and tetracycline was found to be 56% and 44% respectively. This study showed that 54.7% of bacterial isolates were resistant to cotrimoxazole while1.6% of bacterial isolates were resistant to amoxicillin. Of 95% of the isolates in the prophylaxis group showed resistance to sulfamethoxazole, while 61% of resistance was towards trimethoprim. Similarly, in the non-prophylaxis group, the most prevalent resistance was associated with tetracycline(35.7%), while the least resistance was found witherythromycin (3.6%). Resistance to cotrimoxazolein the non-prophylaxis group was 15.5% [162].
At the Plateau State Dental Hospital Jos,74% Streptococcus mutans were associated with dental caries. The molar teeth were more affected with 56% compared to the premolars and the roots with 35% and 9% respectively.The number of cases with dental caries was found to be highest between ages of 21-40 years with more females (54%) than male (46%). Streptococcus mutans wassusceptible to ampicillin (84%), amoxicillin (90%), ciprofloxacin (85%), penicillin (78%), ampicillin/cloxacillin (55%) and streptomycin (30%), but resistant to erythromycin, gentamycin and cefuroxine in this study [163].
Similarly, a study conducted by Kariminik (2015) showed all the isolates of Streptococcus mutans were sensitive to used antibiotics, penicillin, gentamycin, vancomycin, cephalotin [164]. In 2006, the viridans group streptococci (VGS) strains were isolated from carious lesions of 49.3% from children with tooth decay. S. mitis were isolated most frequently from 4-5 year old while S. vestibularis was isolated most often in 12 years old. VGS strains were resistant to erythromycin (23.5%), clindamycin (23.1%), tetracyclines (52%), doxycycline (16%), gentamycin (25.9%) and ciprofloxacin (55.2%) [165].
A study conducted on dental caries by Yadav et al. 2015 at Department of Microbiology, Janaki Medical College, Ramdaiya, Janakpurdham, Nepal reported 66.15% isolates of S. mutans were resistant to Penicillin; 60.76% were to tetracycline and 20% were resistant to cotrimoxazole. S. aureus washighly resistant towards penicillin (91.48%), tetracycline (86.17%) and ampicillin (61.70%). S. mitis was resistant to tetracycline (78.12%), ciprofloxacin (65.62%). Pseudomonas spp were highly resistant to tetracycline followed by cotrimoxazole (90.90%) [4].
Conclusion
Dental caries is seen as a common and costly disease in the world that can greatly affect the health and quality of life. This review highlights significant role of the Streptococcus and Lactobacillus which are acidogenic and aciduric in the development of caries. Other Streptococci, Enterococci, Actinomycetes are also important etiological agents of dental caries. The population interactions are complex and apart from the commonly known mutualism, competitions etc. involve stress responses, adaptation, variation in gene expression, genetic variation and probably quorum sensing. The consumption of easily fermentable carbohydrates which stimulates the growth of oral microbes is the main cause of dental caries. Several risk factors of dental caries are also documented in this review. The mechanism of drug resistance developed by bacteria embedded in biofilm, which are up to 1000- fold more resistant to antibiotics. The increased bacterial resistance to antibiotics currently used in dentistry has a great importance for the prevention of oral bacterial growth, adhesion and colonization. So, it is extremely important to increase the knowledge towards their mechanisms, focusing on prevention and the correct therapeutic approaches usually involve decreasing the growth of S. mutans. The access and efficient use of regular dental care, both preventive and restorative, including optimal use of sealants should be ensured in dealing with this serious dental public health crisis. The antibioitic resistance pattern of the bacterial isolates should be monitored at different time interval regularly.
References
- Thean H, Wong ML, Koh H (2007) The dental awareness of nursing home staff in Singapore -a pilot study. Gerodontology 24: 58-63.
- Yadav K, Prakash S (2016) Dental Caries: A Review. Asian J of Biomed and Pharmaceutical Sci 6: 1-7.
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C (2005) The global burden of oral diseases and risks to oral health. Bull World Health Organ 83: 661-669.
- Yadav K, Prakash S, Yadav NP, Sah RS (2015) Multi-Drug resistance of Bacterial isolates among Dental Caries Patients. Janaki Medical College Journal of Medical Sci 3: 37-44.
- De Marchi RJ, Hugo FN, Padilha DM, Hilgert JB, Machado DB, et al. (2011) Edentulism, use of dentures and consumption of fruit and vegetables in south Brazilian community-dwelling elderly. J Oral Rehabil 38: 533-540.
- Yadav K, Prakash S (2015) Antibiogram profiles against polymicrobial pathogens among dental caries patients at Janaki Medical College teaching hospital, Nepal. Int J Applied Dental Sci 1: 156-162.
- Hartmann JR, Richard W (2008) Ludwig's Angina in Children.
- Cvitkovitch DG (2001) Genetic competence and transformation in oral streptococci. Crit Rev Oral Biol Med 12: 217-243.
- Yadav K, Prakash S (2016) Knowledge, Attitude and Practice on Dental Caries and Oral Hygiene among Medical Students at Janaki Medical College Teaching Hospital. Interna J Medic Biomedi Sci 1: 22-31.
- Sweeney LC, Dave J, Chambers PA, Heritage J (2004) Antibiotic resistance in general dental practice--a cause for concern? J Antimicrob Chemother 53: 567-576.
- Al-Haroni M, Skaug N (2007) Incidence of antibiotic prescribing in dental practice in Norway , its contribution to national consumption. J Antimicrob Chemother 59: 1161-1166.
- Gashaw M, Abtew D, Addis Z (2014) Prevalence and antimicrobial susceptibility pattern of bacteria isolated from mobile phones of health care professionals working in Gondar town health centers. Hindawi Publishing Corporation, ISRN Public Health 10: 1155-1161.
- Kisumbi BK, Kaimenyi JT, Wakiaga JM (1995) Knowledge on treatment modalities and attitude of Nairobi University students towards dental care. Indian J Dent Res 6: 133-136.
- Bader O, Schaller M, Klein S, Kukula J, Haack K, et al. (2001) The KEX2 gene of Candida glabrata is required for cell surface integrity. Mol Microbiol 41: 1431-1444.
- Kear BP (2001) Dental caries in an Early Cretaceous ichthyosaur. Alcheringa: an Australian journal of palaeontol 25: 387-390.
- Kemp A (2003) Dental and skeletal pathology in lungfish jaws and tooth plates. Alcheringa: an Australian journal of palaeontol 27: 155-170.
- Gorrel C (2006) Odontología Veterinaria en la práctica clínica. Ed. Servet, Zaragoza.
- Forrai J (2009) The beginnings of dental caries and its treatments. Rev Clín Pesq Odontol Curitiba 5: 187-192.
- Selwitz RH, Ismail AI, Pitts NB (2007) Dental caries. Lancet 369: 51-59.
- Suddick RP, Harris NO (1990) Historical perspectives of oral biology: a series. Crit Rev Oral Biol Med 1: 135-151.
- ADA Methamphetamine Use (METH MOUTH) (2014) Hosted on the American Dental Association.
- Hoffman-Axthelm W (1981) History of dentistry. Chicago: Quintessence Pub Co.
- MacPhee G (1935) Studies in the aetiology of dental caries. John Bale, Sons & Dainelsson Ltd, London, UK.
- Asbell M (1994) Research in dental caries in the United States: 1820-1920. Compendium 15: 792-8.
- http://www.fauchard.org/dentalworld/2001/DW.08/DWpfaAug01page1.htm
- Kleinberg I (2002) A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 13: 108-125.
- Baehni PC, Guggenheim B (1996) Potential of diagnostic microbiology for treatment and prognosis of dental caries and periodontal diseases. Crit Rev Oral Biol Med 7: 259-277.
- Allukian M Jr (2000) The neglected epidemic and the surgeon general's report: A call to action for better oral health. Am J Public Health 90: S82-S85.
- Newbrun E (1989) Cariology. Quintessence Books, Chicago.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2163-2196.
- http://www.who.int/oral_health/media/en/orh_report03_en.pdf
- World Health Organization (2006).
- http://www.healthypeople.gov.
- Jamison DT, Breman JG, Measham AR (2006) Disease Control Priorities in Developing Countries (2ndedition.). Oxford University Press, New York.
- Touger-Decker R, van Loveren C (2003) Sugars and dental caries. Am J Clin Nutr 78: 881S-892S.
- Africa CW, Nel J, Stemmet M (2014) Anaerobes and Bacterial Vaginosis in Pregnancy: Virulence Factors Contributing to Vaginal Colonisation. Int J Environmental Research, Public Health 11: 6979-7000.
- Plans NAD 2000 Dental Benefits Report. 2000.
- Yadav K (2016) Dental Caries: Bacterial profile of Dental caries. LAP LAMBERT Academic Publishing, OmniScriptum GmbH & Co. KG, Germany. Pp: 120.
- Mjör IA, Toffenetti F (2000) Secondary caries: a literature review with case reports. Quintessence Int 31: 165-179.
- Bowden GHW, Ellwood DC, Hamilton IR (1979) Microbial ecology of the oral cavity. In: Alex, er M (ed.) Advances in Microbial Ecology. Plenum Press, New York. 3: 135-217.
- Theilade E (1990) Factors controlling the microflora of the healthy mouth. In: Hill MJ, Marsh PD (eds.) Human Microbial Ecology. CRC Press, Boca Raton pp: 2-48.
- Bowden GHW (1991) Which bacteria are cariogenic in humans? In: Johnson NW (ed.) Risk Markers for Oral Diseases, Dental Caries. Cambridge University Press, Cambridge 1: 266-86.
- van Houte J (1994) Role of micro-organisms in caries etiology. J Dent Res 73: 672-681.
- Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50: 353-380.
- Nyvad B, Kilian M (1990) Microflora associated with experimental root surface caries in humans. Infect Immun 58: 1628-1633.
- Sarkonen N, Könönen E, Summanen P, Kanervo A, Takala A, et al. (2000) Oral colonization with Actinomyces species in infants by two years of age. J Dent Res 79: 864-867.
- Bowden GH, Ekstrand J, McNaughton B, Challacombe SJ (1990) Association of selected bacteria with the lesions of root surface caries. Oral Microbiol Immunol 5: 346-351.
- Bowden GH (1990) Microbiology of root surface caries in humans. J Dent Res 69: 1205-1210.
- Schüpbach P, Osterwalder V, Guggenheim B (1995) Human root caries: microbiota in plaque covering sound, carious and arrested carious root surfaces. Caries Res 29: 382-395.
- Schüpbach P, Osterwalder V, Guggenheim B (1996) Human root caries: microbiota of a limited number of root caries lesions. Caries Res 30: 52-64.
- Brailsford SR, Lynch EJR, Beighton D (1998) The isolation of Actinomyces naeslundii from sound root surfaces and root carious lesions. Caries Res 32: 100-106.
- Van Ruyven FO, Lingstrom P, van Houte J, Kent R (2000) Relationship among mutans streptococci, ‘low pH’ bacteria and iodophilic polysaccharide-producing bacteria in dental plaque and early enamel caries in humans. J Dent Res 79: 778-84.
- Bowden GH, Hamilton IR (1998) Survival of oral bacteria. Crit Rev Oral Biol Med 9: 54-85.
- Bowden GHW (1999) Oral biofilm an archive of past events? In: Newman HN, Wilson M, eds. Dental Plaque Revisited Oral Biofilms in Health and Disease. Cardiff: Bioline pp: 211-35.
- Li YH, Chen YY, Burne RA (2000) Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ Microbiol 2: 169-177.
- Svensäter G, Sjögreen B, Hamilton IR (2000) Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology 146: 107-117.
- Marquis RE (1995) Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J Ind Microbiol 15: 198-207.
- Sissons CH, Hancock EM (1993) Urease activity in Streptococcus salivarius at low pH. Arch Oral Biol 38: 507-516.
- Rainey PB, Moxon ER, Thompson IP (1993) Intraclonal polymorphism in bacteria. In: Gwynfryn Jones J (ed.) Advances in Microbial Ecology. Plenum Press, New York 13: 263-300.
- Bearson S, Bearson B, Foster JW (1997) Acid stress responses in enterobacteria. FEMS Microbiol Lett 147: 173-180.
- Hecker M, Völker U (1998) Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the sigmaB regulon. Mol Microbiol 29: 1129-1136.
- Segal G, Ron EZ (1998) Regulation of heat-shock response in bacteria. Ann N Y Acad Sci 851: 147-151.
- Marsh PD, Bowden GHW (2000) Microbial community interactions in biofilms. In: Lappin-Scott H, Gilbert P, Wilson M, Allison D (eds.) Community Structure and Co-operation in Biofilms. Society for General Microbiology Symposium series. Cambridge University Press, Cambridge.
- Marsch PD, Martin MV (2009) Oral Microbiology (5thedition.). Elsevier pp: 220-222.
- Tanzer JM, Livingston J, Thompson AM (2001) The microbiology of primary dental caries in humans. J Dent Educ 65: 1028-1037.
- Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, et al. (2002) Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40: 1001-1009.
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, et al. (2008) Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46: 1407-1417.
- Ling Z, Kong J, Jia P, Wei C, Wang Y, et al. (2010) Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol 60: 677-690.
- Hohwy J, Reinholdt J, Kilian M (2001) Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun 69: 6055-6063.
- Islam B, Khan SN, Khan AU (2007) Dental caries: from infection to prevention. Med Sci Monit 13: RA196-203.
- Peterson SN, Snesrud E, Schork NJ, Bretz WA (2011) Dental caries pathogenicity: a genomic and metagenomic perspective. Int Dent J 61: 11-22.
- Merritt J, Qi F (2012) The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol 27: 57-69.
- Ma JK, Kelly CG, Munro G, Whiley RA, Lehner T (1991) Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infect Immun 59: 2686-2694.
- Whittaker CJ, Klier CM, Kolenbrer PE (1996) Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol 50: 513-552.
- Petersen FC, Assev S, Van der Mei HC, Busscher HJ, Scheie AA (2002) Functional variation of the antigen I/II surface protein in Streptococcus mutans and Streptococcus intermedius. Infect Immun 70: 249-256.
- Yu H, Nakano Y, Yamashita Y, Oho T, Koga T (1997) Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptcoccus mutans. Infect Immun 65: 2292-2298.
- Brady LJ, Piacentini DA, Crowley PJ, Oyston CP, Bleiweis SA (1992) Differentiation of salivary agglutininmediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect Immun 60: 1008-1017.
- Crowley PJ, Brady JL, Piacentini DA, Bleiweis SA (1993) Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect Immun 61: 1547-1552.
- Hajishengallis G, Koga T, Russell MW (1994) Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res 73: 1493-1502.
- Nakai M, Okahashi N, Ohta H, Koga T (1993) Saliva-binding region of Streptococcus mutans surface protein antigen. Infect Immun 61: 4344-4349.
- Peterson P, Hayes TE, Arkin CF, Bovill EG, Fairweather RB, et al. (1998) The preoperative bleeding time test lacks clinical benefit: College of American Pathologists' and American Society of Clinical Pathologists' position article. Arch Surg 133: 134-139.
- Schilling KM, Blitzer HM, Bowen HW (1989) Adherence of Streptococcus mutans to glucans formed in situ in salivary pellicle. J Dent Res 68: 1678-1680.
- Rolla G, Ciardi EJ, Eggen K, Bowen HW (1983) Afseth: Free glucosyl-and fructosyltransferase in human saliva and adsorption of these enzymes to teeth in vivo. In Glucosyltransferase, Glucans, Sucrose, and Dental Caries. Doyle RJ, Ciardi JE (Eds.) IRL Press, Washington DC, pp. 21-30.
- Janecek S, Svensson B, Russell RR (2000) Location of repeat elements in glucansucrases of Leuconostoc and Streptococcus species. FEMS Microbiol Lett 192: 53-57.
- Banas JA, Russell RR, Ferretti JJ (1990) Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun 58: 667-673.
- Shah DS, Russell BRR (2002) A novel glucan-binding protein from Streptococcus mutans. In Streptococcal Genetics (6thedition.) Asheville, NC.
- Sato Y, Yamamoto Y, Kizaki H (1997) Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect Immun 65: 668-675.
- Tao L, Tanzer JM (2002) Novel sucrose-dependent adhesion co-factors in Streptococcus mutans. J Dent Res 81: 505-510.
- Russell RR (1979) Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol 112: 197-201.
- Smith DJ, Akita H, King WF, Taubman MA (1994) Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect Immun 62: 2545-2552.
- Colby SM, Russell RRB (1997) Sugar metabolism by mutans streptococci. Soc Appl Bacteriol Symp Ser 26: 80S-88S.
- Ajdiń? D, McShan WM, McLaughlin RE, Saviń? G, Chang J, et al. (2002) Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99: 14434-14439.
- Johnson CP, Gross MS, Hillman DJ (1980) Cariogenic potential in vitro in man and in vivo in the rat of lactate dehydrogenase mutants of Streptococcus mutans. Arch Oral Biol 25: 707-713.
- Fitzgerald RJ, Adams BO, Sandham HJ, Abhyankar S (1989) Cariogenicity of a lactate dehydrogenase-deficient mutant of Streptococcus mutans serotype c in gnotobiotic rats. Infect Immun 57: 823-826.
- Svensäter G, Larsson UB, Greif EC, Cvitkovitch DG, Hamilton IR (1997) Acid tolerance response and survival by oral bacteria. Oral Microbiol Immunol 12: 266-273.
- Wilkins JC, Homer AK, Beighton D (2002) Analysis of Streptococcus mutans proteins modulated by culture under acidic conditions. Appl Environ Microbiol 68: 2382- 2390.
- Hamilton IR, Svensäter G (1998) Acid-regulated proteins induced by Streptococcus mutans and other oral bacteria during acid shock. Oral Microbiol Immunol 13: 292-300.
- Hamilton IR, Buckley ND (1991) Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol 6: 65-71.
- Belli WA, Marquis RE (1991) Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol 57: 1134-1138.
- Dashper SG, Reynolds EC (1992) pH regulation by Streptococcus mutans. J Dent Res 71: 1159-1165.
- Quivey RG Jr, Faustoferri R, Monahan K, Marquis R (2000) Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol Lett 189: 89-92.
- Hudson MC, Curtiss R (1990) Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun 58: 464-470.
- Marsh PD (2004) Dental plaque as a microbial biofilm. Caries Res 38: 204-211.
- Marsh PD (1989) Host defenses and microbial homeostasis: role of microbial interactions. J Dent Res 68:1567-1575.
- Suntharalingam P, Cvitkovitch DG (2005) Quorum sensing in streptococcal biofilm formation. Trends Microbiol 13: 3-6.
- Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, et al. (2002) Communication among oral bacteria. Microbiol Mol Biol Rev 66: 486-505.
- Kolenbrander PE (2000) Oral microbial communities: biofilms, interactions and genetic systems. Annu Rev Microbiol 54: 413-437.
- Bensing BA, Rubens CE, Sullam PM (2001) Genetic loci of Streptococcus mitis that mediate binding to human platelets. Infect Immun 69: 1373-1380.
- Rodríguez VV, Busscher HJ, Norde W, van der Mei HC (2002) Softness of the bacterial cell wall of Streptococcus mitis as probed by micro-electrophoresis. Electrophoresis 23: 2007-11.
- Skovsted IC, Kerrn MB, Sonne-Hansen J, Sauer LE, Nielsen AK, et al. (2007) Purification and structure characterization of the active component in the pneumococcal 22F polysaccharide capsule used for adsorption in pneumococcal enzyme-linked immunosorbent assays. Vaccine 25: 6490-6500.
- Dinesh MD, Uma MS, Meenatchisundaram S, Anjali VM, Athira PS, et al. (2016) Streptococcal Dental Caries-A Short Review. Int J Curr Res Aca Rev 4: 160-170.
- Smith SI, Aweh AJ, Coker AO, Savage KO, Abosede DA, et al. (2001) Lactobacilli in human dental caries and saliva. Microbios 105: 77-85.
- Caufield PW, Li Y, Dasanayake A, Saxena D (2007) Diversity of lactobacilli in the oral cavities of young women with dental caries. Caries Res 41: 2-8.
- Forbes AB, Sahm FD, Weissfelt SA (2007) Bailey and Scott’s Diagnostic Microbiology (12th edition) CV Mosby- Year book Inc, St. Louis Missouri Baltimore pp. 168-187.
- https://arunmktrichy.wordpress.com/2009/02/15/theoriesofdentalcariescausesandetiology/
- http://www.juniordentist.com/acidogenictheoryorchemicoparasitictheoryofdentalcaries.html
- Hurlbutt M, Novy BSM, Young D (2010) Dental Caries: A pH-mediated disease Life Long Learning CDHA J pp: 9-15.
- Reisine S, Douglass JM (1998) Psychosocial and behavioral issues in early childhood caries. Community Dent Oral Epidemiol 26: 32-44.
- Ripa LW (1988) Nursing caries: a comprehensive review. Pediatr Dent 10: 268-282.
- Seow WK (1998) Biological mechanisms of early childhood caries. Community Dent Oral Epidemiol 26: 8-27.
- Rajab LD, Hamdan MA (2002) Early childhood caries and risk factors in Jordan. Community Dent Health 19: 224-229.
- Dimitrova MM, Kukleva MP, Kondeva VK (2002) Prevalence of early childhood caries and risk factors in children from 1 to 3 years of age in Plovdiv, Bulgaria. Folia Med (Plovdiv) 44: 60-63.
- Ayhan H, Suskan E, Yildirim S (1996) The effect of nursing or rampant caries on height, body weight and head circumference. J Clin Pediatr Dent 20: 209-212.
- www.zipheal.com
- https://www.lion.co.jp/en/
- www.rightdiagnosis.com
- Marsh PD, Nyvad B (2001) The oral microflora and biofilms in teeth. In: Fejerskov O, Kidd EAM (eds.) Dental caries. The disease and its clinical management (3rdedn.) Copenhagen: Blackwell Munksgaard pp: 29-48.
- Li Y, Caufield PW, Dasanayake AP, Wiener HW, Vermund SH, et al. (2005) Mode of delivery and other maternal factors influence the acquisition of steptococcus mutans in infants. J Dent Res 84: 806-811.
- Paul J (2000) Oral epidemiological studies in a Swedish adult population. Thesis. Oxford: Oxford University Press. Pp: 1623-38.
- Källestål C, Wall S (2002) Socio-economic effect on caries. Incidence data among Swedish 12-14-year-olds. Community Dent Oral Epidemiol 30: 108-114.
- Julihn A, Barr Agholme M, Grindefjord M, Modéer T (2006) Risk factors and risk indicators associated with high caries experience in Swedish 19-year-olds. Acta Odontol Scand 64: 267-273.
- Poutanen R, Lahti S, Seppä L, Tolvanen M, Hausen H (2007) Oral health-related knowledge, attitudes, behavior and family characteristics among Finnish schoolchildren with and without active initial caries lesions. Acta Odontol Scand 65: 87-96.
- Bolin AK, Bolin A, Jansson L, Calltorp J (1997) Children's dental health in Europe. Swed Dent J 21: 25-40.
- Nyvad B (2003) The role of oral hygiene. In: Fejerskov O, Kidd EAM (eds.) Dental caries: The disease and its clinical management (3rdedn.) Copenhagen: Blackwell Munksgaard 171-177.
- Davies GN (1998) Early childhood caries-a synopsis. Community Dent Oral Epidemiol 26: 106-116.
- Kamal AH, Tefferi A, Pruthi RK (2007) How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time and bleeding time in adults. Mayo Clin Proc 82: 864-873.
- Karger R, Donner-Banzhoff N, Müller HH, Kretschmer V, Hunink M (2007) Diagnostic performance of the platelet function analyzer (PFA-100) for the detection of disorders of primary haemostasis in patients with a bleeding history-a systematic review, meta-analysis. Platelets 18: 249-260.
- http://www.nutricion
- Rosenstiel SF (2000) Clinical Diagnosis of Dental Caries: A North American Perspective. J Dent Educ 65: 979-84.
- Fejerskov O, Kidd E (2008) Dental Caries: the disease and its clinical management. (2ndedition.) Blackwell Munksgaard, Copenhagen pp: 356-360.
- Forbes AB, Sahm FD, Weissfelt SA (2007) Bailey and Scott’s Diagnostic Microbiology (12thedition.), CV Mosby- Year book Inc, St. Louis Missouri Baltimore pp: 168-187.
- Richard J, Lamont MSL, Robert A, Burne D, Blanc LJ (2006) Antibiotics and Treatment of Infectious Diseases. Oral Microbiology and Immunology. ASM Press, Washington, DC pp: 393-422.
- Samaranayake LP (2006) Antimicrobial chemotherapy. Essential Microbiology for Dentistry (1stedition.) Philadelphia, PA. Churchill Livingstone Elsevier pp: 67-76.
- Tong DC, Rothwell BR (2000) Antibiotic prophylaxis in dentistry: a review and practice recommendations. J Am Dent Assoc 131: 366-374.
- Roberts MC (2002) Antibiotic toxicity, interactions and resistance development. Periodontol 2000 28: 280-297.
- Addy LD, Martin MV (2005) Clindamycin and dentistry. Br Dent J 199: 23-26.
- Andersson D (2004) The ways in which bacteria resist antibiotics. Seminar on the Global Threat of Antibiotic Resistance-Exploring Roads towards Concerted Action. pp: 5-7.
- Gaetti-Jardim EC, Marqueti AC, Faverani LP, Gaetti-Jardim EJ (2010) Antimicrobial resistance of aerobes , facultative anaerobes isolated from the oral cavity. J Appl Oral Sci 18: 551-559.
- Rice LB, Bellais S, Carias LL, Hutton-Thomas R, Bonomo RA, et al. (2004) Impact of specific pbp5 mutations on expression of beta-lactam resistance in Enterococcus faecium. Antimicrob Agents Chemother 48: 3028-3032.
- Khosravi AD, Mehdinejad M, Heidari M (2007) Bacteriological findings in patients with ocular infection and antibiotic susceptibility patterns of isolated pathogens. Singapore Med J 48: 741-743.
- Ayla-Nunez NV, Villegas HL, Turrent LC, Padilla C (2009) Silver nanoparticles toxicity and bactericidal effects against meticillin-resistant Staphylococcus aureus: Nanoscale does matter. Nanobiotechnology 5: 2-9.
- Yadav K, Prakash S (2016) Antimicrobial resistance (AMR): A Global problem. Global Journal of Public Health and Epidemiology 3: 120-138.
- Ono T, Shiota S, Hirota K, Nemota K, Tsuchiya T, et al. (2000) Susceptibilities of oral and nasal isolates of Streptococcus mitis and Streptococcus oralis to macrolides and PCR detection of resistance genes. Antimicrob Agents Chemother 44: 1078-1080.
- Olajokun HRE, Folarin AA, Olaniran O, Umo AN (2008) The prevalent bacterial isolates of dental caries in school age children attending the Dental Clinic of Oauthc, ILE-IFE. Afri J Clinic Exper Micro 9: 103-108.
- Daniyan SY, Abalaka ME (2011) Prevalence and Susceptibility Pattern of Bacterial Isolates of Dental Caries in a Secondary Health Care Institution, Nigeria. Shiraz E-Med J 12: 135-139.
- Maripandi A, Kumar AT, Al Salamah AA (2011) Prevalence of dental caries bacterial pathogens, evaluation of inhibitory concentration effect on different tooth pastes against Streptococcus spp. Afric J Microbio Res 5: 1778-1783.
- Fayaz M, Sivakumaar PK, Joe MM (2014) Prevalence and Antibiotic Susceptibility Pattern of Dental Biofilm forming Bacteria. Int J Curr Microbiol App Sci 3: 46-50.
- Omolaja BO, Omolaja EH, Omolaja AT, Adenekan AM (2013) Antibiotic Sensitivity Profiles of Bacteria Isolated from Decayed Teeth. Sch Acad J Pharm 2: 424-428.
- Salman HA, Senthikumar R (2015) Identification and Antibiogram Profile of Streptococcus mutans and Streptococcus sobrinus from Dental Caries Subjects. J App Pharma Sci 5: 54-57.
- Dwivedi D, Kushwah T, Kushwah M, Singh V (2011) Antibiotic susceptibility pattern against pathogenic bacteria causing Dental Caries. South Asian J Exp Biol 1: 31-35.
- Bancescu G, Dumitriu S, Bancescu A, Defta C, Pana M, et al. (2004) Susceptibility testing of Streptococcus mitis group isolates. Indian J Med Res 119: 257-261.
- William B, Rwenyonyi CM, Swedberg G, Kironde F (2012) Cotrimoxazole Prophylaxis Specifically Selects for Cotrimoxazole Resistance in Streptococcus mutans , Streptococcus sobrinus with Varied Polymorphisms in the Target Genes folA and folP. Int J Microbiol 2012.
- Istifanus MF, Oyawoye OM, Caleb CC (2014) The occurrence and effect of some antibiotics on Streptococcus mutans in dental caries in Jos. Int J Curr Microbiol App Sci 3: 16-18.
- Kariminik A, Motaghi MM (2015) Evaluation of Antimicrobial Susceptibility Pattern of Streptococcus Mutans Isolated from Dental Plaques to Chlorhexidine, Nanosil, Common Antibiotics. Internat J of Life Sci 9: 18-21.
- Rozkiewicz D, Daniluk T, Sciepuk M, Zaremba ML, Cylwik-Rokicka D, et al. (2006) Prevalence rate , antibiotic susceptibility of oral viridans group streptococci (VGS) in healthy children population. Adv Med Sci 51: 191-195.
Relevant Topics
- Antibiotics and Resistance
- Antifungal
- Antiviral therapy
- Bacteremia
- Bacterial diseases
- Broad Spectrum of Antibiotics
- Clinical Infectious Diseases
- Diagnosis of Pathogenic microorganisms
- Emerging infections
- Natural Antibiotics
- Opportunistic Pathogens
- Parasitic Diseases
- Pertussis Vaccines
- Prevention of infection
- Septicemia
- Viral Infections
- Viremia
Recommended Journals
Article Tools
Article Usage
- Total views: 45299
- [From(publication date):
April-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 42244
- PDF downloads : 3055