Decrease Amyloid Plaque in Brain Treat Alzheimer Disease
Received: 12-Aug-2022 / Manuscript No. JADP-22-71872 / Editor assigned: 17-Aug-2022 / PreQC No. JADP-22-71872 (PQ) / Reviewed: 31-Aug-2022 / QC No. JADP-22-71872 / Revised: 17-Jan-2023 / Manuscript No. JADP-22-71872 (R) / Published Date: 24-Jan-2023 DOI: 10.4172/2161-0460.23.13.562
Abstract
Background: It is chronic neurodegenerative disease which usually starts slowly and worsens over a period of time. Microscopy of brain in people affected with Alzheimer Disease (AD) show amyloid plaques and neurofibrillary tangles.
Materials and methods: We conducted this research paper by observing the different types of reviews, as well as conducting and evaluating literature review papers.
Results: The treatment currently available to treat Alzheimer disease only addresses symptoms rather than the underlying cause, but research that focuses on the amyloid hypothesis and improves understanding of this proteins role in the disease is hoped to lead to the development of new treatments that may be able to delay or stop disease progression.
Conclusion: Microscopic examination of the brains of Alzheimer's Disease (AD) patients reveals amyloid plaques and neurofibrillary tangles. Amyloid plaques are accumulations of misfolded proteins that form in the spaces between neurons. This research that focuses on the amyloid hypothesis and improves understanding of this protein’s role in the disease is hoped to lead to the development of new treatments that may be able to delay or stop disease progression.
Keywords: Alzheimer; Etiology; Stage; Complication; Diagnosis; Amyloid plaque; Treatment
Introduction
It is chronic neurodegenerative disease which usually starts slowly and worsens over a period of time. It is the cause of 60%-70% cases of dementia. The cause is poorly understood. The disease is characterized by loss of neurons and synapse in the cerebral cortex and certain sub cortical regions. The loss may lead to atrophy of affected regions including degeneration of parietal and temporal lobe and parts of frontal cortex.
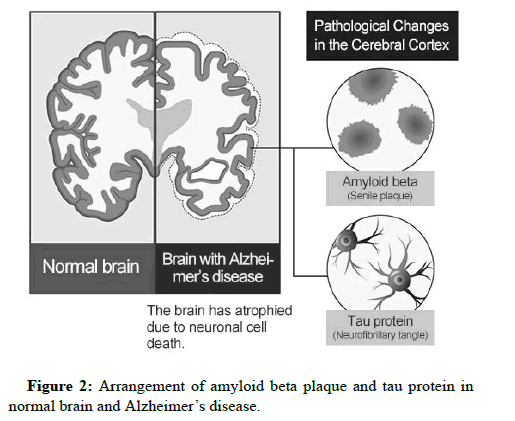
Microscopy of brain in people affected with Alzheimer Disease (AD) show amyloid plaques and neurofibrillary tangles. Plaques are dense, mostly insoluble deposits of beta amyloid peptide. Tangles are aggregates of microtubule associated protein which is hyper phosphor related and accumulates inside the cells [1].
AD is a disease characterized by misfolding of proteins in the brain. The names of proteins are amyloid beta protein. Both proteins form aggregates and disturb transport system of neurons. The exact mechanism of aggregations of protein leading to AD is incompletely understood.
Etiology
Genetics as a cause of AD is indicated. Mutations in one of three genes are linked to AD: The genes are those for the proteins namely, Amyloid Precursor Protein (APP) and presenilins 1 and 2. In addition Genome Wide Association Studies (GWAS) have found 19 areas in genes which are linked to risk of AD.
Following hypothesis have been proposed to explain the cause of AD.
Cholinergic hypothesis: It proposes that reduced synthesis of acetyl choline is the cause of AD. The hypothesis has limited acceptance because medications intended to treat acetylcholine deficiency have limited utility [2].
Amyloid hypothesis: The gene for Amyloid Precursor Protein (APP) is located on chromosome 21. A specific isoform of apo lipoprotein is a risk factor for AD. Apo lipoproteins enhance breakdown of beta amyloid, leading to excess build up in the brain. Plagues of amyloid are said to be cause of AD.
Tau hypothesis: The hypothesis indicates that tau protein abnormalities inities disease cascade of AD. It is argued that hyperphosphorelated tau begins to pair with other threads of tau. Finally they form neurofibrillary tangles inside nerve cell. As a result, microtubules disintegrate destroying structure of cytoskeleton. This finally leads to death of cells.
Biochemical mechanisms and pathophysiology of the disease is not fully understood.
Signs and symptoms
The disease course is divided into following four stages:
Pre dementia: The first noticeable deficit is short memory loss, which shows difficulty in remembering recently learned facts and inability to acquire new information. In addition, problem with attentiveness, planning, flexibility, abstract thinking or impairments in semantic memory i.e., memory of meanings and concept relationship are initial symptoms of AD. At this stage apathy may be observed [3]. Depressive symptoms, irritability and reduced awareness are also common. It has also been termed as Mild Cognitive Impairment (MCI). MCI is a transitional stage between ageing and dementia.
Early stage: Increasing impairment of learning and memory can lead to a definitive diagnosis. In few patients difficulties with language, executive functions, perception i.e., agnosia or execution of movements i.e., apraxia are more prominent than memory problem. AD does not affect all memory of the body on how to do things (implicit memory) are affected to a lesser extent [4].
Language problem lead to loss of word fluency the patients may prefer written language. As the disease progresses, patients of AD may need assistance or supervision with the most cognitively demanding activities.
Moderate stage: Progressive deterioration finally hinders independence. Patients are unable to perform most common activities of daily living. Speech difficulties are evident due to inability to recall vocabulary; it leads to frequent incorrect word substitutions. Reading and writing skills are progressively lost. Complex motor sequences are less coordinated; hence the risk of falling increases. Memory problem so worse that the patients may not be able to recognize close relatives. Long term memory also becomes impaired [5].
Behavioural and neuropsychiatric changes become more prevalent. Common manifestations include wandering, irritability and labile effect, leading to crying, excessive aggression or resistance to care giving. A sizeable patient develops illusionary misidentification and other delusional symptoms. Urinary incontinence may develop. These symptoms create stress for relatives and cares [6]. It is advisable to move the patient from home care to long term care facility.
Advanced stage: During this stage, the patient is completely dependent on care givers. Still patients can understand and return emotional signals. Extreme apathy and exhaustion are common symptoms. Mobility deteriorates to the point that patients are bed ridden and are unable to feed themselves. The cause of death is usually an external factor like infection of pressure ulcers or pneumonia, rather than the disease itself [7].
Complications
In the final stages, a patient with AD may not be able to:
• Communication expression of pain.
• Report symptoms of another illness.
• Follow a prescribed treatment plan.
• Notice or describe medication side effects.
In the terminal stages, brain changes affect physical functions like swallowing, balance, and bowel and bladder control. In such situation, there are additional health problems such as:
• Inhaling food or liquid into lungs.
• Pneumonia and other infections.
• Falls.
• Fracture.
• Bed sores.
• Malnutrition or dehydration.
Alzheimer’s disease diagnosed
There is not one single test or biomarker that diagnoses Alzheimer's. Your doctor will do a comprehensive exam and will look for signs that the symptoms might be caused by a different, reversible problem that can mimic dementia [8].
The evaluation includes cognitive tests, a physical examination and blood tests. The cognitive tests objectively assess how your memory and skills of executive function are working. They may refer you to a neurologist or neuropsychiatric for additional testing.
The specialist might want to view the structure of the brain with a CT scan or MRI imaging tests that show detailed pictures of the brain to rule out other problems that can cause cognitive changes. Imaging may show atrophy (shrinking) of different parts of the brain. Other more detailed testing may be necessary [9,10].
Decrease amyloid plaque in brain: Amyloid plaques are aggregates of misfolded proteins that form in the spaces between nerve cells. These abnormally configured proteins are thought to play a central role in Alzheimer's disease. The amyloid plaques first develop in the areas of the brain concerned with memory and other cognitive functions.
The amyloid hypothesis: Amyloid plaques form when pieces of protein called beta amyloid aggregate. The beta amyloid is produced when a much larger protein referred to as the Amyloid Precursor Protein (APP) is broken down. APP is composed of 771 amino acids and is cleaved by two enzymes to produce beta amyloid. The large protein is first cut by beta secretase and then by gamma secretase, producing beta amyloid pieces that may be made up of 38, 40 or 42 amino acids. The beta amyloid composed of 42 amino acids is chemically “stickier” than the other lengths and therefore is more likely to form plaques. Research has shown that three genetic abnormalities that are associated with early stage Alzheimer’s disease each change the function of gamma secretase in a way that leads to an increased production of beta amyloid 42 [11].
How beta amyloid causes toxic damage to nerve cells is not quite clear, but some research suggests that it may split into fragments and release free radicals, which then attack neurons. Another theory is that the beta amyloid forms tiny holes in neuronal membranes, which leads to an unregulated influx of calcium that can cause neuronal death. Regardless of the exact pathological process through which beta amyloid causes neuronal damage, the result is that neurons die [12].
Plaques then form that are made up of a mixture of these degenerating neurons and the beta amyloid aggregates. These plaques cannot be broken down and removed by the body, so they gradually accumulate in the brain. The accumulation of this amyloid leads to amyloidosis, which is thought to contribute to a number of neurodegenerative diseases.
Amyloid plaques form one of the two defining features of Alzheimer’s disease, the other being neurofibrillary tangles. Beta amyloid is also thought to be responsible for the formation of these tangles, which again damage neurons and cause the symptoms of dementia [13]. Technically, a person may present with all of the characteristics of Alzheimer’s disease but if a brain biopsy or positron emission tomography does not reveal the presence of amyloid plaques or neurofibrillary tangles, a diagnosis of Alzheimer’s disease will not be made.
Treatment
The treatment currently available to treat Alzheimer’s disease only addresses symptoms rather than the underlying cause, but research that focuses on the amyloid hypothesis and improves understanding of this protein’s role in the disease is hoped to lead to the development of new treatments that may be able to delay or stop disease progression.
Several treatments that either remove beta amyloid or interfere with its production from APP are currently being designed and tested. Approaches to preventing beta amyloid production involve targeting the beta secretase and gamma secretase that are required to make it from APP. Some research in animal models has shown preventing the action of these two enzymes to be successful and several drugs based on this mechanism have reached phase III trials. Another approach that has been investigated is preventing the aggregation of beta amyloid in order to prevent plaque formation [14]. Several drug candidates have been identified that seem to prevent this protein clumping and these agents are now due to be tested in animal models of Alzheimer’s disease.
Another approach currently being investigated is the removal of the amyloid plaques or proteins that form in the brain. In 2014, researchers from Stanford university published the ground breaking results of a study that investigated the function of microglia in amyloid plaque formation. Microglia’s are resident macrophages in the central nervous system that are responsible for removing bacteria, viruses and abnormal deposits from the brain in order to maintain its function. The researchers found that nerve cells die when the microglia stop working (which tends to occur as people age) and a protein referred to as EP2 stops the microglia from functioning efficiently. By blocking this protein, the team found the normal function of microglia was restored, which allowed them to clean up the sticky amyloid plaques which accumulate in Alzheimer’s disease [15,16]. When a drug was used to block EP2 in mice, the researchers found that memory loss was reversed in the animals as well as many other symptoms of the disease.
Overall, laboratory research has shown promising evidence that it may be possible to prevent the formation of amyloid plaques or to boost the brain’s immune response to clear the deposits once they have formed. Results from trials in humans over the next five years should indicate whether a cure for Alzheimer’s disease is indeed a realistic possibility in the future [17].
Materials and Methods
We conducted this research paper by observing the different types of reviews, as well as conducting and evaluating literature review papers.
Results and Discussion
The treatment currently available to treat Alzheimer disease only addresses symptoms rather than the underlying cause, but research that focuses on the amyloid hypothesis and improves understanding of this proteins role in the disease is hoped to lead to the development of new treatments that may be able to delay or stop disease progression (Figure 1).
Amyloid plaques are aggregates of misfolded proteins that form in the spaces between nerve cells [18]. The amyloid plaques first develop in the areas of the brain concerned with memory and other cognitive functions. Amyloid plaques form when pieces of protein called beta amyloid aggregate. APP is composed of 771 amino acids and is cleaved by two enzymes to produce beta amyloid. Several treatments that either remove beta amyloid or interfere with its production from APP are currently being designed and tested. Approaches to preventing beta amyloid production involve targeting the beta secretase and gamma secretase that are required to make it from APP (Figure 2).
Some research in animal models has shown preventing the action of these two enzymes to be successful and several drugs based on this mechanism have reached phase III trials (Figure 3) [19].
Another approach that has been investigated is preventing the aggregation of beta amyloid in order to prevent plaque formation. Several drug candidates have been identified that seem to prevent this protein clumping and these agents are now due to be tested in animal models of Alzheimers disease [20]. Another approach currently being investigated is the removal of the amyloid plaques or proteins that form in the brain. Overall, laboratory research has shown promising evidence that it may be possible to prevent the formation of amyloid plaques or to boost the brain’s immune response to clear the deposits once they have formed.
Conclusion
Microscopic examination of the brains of Alzheimer's Disease (AD) patients reveals amyloid plaques and neurofibrillary tangles. Amyloid plaques are accumulations of misfolded proteins that form in the spaces between neurons. This research that focuses on the amyloid hypothesis and improves understanding of this protein’s role in the disease is hoped to lead to the development of new treatments that may be able to delay or stop disease progression. The 42 amino acid beta amyloid is chemically more “sticky” than other lengths, making it more likely to form plaques. Studies have shown that each of the three genetic abnormalities associated with early Alzheimer's disease alters gamma secretase function, resulting in increased beta amyloid production 42. Although it is not entirely clear how beta amyloid causes toxic nerve damage in cells, some studies have shown that beta amyloid breaks down into fragments releasing free radicals, which then kill neurons. It is suggested that they may attack. Plaques then form, consisting of a mixture of these degenerated neurons and beta amyloid aggregates. The amyloid plaques form one of the two hallmarks of Alzheimer's disease, the other being neurofibrillary tangles. It is also thought that beta amyloid is involved in the formation of these tangles, which damage neurons and cause symptoms of dementia. It is to remove amyloid plaques or proteins that from within by blocking this protein, the team found that microglia could restore normal function and clear the sticky amyloid plaques that accumulate in Alzheimer's disease. Overall, laboratory studies provide promising evidence that it is possible to prevent the formation of amyloid plaques or to boost the brain's immune response to clear plaques that do form.
Acknowledgment
We grateful thanks to all the sincere and extremely helpful friends for their support and help for the completion of work. Last but not the least, we thankful to all those who cooperated and helped me directly or indirectly to carry out this work.
Ethical Approval
Ethical approval was not required for this letter. All data used is publicly accessible.
Funding
There were no external sources of funding for this research.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
All authors are declaring that they have no conflicts of interest.
References
- Chandrashekar K, Vinayak M, Saritha MK (2013) Recent advances in the management of Alzheimer’s disease. Int J Pharm Bio Sci 4: 519523.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: 353–356.
- Klein W (2002) Aβ toxicity in Alzheimer’s Disease: Globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int 41: 345‑352.
- Solomon B, Koppel R, Hanan E, Katzav T (1996) Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proc Natl Acad Sci USA 93: 452-455.
- Solomon B, Koppel R, Frenkel D, Hanan-Aharon E (1997) Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proc Natl Acad Sci USA 94: 4109-4112.
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, et al. (1999) Immunization with amyloid-β attenuates Alzheimer disease like pathology in the PDAPP mouse. Nature 400: 173–177.
- Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, et al. (2000) Nasal Aβ treatment induces anti-Aβ antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann NY Acad Sci 920: 328–331.
- Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, et al. (2000) Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurol 48: 567–579.
- Das P, Murphy M, Younkin L, Younkin S, Golde T (2001) Reduced effectiveness of Aβ1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging 22: 721–727.
- Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T (2001) Immunization with a nontoxic/nonfibrillar amyloid-β homologous peptide reduces Alzheimer’s disease associated pathology in transgenic mice. Am J Pathol 159: 439–447.
- Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, et al. (2006) Short amyloid-β (Aβ) immunogens reduce cerebral Aβ load and learning deficits in an Alzheimer's disease mouse model in the absence of an Aβ-specific cellular immune response. J Neurosci 26: 4717-4728.
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, et al. (2000) Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 408: 979-982.
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, et al (2000) Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 408: 982–985.
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, et al. (2006) Reduction of soluble Aβ and tau, but not soluble Aβ alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem 281: 39413–39423.
- Head E, Pop V, Vasilevko V, Hill M, Saing T, et al. (2008) A two-year study with fibrillar β-amyloid (Aβ) immunization in aged canines: effects on cognitive function and brain Aβ. J Neurosci 28: 3555–3566.
- Lemere CA, Maron R, Selkoe DJ, Weiner HL (2001) Nasal vaccination with β-amyloid peptide for the treatment of Alzheimer’s disease. DNA Cell Biol 20: 705–711.
- Town T, Tan J, Sansone N, Obregon D, Klein T, et al. (2001) Characterization of murine immunoglobulin G antibodies against human amyloid-β1–42. Neurosci Lett 307: 101–104.
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, et al. (2002) Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med 8:1263–1269.
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, et al (2003) Adjuvant dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int Immunol 15:505–514.
- Monsonego A, Maron R, Zota V, Selkoe D, Weiner H (2001) Immune hyporesponsiveness to amyloid-β peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci USA 98:10273-10278.
Citation: Chouhan AS, Sharma K (2023) Decrease Amyloid Plaque in Brain Treat Alzheimer Disease. J Alzheimers Dis Parkinsonism 13:562. DOI: 10.4172/2161-0460.23.13.562
Copyright: © 2023 Chouhan AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1478
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1221
- PDF downloads: 257