Research Article Open Access
Decontamination of Domestic Wastewater Using Suspended Individual and Mixed Bacteria in Batch System
| Ebtesam El Bestawy1,2*, Ahmed AL-Hejin2, Ranya Amer3 and Rzaz Abdulrazaq Kashmeri2 | |
| 1Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, Alexandria, Egypt | |
| 2Department of Life Sciences, Faculty of Science, King Abdul Aziz University, Jeddah 21551, Kingdom of Saudi Arabia | |
| 3Department of Environmental Biotechnology, City for Scientific Research and Technology Applications, Alexandria, Egypt | |
| Corresponding Author : | Ebtesam El Bestawy Department of Life Sciences, Faculty of Science King Abdul Aziz University Jeddah 21551, Kingdom of Saudi Arabia Tel: 203-4295007 Fax: 203-4285793 E-mail: ebtesamelbestawy@yahoo.com |
| Received May 16, 2014; Accepted June 16, 2014; Published June 20, 2014 | |
| Citation: El Bestawy E, AL-Hejin A, Amer R and Kashmeri RA (2014) Decontamination of Domestic Wastewater Using Suspended Individual and Mixed Bacteria in Batch System. J Bioremed Biodeg 5:231. doi:10.4172/2155-6199.1000231 | |
| Copyright: © 2014 El Bestawy E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The study aimed to investigate the ability of indigenous and/or exogenous free living bacteria either individual or as mixed culture to decontaminate raw domestic wastewater. Seven indigenous and two exogenous bacteria were selected and identified using traditional as well as molecular characterization, then used in the batch remediation system for seven days. Results indicated that the raw wastewater was relatively of high strength according to the levels of all the tested parameters. Treatment efficiency was time and bacterial species dependent. In general, the mixture of the tested bacteria considered the most efficient for the removal of all the tested parameters. Pseudomonas stutzeri (PS) was perfect for removing organic matter (BOD and COD) while the mixed culture considered the most efficient for removing fecal coliform (≈100%) brought them to safe (60, 100 mg/l and ≈ 0.0 CFU/ml respectively) discharge limits (MPL) stated by the Egyptian and Saudi Environmental laws that regulate discharging of domestic and industrial wastewater into fresh and saline open water. In addition, high removal efficiencies of TSS, FOG and TC recording 39.1, 90.0 and 99.0% respectively were achieved by B. amyloliquefaciens (S1), E. coli (Rz6) and the mixed culture respectively. However, their residuals still higher (23.3, 20 and 200 fold respectively) than their MPLs for the safe discharge due to the short treatment course. Therefore, longer treatment time and/or using biofilm of the selected bacteria are highly recommended to bring the contaminated domestic effluent it to the safe limits for the environment. The present study confirmed the ability of the selected bacteria for the removal of the target contaminants especially pathogenic bacteria (coliform) and thus can be manipulated efficiently to decontaminate polluted systems providing the optimum degradation conditions.
Domestic sewage can be treated physically, chemically or biologically to remove the included contaminants [4]. It can be treated close to where it is created (in septic tanks, biofilters or aerobic treatment systems), or collected and transported via a network of pipes and pump stations to a municipal treatment plant. Industrial sources of wastewater often require specialized treatment processes [5,6]. Although conventional sewage treatment involves primary, secondary and tertiary treatment stages, secondary (biological) treatment considered the main process where it removes dissolved and suspended biological matter and is typically performed by indigenous, water-borne microorganisms in a managed habitat [4,7]. In that stage, bacteria and protozoa consume biodegradable soluble organic contaminants (e.g. sugars, fats, organic short-chain carbon molecules, etc.) and bind much of the less soluble fractions into floc. Secondary treatment systems may be designed as fixed-film or suspended growth secondary treatment systems (activated sludge and surface aerated basins) [8,9].
Bioremediation is a superior destruction technology for some types of hazardous wastes (particularly those contaminated with organic chemicals). It is also seen as environmentally friendly, cost effective and low energy/chemicals consuming technology. The main objective of present study was to investigate the ability of some free-living indigenous and exogenous bacteria (pure and/or mixed cultures) for remediation and disinfection of the raw sanitary effluent of Jeddah City before discharging into the open environments.
Removal Efficiency(RE%)=C0-RC/C0X100
Where C0=Initial Concentration before Treatment (Zero Time);
RC=Residual Concentration after Treatment at each Exposure Time.
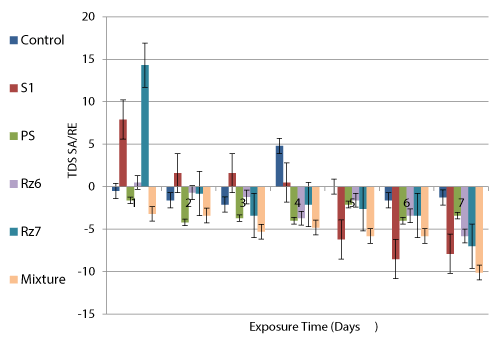
Concerning salt removal, some of the tested species showed salt removal especially at the beginning of the treatment process which were quickly shifted to add salts as a result of contaminants transformation. In that regard, RE of the TDS ranged between 0.5% (S1 after 4 days) and 14.3% (Rz7 after one day). However, control wastewater showed lower SA (0.5-2.1%) compared to the seeded samples while it showed also 4.8% RE after 4 days confirming low biodegradation activity.
Removal efficiency of the BOD increased regularly in all the seeded wastewater samples until the last exposure day. PS exhibited the highest RE of BOD followed by the mixed culture, Rz7, Rz6 and finally S1 with the least RE recording 87.5, 78.9, 75.0, 69.8 and 31.9% respectively after 7 days. However, control wastewater did not achieve any removal but exhibited BOD addition (i.e. 2.49 fold of the initial BOD) after the same time.
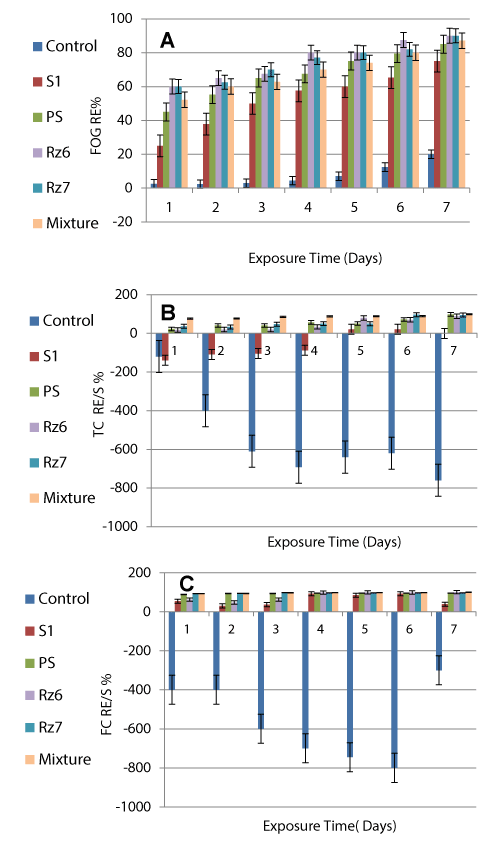
Raw wastewater showed high counts for both TC (1.0-9.6x109 CFU/ml) and FC (1.0-69.0x108 CFU/ml). Augmented bacteria showed remarkable ability for TC and FC removal from polluted wastewater. The mixed culture exhibited the highest RE% of TC (99.0%) followed by PS (98.1), Rz7 (95.2) and Rz6 (88.0%) at the 7th day of exposure. The same pattern was recorded for the removal efficiency % of FC with almost 100% removal recorded by the mixed culture and Rz6 at the last day of exposure.
However, S1 showed reverse trend where it had no or low antimicrobial effect on TC bacteria while it showed more positive role in the removal of FC with no increases in the FC count indicating that it has selective antagonistic effect on FC only. RE of FC by S1 was fluctuated between a minimum of 38.5% and a maximum of 92.3% confirming the effect of the toxic components on that strain which directly affected its performance up and down. Control wastewater sample showed the opposite trend with continuous increase in the TC starting from 120% after one day to 760% after 7 days and continuous increase in the FC ranged between a minimum of 300% and maximum of 800% indicating the absence of antagonistic bacteria that can kill and assimilate coliform bacteria.
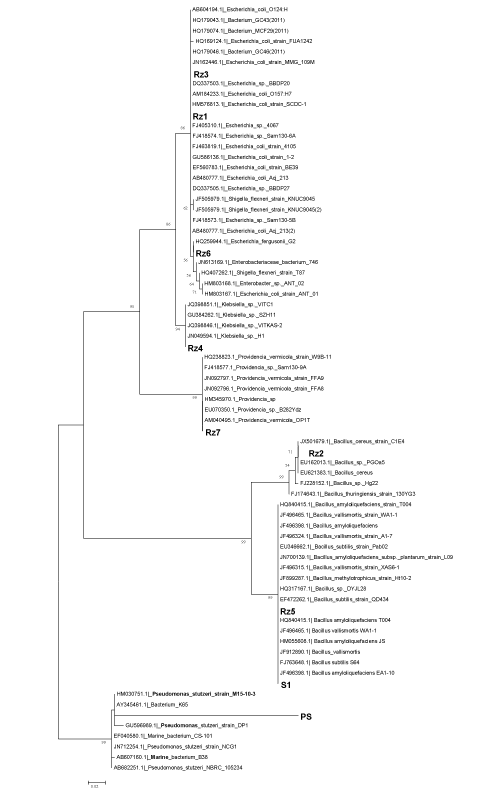
Pseudomonas stutzeri (PS) is a Gram-negative, rod-shaped, motile, single polar-flagellated, soil denitrifying bacterium with superior biodegradation and transformation ability for many environmental pollutants [27]. Active and dead masses of Pseudomonas spp. are well known as biodegraders for pesticides [10,21], crude [9] and vegetable oil [12].
Wastewater treated in the present study can be classified as moderately strong depends on levels of contaminants it contains that required powerful treatment to minimize its pollution load and discharge it safely. Batch treatment using free living individual and mixed selected bacteria aimed to design an efficient treatment process for eliminating or minimizing chemical, biological and organic load from the drainage network in Jeddah City to protect the receiving ecosystem. It was time and species dependent and subsequently resulted in varied removal efficiencies of contaminants.
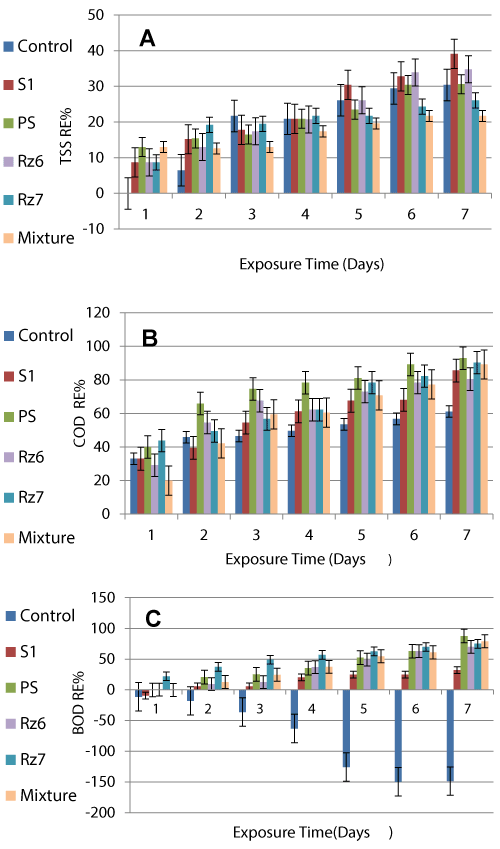
Total suspended solids (TSS), biological oxygen demand (BOD) and chemical oxygen demand (COD) are three major waste characteristics determining the pollution levels in wastewater. They are also the main identification indicators for determining the efficiency of any proposed treatment system. The maximum permissible limits (MPLs) of these indicators are specified nationally by laws and regulations as those of Egypt and Saudi Arabia to provide a safe discharge. PS considered the most efficient for removing organic matter (BOD and COD) brought them to safe discharge limits in the batch treatment. However, S1, Rz6, Rz7 and the mixed culture showed the highest removal of TSS, FOG, TC and FC respectively but their residuals still above their MPLs for the safe discharge (23.3, 20 and 200 fold for TSS, FOG and TC respectively).
Microbial degradation requires optimum pH and temperature to proceed. In the present study pH range (5.60 to 6.90) and optimum temperature of 37°C encourage all the selected bacteria to remove organic matter and other included pollutant which is supported by other workers [28]. However, pH of the treated domestic effluent lies in the permissible limits (6-9) stated by the Presidency Meteorology and Environment Organization (PME), KSA. Environmental laws stated that DO content of water and wastewater should not decrease below 5 mg/l to be safely discharged. Raw wastewater recorded 3.5 mg/l DO that is lower than DO MPL (5 mg/l). More oxygen is required for degradation of the included organic matter to the extent that wastewater may be depleted of oxygen during stabilization. Therefore, high amounts of oxygen in addition to powerful bacterial strains are needed for degradation of the contaminants. Total suspended solids TSS include settle able and colloidal fractions. The colloidal fraction cannot be removed by settling. Generally, biological oxidation or coagulation, followed by sedimentation, is required to remove these particles from suspension. The highest TSS RE recorded was 39.1% achieved using the free living Bacillus amyloliquefaciens (S1) after 7 day which is 23.3 fold higher than its maximum permissible limit (MPL) of 60.
Pseudomonas stutzeri (PS) achieved the highest RE of BOD (87.5%) after 7 days produced an effluent with good quality (55 mg BOD/l) that can safely be discharged into open systems. BOD is removed mainly by the powerful ability of the augmented bacteria that metabolize organic matter found in wastewater transforming them into harmless by-products such as carbon dioxide and water. During the first 24 h, BOD of wastewater augmented with B. amyloliquefaciens, P. stutzeri and the mixed culture as well as the control increased at varies levels (9.5, 0.4, 0.4 and 11.3% respectively). The control continued to have higher BOD reaching the highest increase (-148.7%) after 7 exposure days. These increases reflect toxicity of the wastewater that led to the death of some bacterial cells with the addition of their organic matter to the wastewater as BOD. It is also reported that unless the microbial cells produced during organic matter decomposition are removed from the solution, complete treatment will not be accomplished because biomass of these cells will be measured as BOD in the effluent. In such case the only treatment that has been achieved is that associated with the bacterial conversion of a portion of the organic matter originally present to various gaseous ends by products [29].
Raw wastewater recorded relatively high COD level (925 mg/l) at the zero time that was regularly decreased till the 7th exposure day reaching 65 mg/l (93.0% RE) achieved by Pseudomonas stutzeri (PS). The efficiency of P. stutzeri (PS) in removing COD load is also supported by Ramteke et al. [30] for heavily polluted tannery effluents. According to the MPL of the COD (100 mg/l in the Egyptian regulations) the lowest recorded RC (65 mg/l) of the COD is much lower and compiles with the law for safe discharging into open environments.
Raw wastewater recorded very high FOG level (2000 mg/l) at the zero time that was regularly decreased till the 7th exposure day reaching 200 mg/l (90.0% RE) achieved by Escherichia coli (RZ6) indicating high capability of the selected bacteria to use fatty organic matter as a source of carbon and energy [11]. The lowest FOG residue recorded 200 mg/l which is 20 fold higher than the MPL of FOG in the final treated effluent (10 mg/l Egyptian Limits) and 1.7 fold higher than Saudi Limit (120 mg/l) stated for the discharge into Central Treatment Unit [31,32].
Toxicity of wastewater on the selected bacteria was determined as growth inhibition (GI) shown in the total viable count of bacteria (TVC). GI gradually increased reaching the highest GI after 7th day. In that respect, mixed culture recorded the highest (99.1%) growth inhibition after the 7th day. Control wastewater showed relatively lower GI (75% after 7 days) compared to the seeded wastewater. These results are attributed to higher toxicity of wastewater posed on the exogenous bacteria that deal with the included contaminants compared to indigenous bacteria that are inactive towards those contaminants. However, bacteria survived, although of low densities, acquired high resistance against wastewater toxic pollutants [33,34].
Raw wastewater contains huge amount of total coliform (TC) (1.0-9.6x109 CFU/ml). Augmented bacteria showed remarkable ability for TC removal from polluted wastewater. Again, the mixed culture exhibited the highest RE% of TC (99.0%). In contrast control sample showed the opposite trend with continuous increase in the TC starting from 120% after one day to 760% after 7 days indicating the absence of antagonistic bacteria that can kill and assimilate coliform bacteria [35,36]. Also, very high fecal coliform (FC) density (1.0-6.9x109 CFU/ml) was recorded in the raw wastewater. However, augmented bacteria showed remarkable ability for FC removal from polluted wastewater with the mixed culture exhibited the highest (almost 100%) removal of FC. Control wastewater FC kept the same trend as for the TC where it recorded continuous increase in the FC ranged between a minimum of 300% and maximum of 800% due to the absence of antagonistic bacteria [37]. TC that includes both fecal and non-fecal coliform in the treated sample was slightly higher (0.1x109 CFU/ml or 1x108, i.e. 1000x105 CFU/ml) which means 200 fold higher than the MPL of the TC. However, such increase is mainly composed of non-pathogenic coliform bacteria which represents much lower environmental risk and can be easily removed by the traditional chlorination in a subsequent disinfection stage.
Results of the present study confirmed the ability of the selected bacteria for the removal of the target contaminants including antagonistic effect against pathogenic bacteria (coliform) and thus can be manipulated efficiently to decontaminate polluted systems providing the optimum degradation conditions. Removal of such contaminants was controlled by microbial species, concentration of pollutants in the tested wastewater and finally the contact time between wastewater and the bacterial cells.
References
- PUB (Singapore National Water Agency) (2011) NEWater: History.
- Shannon KE, Lee DY, Trevors JT, Beaudette LA (2007) Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Sci Total Environ 382: 121-129.
- Melosi MV (2010) The Sanitary City: Environmental Services in Urban America from Colonial Times to the Present. University of Pittsburgh Press, 110.
- SriuNaik S, PydiSetty Y (2011) Biological Denitrification of wastewater in aFBBRd by immobilization of Pseudomonas stutzeri using poly propylene granules. International Journal of Biotechnology Applications 3: 106-109.
- Ting C, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renewable and Sustainable Energy Reviews 19: 360-369.
- Sharma SK, Sanghi R (2012) Advances in Water Treatment and Pollution Prevention. Dordrecht, New York: Springer.
- Tilley DF (2011) Aerobic Wastewater Treatment Processes: History and Development. IWA Publishing.
- Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) (2008) Biological Wastewater Treatment Principles, Modelling and Design. IWA Publishing, 528.
- Benidickson J (2011) The Culture of Flushing: A Social and Legal History of Sewage. Univ of British Columbia (UBC) Press.
- El-Bestawy E, Ibrahim HZ (2005) Bioremediation of carbofuran from contaminated media using soil bacteria. Assiut University J Botany 34: 181-195.
- El-Bestawy E, Hussein H, Baghdadi HH, El-Saka M (2004) Integration approach for nutrients removal via combined biological-chemical treatment of wastewater. Egyptian Journal of Biotechnology 16: 292-307.
- El-Bestawy E, El-Masry MH, El-Adl N (2005). The potentiality of free Gram-Negative bacteria for removing oil and grease from contaminated industrial effluents. World Journal of Microbiology and Biotechnology 21: 815-822.
- El-Bestawy E, Hussein H, Baghdadi HH, El-Saka MF (2005) Comparison between biological and chemical treatment of wastewater containing nitrogen and phosphorus. J IndMicrobiolBiotechnol 32: 195-203.
- El-Bestawy E, El-Sokkary I, Hussein H, Farouk A (2008) Pollution control in pulp and paper industrial effluents using integrated chemical-biological treatment sequences. Journal of Industrial Microbiology and Biotechnology 35:1517-1529.
- Clesceri LS, Greenberg CG, Eaton AD (1999) Standard Method for the Examination of Water and Wastewater, 20thedn. American Public Health Association (APHA), Washington, 1325.
- Ma YJ, Dissen GA, Rage F, Ojeda SR (1996) RNase Protection Assay Methods 10: 273-278.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J MolBiol 215: 403-410.
- Hall BK (1999) Homology. Introduction. Novartis Found Symp 222: 1-4.
- El-Bestawy E, Mansy AH, Mansee AH, El-Koweidy AH (2000) Biodegradation of selected chlorinated pesticides from Lake Mariut ecosystem. Pakistan Journal of Biological Sciences 3: 1673-1680.
- El-Bestawy E, Mansy AH, El-Koweidy AH (2002) The potential use of Bacillus spp. in the bioremediation of highly persistent chlorinated pesticides. Al-Azhar Journal of Microbiology 55: 320-335.
- El-Bestawy E, Albrechtsen HJ (2007) Effect of nutrient amendments and sterilization on mineralization and/or biodegradation of 14C-labeled MCPP by soil bacteria under aerobic conditions. International Biodeterioration and Biodegradation 59: 193-201.
- Emmert EA, Handelsman J (1999) Biocontrol of plant disease: a (gram-) positive perspective. FEMS MicrobiolLett 171: 1-9.
- Greene JK, Turnipseed SG, Sullivan MJ, May OL (2001) Treatment thresholds for stink bugs (Hemiptera: Pentatomidae) in cotton. J Econ Entomol 94: 403-409.
- Nuñez-Valdez M, Sánchez J, Lina L, Güereca L, Bravo A (2001) Structural and functional studies of alpha-helix 5 region from Bacillus thuringiensis Cry1Ab delta-endotoxin. BiochimBiophysActa 1546: 122-131.
- Sabir J, El-Bestawy E (2009) Enhancement of alkaline protease production in Bacillus circulans using plasmid transformation. World Journal of Microbiology and Biotechnology 25: 2021-2027.
- Fiechter A (1992) Biosurfactants: moving towards industrial application. Trends Biotechnol 10: 208-217.
- Lalucat J, Bennasar A, Bosch R, García-Valdés E, Palleroni NJ (2006) Biology of Pseudomonas stutzeri. MicrobiolMolBiol Rev 70: 510-547.
- Kongjao S, Damronglerd S, Hunsom M (2008) Simultaneous removal of organic and inorganic pollutants in tannery wastewater using electro coagulation technique. Korean J ChemEng 25: 703-709.
- Haydar S, Aziz J A, Ahmad M S (2007) Biological treatment of tannery wastewater using activated sludge process. Pak J Eng Applied Sci 1: 61-66.
- Ramteke PW, Awasthi S, Srinath T, Joseph B (2010) Efficiency assessment of Common Effluent Treatment Plant (CETP) treating tannery effluents. Environ Monit Assess 169: 125-131.
- Thomas O, El Khorassani H, Touraud E, Bitar H (1999) TOC versus UV spectrophotometry for wastewater quality monitoring. Talanta 50: 743-749.
- Stoll MH, Bakker K, Nobbe GH, Haese RR (2001) Continuous-flow analysis of dissolved inorganic carbon content in seawater. Anal Chem 73: 4111-4116.
- Kelly CJ, Tumsaroj N, Lajoie CA (2004) Assessing wastewater metal toxicity with bacterial bioluminescence in a bench-scale wastewater treatment system. Water Res 38: 423-431.
- Narmadha D, Kavitha MSVJ (2012) Treatment of domestic wastewater using natural flocculants. International J of Life Sciences Biotechnology and Pharma Research 1: 206-213.
- Zhao H, Mavinic D, Oldham W, Koch F (1999) Controlling factor for simultaneous nitrification and denitrification in a two-stage intermittent aeration process treating domestic sewage. Water Res 33: 961-970.
- Prenaven R, Visvanathan LP, Adinarayana K, Suren S (2005) Degradation of pulp and paper-mill effluent by thermophilic micro-organisms using batch systems. Water SA 31.
- Gerardi MH (2006) Wastewater Microorganisms, in: Wastewater Bacteria. John Wiley & Sons, Inc., Hoboken, NJ, USA.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16744
- [From(publication date):
September-2014 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 11942
- PDF downloads : 4802
