DCF (DOC+CDDP+5-FU) Therapy Led to Curative Resection in a Patient with Advanced Esophageal Carcinoma after the Failure of CF Therapy
Received: 12-Dec-2017 / Accepted Date: 21-Dec-2017 / Published Date: 29-Dec-2017
Abstract
In Japan, two courses of CF therapy followed by surgery are accepted as a standard treatment for stage II/III oesophageal cancer according to the result of the JCOG9907 trial. However, some cases experience insufficient anti-tumour effects with CF therapy. We experienced a case with oesophageal cancer that underwent curative resection and DCF therapy after the failure of CF therapy. The patient was a 51-year-old male who visited our department due to heart burn, back pain, and weight loss since July 2014. He was diagnosed with stage III (T3N2M0) oesophageal carcinoma and started neo adjuvant CF therapy on September 19, 2014. After the first course of CF, a CT scan showed enlarged lymph node swelling and swallowing disturbance was worsened. We attempted to start DCF therapy on October 29, 2014. The feasibility of this therapy was high, with manageable grade 2 diarrhoea and a transient high fever. Swallowing disturbance further subsided during the course of treatment. After DCF treatment, a CT scan showed shrinkage of LN swelling, as well as the primary lesion. We performed videoassisted thoracoscopic oesophagectomy and 3-field LN dissection. Pathological findings showed a grade 2 histological therapeutic effects by neoadjuvant chemotherapy and down staging of the tumour to stage II (T2N2M0) was attained, which indicated curative resection was achieved. If there is a discrepancy in the anti-tumour effect between the primary lesion and LNs by neoadjuvant CF therapy for stage II/III oesophageal cancer, then conversion to DCF therapy might be one of the treatment choices.
Keywords: DCF; Oesophageal cancer; Oncology
Introduction
More than 95% of oesophageal cancer comprises squamous cell carcinoma (SCC) in Japan, in contrast with more than 60% of adenocarcinoma in Western countries. In Japan, cisplatin (CDDP) +5- fluorourasil (5-FU) combination chemotherapy (CF) followed by surgery is regarded as one of the standard treatments for stage II/III advanced disease [1,2]. However, here are some cases with insufficient anti-tumour effects following CF therapy, particularly stage III cases [1]. We herein report the findings of a patient successfully managed by the addition of docetaxel (DOC) to CF therapy (DCF) and present a review of the pertinent literature.
Case Report
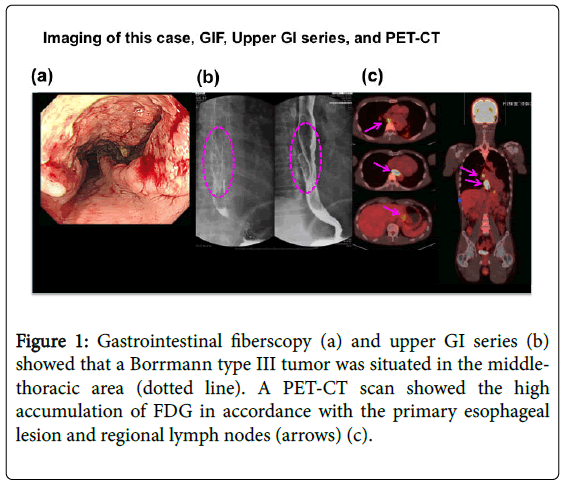
The patient was a 51-year-old man who visited our department due to heart burn, back pain, and weight loss since June 2014. His family and past history was unremarkable. He had experienced smoking for 15 years (20-36 years of age, but quit 15 years prior to admission). However, he consumed alcohol every day (equivalent to 1.5 litres of beer/day). His height was 166.4 cm and body weight 56.6 kg (BMI: 19.2). His vital signs were as follows: BP104/76 mmHg, HR64/min regular, BT36.2°C, and ECOG-PS: 1. On physical examination, no abnormal findings were noted. All laboratory data including tumour markers were within normal limits. An upper GI series and gastrointestinal fibre Figure 1a and 1b showed a Bormann type III advanced tumour at the middle thoracic oesophagus with SCC according to a biopsy. A CT scan showed swelling of the regional lymph node (LN) s and the absence of distant metastasis (Figure 2a) in accordance with a high FDG accumulation by PET-CT (Figure 1c).
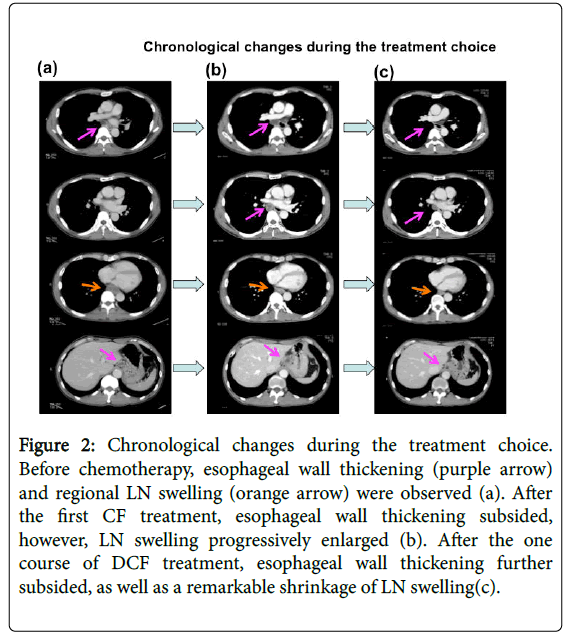
Figure 2: Chronological changes during the treatment choice. Before chemotherapy, esophageal wall thickening (purple arrow) and regional LN swelling (orange arrow) were observed (a). After the first CF treatment, esophageal wall thickening subsided, however, LN swelling progressively enlarged (b). After the one course of DCF treatment, esophageal wall thickening further subsided, as well as a remarkable shrinkage of LN swelling(c).
According to these findings, we diagnosed the patient with stage III (T3N2M0) border line respectable oesophageal carcinoma. Although he had swallowing disturbances, he could orally intake food. Therefore, we started neoadjuvant (NAC) CF therapy on September 19, 2014 according to the Japanese guidelines for the diagnosis and treatment of oesophageal cancer [2]. The treatment regimen was Div of CDDP: 80 mg/m2 on day 1, and continuous infusion of 5-FU: 700 mg/m2 from day 1-day 5. Grade 2 oral mucositis, appetite loss and nausea was observed.
After the first course of CF, a CT scan showed the progression of LN swelling (Figure 2b) enlargement and his swallowing disturbance worsened. Although we typically transition to surgery in cases of disease progression after the first course of NAC, we initiated DCF therapy DOC: 60 mg/m2: day 1, CDDP: 50 mg/m2: day 2, 5-FU700 mg/m2/day: days 2-6 on October 29, 2014 due to the possibility of non-curative operation and remarkable shrinkage of the primary lesion. Therefore the addition of DOC, which is not cross-resistant to CF, might be effective for the LNs.
The feasibility of this therapy was high, with manageable grade 2 diarrhoea and a transient high fever (38.5°C) without leukopenia. Swallowing disturbance further subsided after 3 days of DCF administration. Grade 2 diarrhoea during the course of this treatment and a transient high fever (38.5°C) without leukopenia and grade 2 alopecia after discharge occurred but were manageable.
The patient has remained well 10 months after surgery, and no evidence of recurrence was found by a follow-up CT scan.
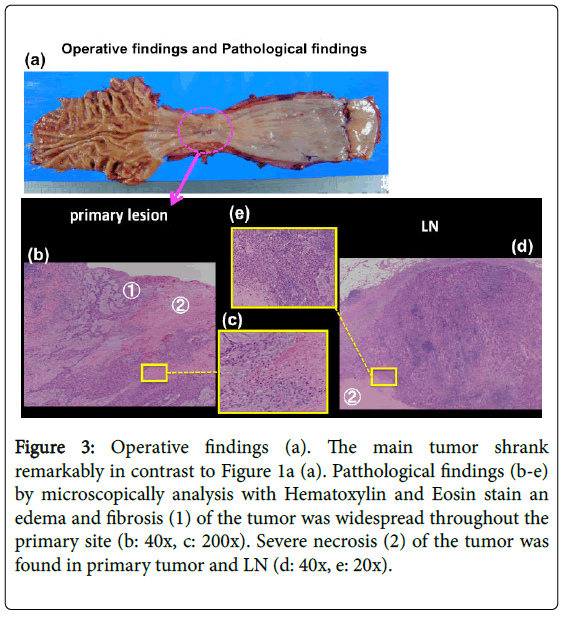
After DCF treatment, a CT scan showed shrinkage of LN swelling as well as the primary lesion (Figure 2c). We performed video-assisted thoracoscopico esophagectomy and 3-field LN dissection on January 15, 2015. A histological analysis demonstrated SCC, moderately differentiated, post-chemotherapy, lower oesophagus, and surgical findings [Mt, type 3, 12 × 10 mm, pT2 (MP), INFβ, ly0 (D2-40), v0 (EVG), pN2 (1/60), pPM0 (85 mm), pDM0 (60 mm), pEM0 (2 mm), therapeutic effect: Grade 2] according to. These findings showed that grade 2 histological therapeutic effects by NAC and down staging to stage II (T2N2M0) was obtained (Figure 3), which indicated that curative resection was achieved.
Figure 3: Operative findings (a). The main tumor shrank remarkably in contrast to Figure 1a (a). Patthological findings (b-e) by microscopically analysis with Hematoxylin and Eosin stain an edema and fibrosis (1) of the tumor was widespread throughout the primary site (b: 40x, c: 200x). Severe necrosis (2) of the tumor was found in primary tumor and LN (d: 40x, e: 20x).
Discussions
The treatment strategy of potentially respectable stage II/III oesophageal carcinoma has gradually developed from surgery alone to surgery plus adjuvant CF (JCOG9204) [3] to NA followed by surgery (JCOG9907) [1].
However, to achieve a good prognosis, the addition of DOC to CF (DCF) was attempted in some phase II clinical trials with promising results [4,5]. The anti-tumour effect of DOC is depolymerisation of tubulin and microtubules, which is quite different from that of CDDP and 5-FU [4]. Additionally, a phase III trial showed that DCF was superior to CF in the treatment of head and neck cancer [6], which was similar to SCC-like Japanese type oesophageal cancer. Two of phase III trials (JCOG1109, 1304) including DCF for oesophageal cancer are currently on-going [7,8].
If progressive disease following the first course of chemotherapy is observed, then the second course is cancelled and transition to surgery is the most common measure so as not to miss the opportunity for curative resection in the JCOG9907 protocol [1]. The regimen change from CF to DOC alone is also considered to be valid.
As a remarkable difference in the chemo-sensitivity between the primary site and LNs was observed in our case, we thought that DOC, which is not cross-resistant to CF [4], might be effective for LNs. We prepared salvage surgery as soon as progressive disease was observed.
Our case demonstrated a quite different anti-tumour effect between the primary tumour and LNs and was thought to be rare; we could not find any other cases with successful resection by the addition of other agents in a search of the pertinent literature.
This case was also the first experience of DCF therapy for the treatment of oesophageal cancer in our hospital. Following this successful experience, we have aggressively used DCF therapy in cases with stage III borderline respectable oesophageal cancer with a good PS.
Conclusions
In conclusion, if there is a discrepancy in the anti-tumour effect between the primary lesion and LNs by NAC therapy for stage II/III oesophageal cancer, then conversion to DCF therapy might be one of the treatment choices other than the transition to surgery.
Acknowledgement
Any substantial contribution when provided by a person different from the author and list all other persons who do not fulfil authorship criteria. We thank for the assistance by the medical writing expert, Brian Quinn in the Japan Medical Communication. This study has been accomplished by only all the authors contributions. The authors declare that they have no conflict of interest. The findings of this study was ordinary clinical practice covered by national medical insurance, thus we do not receive any funds nor any financial support.
References
- Ando N, Kato H, Igaki I HH, Shinoda M, Ozawa S, et al. (2012) A Randomized Trial Comparing Postoperative Adjuvant Chemotherapy with Cisplatin and 5-Fluorouracil Versus Preoperative Chemotherapy for Localized Advanced Squamous Cell Carcinoma of the Thoracic Oesophagus (JCOG9907) Ann Surg Oncol 19: 68-74.
- Â Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, et al. (2015) Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Oesophageal Society Oesophagus 12: 1-30.
- Â Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, et al. (2003) Surgery Plus Chemotherapy Compared With Surgery Alone for Localized Squamous Cell Carcinoma of the Thoracic Oesophagus: A Japan Clinical Oncology Group Study JCOG9204 J Clin Oncol 2: 4592-4596.
- Â Osaka Y, Shinohara M, Hoshino S, Ogata T, Takagi Y, et al. (2011) Phase II Study of Combined Chemotherapy with Docetaxel, CDDP and 5-FU for Highly Advanced Oesophageal Cancer ANTICANCER RESEARCH 31: 633-638.
- Hironaka S, Tsubosa Y, Mizusawa J, Kii T, Kato K, et al. (2014) Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic oesophageal cancer (JCOG0807) Cancer Sci. 105: 1189-1195.
- Â Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, et al. (2007) Cisplatin, Fluorouracil, and Docetaxel in Unrespectable Head and Neck Cancer N Engl J Med 357:1695-704.
- Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, et al. (2013) Three-arm Phase III Trial Comparing Cisplatin Plus 5-FU (CF) Versus Docetaxel, Cisplatin Plus 5-FU (DCF) Versus Radiotherapy with CF (CF-RT) as Preoperative Therapy for Locally Advanced Oesophageal Cancer (JCOG1109, NExT Study) Jpn J Clin Oncol 43: 752-755.
- Â Kataoka K, Tsushima T, Mizusawa J, Hironaka S, Tsubosa Y, et al. (2015) A randomized controlled Phase III trial comparing 2-weekly docetaxel combined with cisplatin plus fluorouracil (2-weekly DCF) with cisplatin plus fluorouracil (CF) in patients with metastatic or recurrent oesophageal cancer: rationale, design and methods of Japan Clinical Oncology Group study JCOG1314 (MIRACLE study) Jpn J Clin Oncol, 45: 494-498.
Citation: Kobayashi K, Yamaguchi S, Fujita T, Ikeda T, Ishii A, et al. (2017) DCF (DOC+CDDP+5-FU) Therapy Led to Curative Resection in a Patient with Advanced Esophageal Carcinoma after the Failure of CF Therapy. J Oncol Res Treat 2: 118.
Copyright: © 2017 Kobayashi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4895
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 4066
- PDF downloads: 829