Cytokine Levels in Plasma and PHA Activated T Cells in Head and Neck Cancer Patients
Received: 13-Sep-2019 / Accepted Date: 24-Sep-2019 / Published Date: 30-Sep-2019
Abstract
Cytokines play a pivotal role in cancer, as these act as mediators in response to several physiological factors, caused by disease burden or interventions like surgery, chemotherapy and radiation. The levels of these cytokines can also act as biomarkers, and help in predicting a relapse episode or by analysing the efficacy of an intervention. Combination approaches based on the Cellular Immunotherapy are now being considered to reset the immune system thereby help achieve better prognosis in cancer treatment. One such approach is Autologous Activated T Cells Therapy (ATC), which entails in vitro activation of patient's own T cells from the peripheral blood and infusion of these activated T cells back into the patient. This helps in resetting the immune system which makes it conducive for better antigen presentation and a TH1 mediated anti-tumour response.
Since there is an immuno-suppressive milieu pre-existing in the body, it is important to check if the Autologous activation of T cells produces immunosuppressive cytokines, which would then undermine the intent of this approach.
In this study, we have measured the cytokines level in plasma and PHA stimulated mononuclear cells in control healthy population and head and neck cancer patients. Interleukin 10 (IL 10) and Transforming growth factor-beta (TGFβ) which fall under the anti-inflammatory category (pro-tumour) and Interferon-gamma (IFNγ) and Tumour necrosis factor-alpha (TNFα) which fall under the category of pro-inflammatory (anti-tumor) have been studied by ELISA.
Results show a significant decrease in plasma TGFβ levels in patients when compared to healthy controls. There is no increase in TGFβ levels on PHA stimulation, which is comparable to the healthy controls. There is an increase in plasma IL 10 levels in patients as compared to controls, indicating slight immunosuppression, although no significant increase in IL 10 is observed on PHA stimulation. There is a slight increase in plasma TNFα levels in patient population. On PHA stimulation, patient T cells produce significantly less TNFα when compared to control. There is a slight elevation in plasma IFNγ levels in patients, while on PHA stimulation; patient T cells produce comparable amounts of IFNγ, when compared to control.
These results indicate a) that Autologous Activated T Cell (ATC) does not result in amplification of TGFβ, and IL10 cytokines, b) Patients have the ability to produce IFNγ on PHA stimulation, indicating a functional immune system, c) PHA stimulation in patient results in less TNFα when compared to control, indicating a compromise in the T cell compartment.
Keywords: Cytokines; Head and Neck cancer; PHA; Activated T cells; Anti-inflammatory; Pro-inflammatory.
Introduction
Cytokines are small non-structural proteins which are released by cells and have a prominent role to play as mediators between cell to cell interactions and communication [1]. Cytokines are crucial in any immunological reaction as these proteins have a profound effect on the effector cells and also decide the fate of an immunological cascade [2]. Cytokines control differentiation, proliferation and survival of leukocytes and are important mediators in cancer. Cancer progression is deeply connected with the orchestra of cytokines in the body and their modulation by tumours in vivo [3]. Cytokines mainly fall under 2 categories: TH1 and TH2. CD4 helper T cells produce polarized TH1 and TH2 responses which evoke cell mediated immunity and strong antibody responses, respectively.[4]. TH1 cells produce IFNγ and TNFα amongst many others and are pro-inflammatory and anticancer by function whereas TH2 cells produce IL10 in addition to IL4, IL5, IL6, IL9, and are pro-tumorigenic, immuno-suppressive and antiinflammatory. TGFβ is produced by many hematopoietic cell subtypes and is a pleiotropic growth factor which controls homeostasis, differentiation and peripheral tolerance in immune cells [5,6]. TGFβ is known to have diverse effects on cells based on its levels in blood. At higher concentrations they promote cancer whereas at lower concentrations show anticancer properties [7].Cytokine levels in cancer patients show higher TH2 cytokine patterns vis a vis TH1 profiles, thereby inducing a more immuno-suppressive milieu. This subverts the presentation of tumour antigens by dendritic cells, thereby tolerizing these antigens and hindering the body's ability to mount an immune response. Treatment modalities to increase the efficiency of standard therapies could include cytokine infusions prior or during standard interventions thereby altering the immune status which could lead to a favourable outcome.
Combination approaches, like Activated T cells (ATC) are now being explored on the platform of Cellular Immunotherapy. This entails infusion of PHA activated T cells (after in vitro activation) into patients, which would modulate the immune suppressed environment and help the body fight the disease, resulting in optimal antigen presentation, furthered by reduced incidence of relapse. In patients, studies indicate an increased plasma TGFβ levels and TH2 type cytokine profile when compared to other TH1 type cytokines [7]. PHA stimulation of T cells could inadvertently activate these immunosuppressive cytokines, undermining the objective of Autologous ATC therapy. In this project, therefore we have evaluated the cytokine profiles of Head and Neck cancer patients in plasma and PHA activated T cell supernatants and compared them with corresponding healthy population, to objectively evaluate the merits of Autologous ATC therapy. Ethical clearances were obtained prior to the commencement of the study.
Patient derived PHA activation of T cells did not result in increase in TGFβ levels and IL 10 when compared to healthy control group, making autologous ATC a viable option for treatment under the ambit of Cellular Immunotherapy platform.
Materials and Methods
The population was selected under the framework of the research regulations of the Central Ethics Committee (CEC) of Health Care Global (HCG) Hospitals, Bangalore, India. For the study population to be selected, ethical clearance was obtained under reference number EC/363/17/09 and all the subjects whose blood samples were used for the study were given informed consent prior to their enrolment in the investigation.
A study group of 15 patients (30-60 years) diagnosed with Head and Neck cancer was selected for TGFβ, IL 10, IFNγ and TNFα level analysis. Additionally, a group of 25 healthy subjects were selected as a control group. It was ensured that all the healthy subjects fulfilled the criteria for blood donation, as set by the Blood Bank guidelines and were free of autoimmune and chronic diseases as well as infectious diseases during the course of the investigation. TGFβ, IL 10, IFNγ and TNFα cytokine levels were subsequently measured from the subjects’ plasma as well as PHA stimulated T-cell supernatants, obtained from the blood samples.
Blood collection
15 ml of blood was collected from 25 healthy subjects via venous puncture, according to the standard Blood Bank collection procedure, and from the 15 Head and neck cancer patients during surgery, in EDTA vacutainers. The collected blood samples were used for plasma separation and mononuclear cell isolation for cytokine analysis.
Plasma separation and mononuclear cell isolation
The blood samples were centrifuged at 1500 rpm for 10 minutes, leaving the lighter plasma suspended above the denser cells. The plasma was isolated after centrifuging and stored at -800˚C until required. The remaining blood was diluted 3-fold with saline and mononuclear cells were isolated from the samples using ficoll-hypaque density gradient centrifugation for 20 minutes at 1500 rpm. The buffy coat was collected using a micropipette, washed with saline and counted. 2 million cells/ml were cultured in DMEM (Gibco, Invitrogen) with 10% bovine foetal serum and 100 ng/ml PHA (phytohaemagglutinin, Gibco) in vitro and incubated for 72 hours to allow T cell activation by PHA. After 72 hours, the T cell supernatant was collected and stored at -80˚C until required.
Cytokine level analysis
The levels of 4 cytokines, TGFβ, IL 10, IFNγ and TNFα were analysed by using Enzyme Linked Immuno Sorbent Assay (ELISA) kits, according to the manufacturer’s (Ray BioTech) instructions. The concentration of these cytokines in the samples was determined by comparing them to standard curves for absorbance against concentration for each of the four cytokines.
Statistical analysis
The concentrations were expressed as mean values, with standard error mean used to express uncertainty in the mean values and bar graphs were constructed to visually depict the difference in cytokine levels. An unpaired Student’s t-test was conducted between healthy volunteers and patients’ cytokine levels in plasma and PHA stimulated T-cell supernatants and p value was obtained to determine the significance of the data using Graph Pad Prism software. p values<0.001 were considered to be significant.
Results
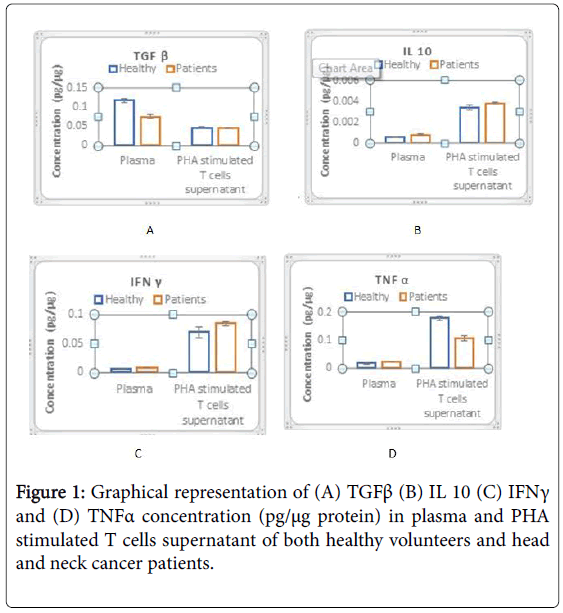
TGFβ, IL 10, IFNγ and TNFα levels were analysed in 15 Head and neck cancer patients and 25 healthy subjects were included in the study, with proper consents. The plasma levels and PHA stimulated T cell supernatants showed distinct patterns which was measured and analysed (Table 1).
| Cytokines | Groups | Plasma levels (pg/µg protein) | PHA stimulated T cells supernatant (pg/µg protein) |
|---|---|---|---|
| TGFß | Healthy | 0.118 ± 0.005 | 0.046 ± 0.003 |
| Patients | 0.075 ± 0.005 | 0.046 ± 0.001 | |
| IL 10 | Healthy | 0.00057 ± 0.00007 | 0.0034 ± 0.0002 |
| Patients | 0.00078 ± 0.00008 | 0.0038 ± 0.0001 | |
| IFNγ | Healthy | 0.006 ± 0.0003 | 0.07 ± 0.0094 |
| Patients | 0.009 ± 0.0005 | 0.085 ± 0.0033 | |
| TNFα | Healthy | 0.017 ± 0.0026 | 0.178 ± 0.0078 |
| Patients | 0.024 ± 0.0015 | 0.106 ± 0.008 |
Table 1: Levels (pg/µg protein) of TGFß, IL 10, IFN?, TNFa in plasma and PHA stimulated T cells supernatant of healthy volunteers and head and neck cancer patients.
Unlike IL 10, TGFβ levels in patients were significantly less (p<0.001) as compared to healthy subjects. On PHA stimulation, there was no increase in TGFβ and IL 10 levels indicating T cells in patients do not produce drastically elevated levels TGFβ (Figures 1A and 1B).
Although TNFα and IFNγ levels were slightly higher in healthy subjects, showed comparable levels of IFNγ after activation of T cells whereas a significant increase (p<0.001) in TNFα levels was observed in patients’ ATC (Figures 1C and 1D).
Discussion
Head and Neck cancer arises in the oral cavity, pharynx, larynx, salivary glands and has the highest incidence in Indian population, which is also associated with high morbidity, metastasis and increased relapse rate. Cytokine profiles serve as biomarkers in such cases as tumour burden causes imbalances in the cytokine levels causing exacerbation of the disease and early relapse episode.
TGFβ is a pleiotropic cytokine with multifunctional effects on target cells. TGFβ signaling can induce anti-tumour as well as pro tumour response. TGFβ induces Epithelial to Mesenchymal Transition (EMT) and allows migration of suppressor cells like Macrophage Derived Suppressor Cells (MDSC) to migrate in the tumour microenvironment causing immune suppression.
When expressed and secreted, TGFβ is sequestered in the extracellular complex till it gets activated subsequently. Hence, there is a definite role of TGFβ in the tumour environment. TGFβ has been shown to be up-regulated in breast, colon, oesophageal, gastric, liver, lung, pancreas [8,9]. Serum concentrations in patients [10-20] have shown either increased levels [14,15,21] or decreased levels [22], indicating that there could be other factors contributing to changes in levels. Association of levels of TGFβ and stage of cancers have been explored, while some showing correlation [23,10,11] while others failing to show any correlation [12,13].
In a study in thyroid cancer patients [7], serum concentration levels of TGFβ were reported to be high in both healthy and patient population, although when compared with control, TGFβ levels measured less, although statistically not significant. This is similar to our findings indicating that cytokine levels in patients make a small contribution to the global cancer-immune interaction and the effects of TGFβ could be more profound in the tumour microenvironment. Moreover, decrease in TGFβ levels could indicate a loss of homeostasis, which could have far reaching consequences in a disease burdened person. Also, PHA stimulated T cells produced significantly less TGFβ when compared to healthy controls, which was not observed in our study, wherein no difference was reported. Lastly, as serum samples will have significant contribution of TGFβ from platelets, it is better to use plasma samples than serum samples [24-27]. This could be an important differential where high TGFβ serum levels are reported in other studies.
IL 10 is a TH2 type cytokine with immunosuppressive activity on immune cells. In our studies, we found increased levels of IL 10 in plasma levels of patients as compared to healthy subjects. Although no significant increase of IL 10 levels was found on PHA stimulation. Elevated IL 10 serum concentrations in Head and Neck cancer patients have been reported. It is elevated in stage III/IV than stage I/II of the disease and is an adverse prognostic factor [28]. Although not many reports have studied IL 10 levels in PHA stimulated T cells, this is interesting to note that there is no increase in IL 10 levels following PHA stimulation.
Levels of TNFα in plasma of patients were slightly elevated, PHA stimulation resulted in an increase in TNFα but this was lower than that in healthy subjects. This indicates a compromise in the T cell compartment in patients, although the role of TNFα as antitumourogenic is conflicting, as explained later in the paper.
In a similar study, conditioned media of head and neck cancer cell lines were tested on PBMCs, which on stimulation showed a significant increase in TNFα production just 6 hours post stimulation. Particularly, conditioned media from KB16 and HEP cells induced significantly increased levels of TNFα in PBMCs [29].
Currently the role of TNFα in Head and Neck cancers with respect to immune cells have not been fully elucidated [30-32]. TNFα has a paradoxical role to play in cancer. At high concentrations, TNFα has powerful anti-angiogenic and anti-cancer effects [33], however TNFα also has been shown to induce angiogenic factors resulting in tumour growth. TNFα is also shown to be involved in stroma modelling, induce DNA damage, and selection of resistant clones [32,34-36].
Our results on TNFα levels indicate a reduced ability of patients' T cells to produce TNFα, which could impinge the body's ability to mount an anti-tumour response, although considering the yin/yang nature of most of cytokines, including TNFα, more studies are required to conclude the findings.
Plasma IFNγ levels and the respective activated counterparts are comparable in both groups, indicating no difference in their respective levels. IFNγ is an integral TH1 cytokine and is attributed for its potent anti-tumoral activity. IFNγ signaling induces cell cycle arrest in tumour cells, inhibits angiogenesis, activating antigen presentation, and inhibiting migration of tumour suppressive cells in the tumour environment [37,38].
Conclusion
Our results thus indicate that the patient population under study are not compromised in their ability to produce IFNγ, but also indicates other factors could contribute to immune suppression. Autologous ATC therapy could be beneficial to such patients as their T cells retain their ability to produce IFNγ and hence render anti-tumour benefits.
Results obtained indicate that Autologous T cells (ATC) therapy can be considered as a viable treatment option as IL 10 and TGFβ are not amplified upon PHA stimulation when compared to healthy controls. These findings resonate with our earlier results wherein reference levels in healthy subjects showed no increased production of TGFβ.
References
- Zhang JM, An J (2007) Cytokines, inflammation and pain. Int Anesthesiol Clin 45:Â 27-37
- Waldmann TA (2018) Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol 10: Â a028472.
- Goldstein  D, Laszio J (1988) The role of interferon in cancer therapy: A current perspective. CA Cancer J Clin 38: 258– 277.
- Romagnani S (2000) T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol 85: 9-21.
- Sanjabi S, Oh SA, Li MO (2017) Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol 9: a022236.
- Travis MA, Sheppard D (2014) TGF-β activation and function in immunity. Annu Rev Immunol 32: 51-82.
- Zivancevic-Simonovic S, Mihaljevic O, Mihajlovic D, Milosevic-Djordjevic O, Jovanovic Z, et al. (2016) Transforming growth factor beta 1 (TGF-β1) in thyroid cancer patients: a view from the peripheral blood. Ann Clin Lab Sci 46: 401-406.
- Levy L, Hill CS (2006) Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17: 41-58.
- Bierie B, Moses HL (2010) Transforming growth factor beta (TGF-β) and inflammation in cancer. Cytokine Growth Factor Rev 21: 49-59.
- Lin Y, Kikuchi S, Obata Y, Yagyu K, Tokyo Research Group on Prevention of Gastric Cancer (2006) Serum levels of transforming growth factor β1 are significantly correlated with venous invasion in patients with gastric cancer. J Gastroenterol Hepatol 21: 432-437.
- Suda A, Saito N, Seshimo A, Kameoka S, Kobayashi M (2009) Â Examination of transforming growth factor beta1 expression in the serum and tumor tissue of gastric cancer. Int Surg 94: 182-188.
- Lin Y, Nakachi K, Ito Y, Kikuchi S, Tamakoshi A, et al. (2009) Â Lack of association between serum transforming growth factor-beta 1 and cancer mortality risk in a nested case-control study in Japan. Asian Pac J Cancer Prev 10: 273-278.
- Lebrecht A, Grimm C, Euller  G, Ludwig E, Ulbrich E, et al. (2004) Transforming growth factor beta 1 serum levels in patients with preinvasive and invasive lesions of the breast. Int J Biol Markers 19: 236-239
- Coban S, Yueksel O, Kockar MC, Köklü S, Basar O, et al. (2007) The significance of serum transforming growth factor beta 1 in detecting of gastric and colon cancers. Hepatogastroenterology 54: 1472-1476.
- Astl J, Vesely D, Matucha P, MartÃnek J, Kucera T, et al. (2004) Serum levels of growth factors HGF (Hepatocyte Growth Factor), TGFb1 (transforming growth factor b1) and IGF-I (Insulin Like Growth Factor I) in parathyroid tumors. Neuro Endocrinol Lett 25: 356-360.
- Balcan E, Demirkiran F, Aydin Y, Sanioglu  C, Bese T, et al. (2012)  Serum levels of epidermal growth factor, transforming growth factor, and c-erbB2 in ovarian cancer. Int J Gynecol Cancer 22: 1138-1142.
- Ciftci R, Tas F, Kilic L, Vatansever S, Karabulut S (2014) Clinical significance of serum transforming growth factor beta 1 (TGFB1) level in breast cancer. J Clin Oncol 32:e11526-e11526.
- Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ (2001) Serum levels of transforming growth factor β1 in patients with breast cancer. Arch Surg 136: 937-940.
- Yu Y, Dong W, Zhou X, LiS (2004) The significance of serum soluble intercellular adhesion molecule 1 and transforming growth factor α in patients with nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg: 130: 1205-1208.
- Saltzman BS, Yamamoto JF, Decker R, Yokochi L, Theriault AG, et al. (2008) Association of genetic variation in the transforming growth factor β-1 gene with serum levels and risk of Colorectal Neoplasia. Cancer Res 68: 1236-1244.
- Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, et al. (2003) Increased serum transforming growth factorâ€Î²1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol 134: 270-278.
- Wu HS, Li YF, Chou CI, Yuan CC, Hung MW, et al. (2002) The concentration of serum Transforming Growth Factor beta-1 (TGF-β1) is decreased in cervical carcinoma patients. Cancer Invest  20: 55-59.
- Elenkova A, Atanassova I, Kirilov G, Vasilev V, Kalinov K, et al. (2013) Transforming growth factor β1 is not a reliable biomarker for valvular fibrosis but could be a potential serum marker for invasiveness of prolactinomas (pilot study). Eur J Endocrinol: 169: 299–306.
- Kropf J, Schurek JO, Wollner A, Gressner AM (1997) Immunological measurement of transforming growth factor-beta 1 (TGF-β1) in blood; assay development and comparison. Clin Chem 43: 1965-1974
- Chen H, Chang Y, Lai Y, Chen Y, Huang M, et al. (2005)  Change of plasma transforming growth factor-β1 levels in nasopharyngeal carcinoma patients treated with concurrent chemo-radiotherapy. Jpn J Clin Oncol 35: 427-432.
- Coupes BM, Williams S, Roberts I S, Short CD, Brenchley PE (2001) Plasma transforming growth factor β1 and platelet activation: Implications for studies in transplant recipients. Nephrol Dial Transplant 16: 361-367.
- Fukuchi M, Miyazaki T, Fukai Y, Nakajima M, Sohda M, et al. (2004) Plasma level of Transforming Growth Factor 1 measured from the Azygos Vein predicts prognosis in patients with esophageal cancer. Clin Cancer Res 10: 2738-2741.
- Mitra F, Jannan G, Azadesh AT (2012) Serum level of interleukin-10 in patients with head and neck squamous cell carcinoma. Aust J Basic Appl Sci 6: 282-286.
-  França CM, Barros FM, Lotufo MA, Fernandes KP, Borra RC (2011) Response of peripheral blood mononuclear cells to conditioned medium from cultured oral squamous cell carcinomas. Braz Oral Res 25: 414-20
- Müller-Hermelink  N, Braumüller H, Pichler B, Wieder T, Mailhammer R, et al. (2008) TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer cell 13: 507-518.
- Romero-Reyes M, Head C, Cacalano NA, Jewett A (2007) Potent induction of TNF-α during interaction of immune effectors with oral tumors as a potential mechanism for the loss of NK cell viability and function. Apoptosis 12: 2063-2075
- Saito T, Kuss I, Dworacki G, Gooding W, Johnson JT et al. (1999) Spontaneous ex vivo apoptosis of peripheral blood mononuclear cells in patients with head and neck cancer. Clin Cancer Res 5: 1263-1273
- Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9: 361-371.
- Sparano A, Lathers DM, Achille N, Petruzzelli GJ, Young MR (2004) Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 131: 573-576.
- Teruel A, Romero M, Cacalano NA, Head C, Jewett A (2008) Potential contribution of naive immune effectors to oral tumor resistance: role in synergistic induction of VEGF, IL-6, and IL-8 secretion. Cancer Immunol Immunother 57: 359-366.
- Woods KV, El-Naggar A, Clayman GL, Grimm EA (1998) Variable expression of cytokines in human head and neck squamous cell carcinoma cell lines and consistent expression in surgical specimens. Cancer Res 58: 3132-3141.
- Ni L, Lu J (2018) Interferon gamma in cancer immunotherapy. Cancer Med 7: 4509-4516.
- Aakash M, Ankita U, Gururaj AR, Jyothsna AR (2018) Cytokine levels in plasma and PHA stimulated T cells in healthy Indian subjects. J Cytokine Biol 3: 122.
Citation: Kambdur N, Umrao A, Rao VUS, Rao GA, Rao JA (2019) Cytokine Levels in Plasma and PHA Activated T Cells in Head and Neck Cancer Patients. J Cytokine Biol 4: 129.
Copyright: © 2019 Kambdur N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.