Cytokine Dynamics of �?³�?´ T Cells: A Double Edged Sword in Osteoclastogenesis
Received: 28-Jun-2016 / Accepted Date: 28-Sep-2016 / Published Date: 05-Oct-2016 DOI: 10.4172/2576-3881.1000111
Keywords: γδ T cells; Aminobisphosphonates; Cytokines; Osteoclastogenesis; IL6; IFNγ.
6530Short Communication
Bone remodelling is necessary to maintain mineral homeostasis and structural integrity of bone. It is a continuous and highly coordinated process, which is essentially carried out by osteoblasts (bone forming cells) and osteoclasts (bone resorbing cells) through release of cytokines or soluble factors. Number of reports suggests that, tumor cells, immune cells and bone cells share cytokines, chemokines and signalling molecules. Conventional T cells (αβ T cells) are known to enhance osteoclast generation and function through release of proosteoclastogenic factors like IL17 and RANKL. Although the presence of γδ T cells in bone microenvironment has been reported, their role in bone biology is not well understood. Studies from our lab suggest that, depending on the activation status and cytokine dynamics, γδ T cells can function in a pro or anti-osteoclastogenic manner. Activated γδ T cells secrete higher levels of IFNγ (anti-osteoclastogenic cytokine) and inhibit the process of osteoclastogenesis, while non-activated γδ T cells produce increased levels of IL6 (anti-osteoclastogenic cytokine) and were found to enhance osteoclast generation and function. Aminobisphosphonate Zoledronate has potent antiresorptive activity and is used for the treatment of postmenopausal osteoporosis and skeletal malignancies associated with metastatic cancer. Zoledronate is also known be a potent activator of γδ T cells. Aminobisphosphonates are embedded in bone due to their high affinity for calcium and get released in the bone microenvironment by resorbing osteoclasts. These aminobisophsopnates activate γδ T cells, which have antitumor and antiresorptive activity. The present review highlights the new role played by aminobisphosphonates in cancer patients through activation of effector functions of γδ T cells and other immune cells, which extends beyond their well-defined antiresorptive function.
Bone is a dynamic structure and is continuously remodelled. To maintain the quality of the bone, it is necessary to replace old / damaged bone with new bone and this process is carried out by osteoblasts and osteoclasts. Osteoblasts are bone forming cells, which differentiate from mesenchymal stem cells, while osteoclasts are bone resorbing cells which are formed by fusion of monocyte-macrophage precursor cells under the influence of macrophage colony stimulating factor (MCSF) and receptor activator of nuclear factor kappa B ligand (RANKL). MCSF is crucial for proliferation and survival of macrophages and osteoclast precursor cells [1] while RANKL is essential for differentiation of osteoclasts [2]. Osteoblasts and osteoclasts work in a tightly regulated manner to maintain the normal bone physiology, while imbalance results in pathological conditions such as osteopetrosis, osteoporosis, Paget’s diseases, rheumatoid arthritis (RA), periodontal disease [3,4]. The process of bone remodelling is regulated by cytokines present in the bone microenvironment.
There is enough data suggesting that immune cells influence skeletal system through cytokines, chemokines, signalling molecules and surface receptors [5]. Cytokines and chemokines released by macrophages, T lymphocytes, bone marrow cells and B cells present in the bone microenvironment mediate crosstalk between immune cells and bone cells. There is an increasing interest in studying how T cells are involved in bone metabolism and how they influence the generation and resorptive activity of osteoclasts [2,6,7]. Immune cells secrete an array of cytokines like IL6, IL17, TGFβ, TNFα and RANKL which induce osteoclast formation and function, while cytokines like IL4, IL10, IL12, IL13, IL18 and IFNγ inhibit the process of osteoclastogenesis [8]. Conventional CD4+ T cells upon activation increase expression of RANKL and are known to be proosteoclastogenic [9]. IL17 producing CD4+T cells cause bone destruction by inducing RANKL expression on synovial fibroblasts and osteoblasts. Although αβ T cells are studied with respect to their role in bone biology, role of γδ T cells has not been explored in detail.
γδ T cells are a unique subset of T cells, which harbor properties of both innate and adaptive immune cells. They represent <10% of the total T cell population, where >90% population resides in peripheral blood and expresses Vγ9Vδ2 TCR. γδ T cells possess unique properties with respect to antigen recognition, tissue tropism, MHCindependent antigen recognition and antitumor response. γδ T cells recognizes unique antigens, different from conventional αβ T cells, which include small phosphoantigens such as isopentenyl pyrophosphate (IPP) and (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), phospholipids, heat shock proteins, alkyl amines and amino bisphosphonates [10]. IPP is an intermediate molecule in eukaryotic mevalonate/ cholesterol pathway, while HMBPP is an intermediate molecule in bacterial non-mevalonate/ rohmer pathway. Amino bisphosphonates (Zoledronate), a class of potent antiresorptive drug, are used as a standard treatment modality to treat postmenopausal osteoporosis and cancer patients with bone metastasis [11,12]. γδ T cells are Th1 type cells but are extensively plastic and differentiate into different subsets like Th2, Th17, Tfollicular helper and T-regulatory cells under different pathological conditions, producing different sets of cytokines [13]. γδ T cells have been appreciated for their role in antitumor cytotoxicity [14,15], wound healing and tissue repair [16-18] and thus have generated much interest in recent years. These cells produce several cytokines like IL17, IL6, IL10, TNFα, IFNγ and RANKL depending on their activation status. Many of these factors are known to influence bone metabolism. Most of the γδ T cell based studies, in context of osteoimmunology, have been done in patients of rheumatoid arthritis (RA) and multiple myeloma [19,20]. Their presence has been demonstrated in bone microenvironment, in synovial fluids of rheumatoid arthritis patients and at bone fracture sites [19,21]. Various groups have also shown presence of chemokine receptors like CCL5 and CCR1 on γδ T cells, which indicate their propensity to migrate to bone. It has been reported that, Th17+ cells, and not IL17+ γδ T cells drive arthritic bone destruction in RA patients [19]. In contrast, another report shows that, IL17+ producing γδ T cells are increased in synovial fluids and peripheral blood of RA patients [21]. Also, RA patients have shown changes in γδ T cell subpopulations and their phenotypes [22,23]. Recently, IL17 producing γδ T cells were shown to promote bone formation and facilitate bone fracture healing [24]. However, role of γδ T cells in fracture healing has remained controversial as γδ T cell deficient mice had shown stable fracture repair and better biochemical strength of bone [25].
We believe that the functional differences observed with γδ T cells may be attributed to their activation status. Studies from our lab investigated the role of non-activated and activated γδ T cells in osteoclastogenesis. We have shown that γδ T cells behave in both pro or anti-osteoclastogenic manner and it is the activation status (expression of CD69, CD25 and RANKL) and cytokine dynamics of γδ T cells which dictates their ultimate behaviour [26]. Non-activated γδ T cells produced higher levels of IL6 and were found to enhance osteoclastogenesis [26]. IL6 is a potent stimulator of osteoclast differentiation and activity [8]. IL6, TNFα and IL1β work in a synergistic manner to stimulate osteoclast differentiation [27]. IL6 together with IL11 supports osteoclast formation and resorption [28]. Non-activated γδ T cells were also found to secrete higher levels of TNFα, a proinflammatory cytokine, which directly and indirectly enhances osteoclast generation and its resorptive activity [29,30]. TNF along with RANKL increases expression of RANK on osteoclast precursor cells [31]. TNFα and TGFβ synergistically can induce osteoclastgenesis in the absence of TRAF6 or RANK, which explains potential role of TNFα in bone pathologies [29]. TNF and IL1β synergistically promote expression of osteoprotegerin ligand (osteoprotegerin is a decoy receptor for RANKL) in osteoblasts [32], up regulates expression of RANKL on osteoblasts and stromal cells, stimulates differentiation of osteoclast precursor cells and increases activity and survival of osteoclasts by preventing apoptosis [33]. TNFα stimulates production of IL6 in osteoblasts and osteoblast-like osteosarcoma cells [34].
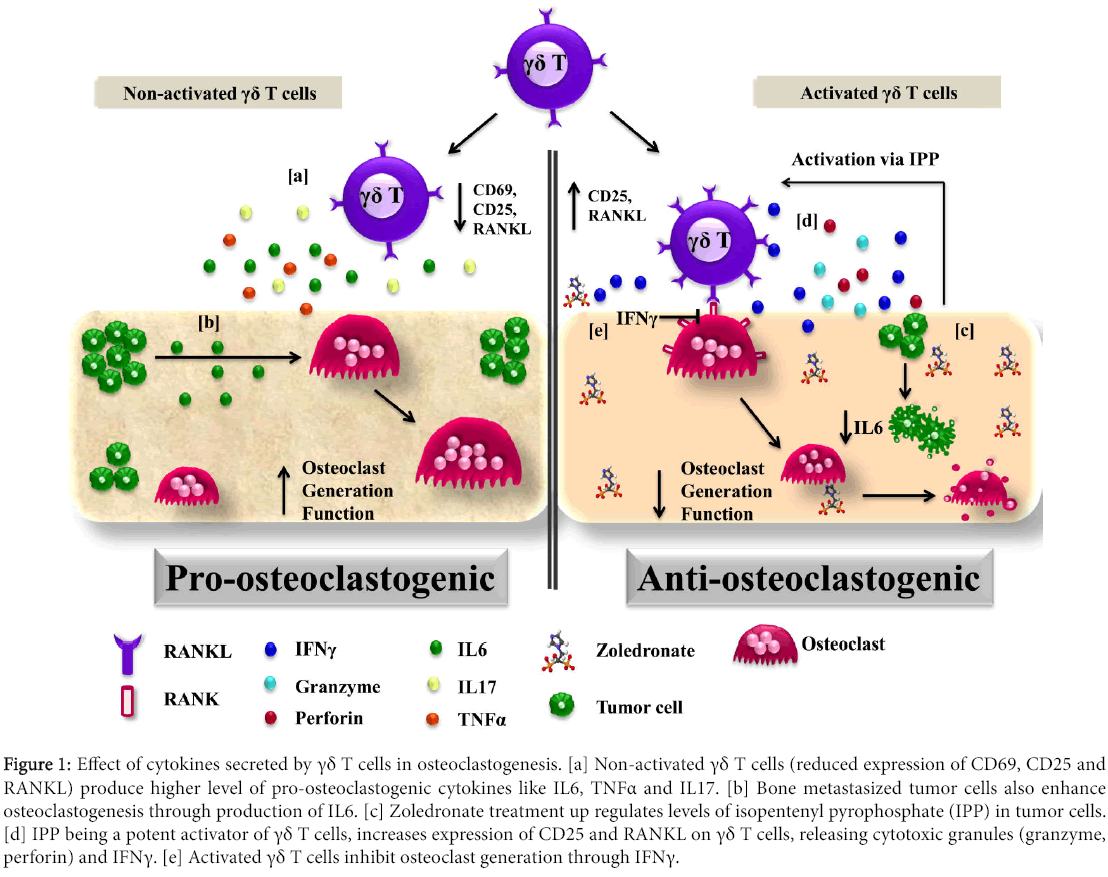
We demonstrated that, activated γδ T cells inhibited osteoclast generation and function through secretion of increased levels of IFNγ [26]. IFNγ inhibits generation (degradation of TRAF6 adaptor protein in RANKL signalling) and function (down regulated expression of cathepsin-k) in osteoclasts [35]. Stimulation of γδ T cells with phosphoantigens (bromohydrin pyrophosphate and Zoledronate) increased the expression of RANKL along with increase in IFNγ secretion. Increased RANKL expression on activated γδ T cells assists their interaction with osteoclasts; while increased IFNγ disrupts RANK-RANKL signalling thus inhibiting osteoclast survival (Figure 1).
Figure 1: Effect of cytokines secreted by γδ T cells in osteoclastogenesis. [a] Non-activated γδ T cells (reduced expression of CD69, CD25 and RANKL) produce higher level of pro-osteoclastogenic cytokines like IL6, TNFα and IL17. [b] Bone metastasized tumor cells also enhance osteoclastogenesis through production of IL6. [c] Zoledronate treatment up regulates levels of isopentenyl pyrophosphate (IPP) in tumor cells. [d] IPP being a potent activator of γδ T cells, increases expression of CD25 and RANKL on γδ T cells, releasing cytotoxic granules (granzyme, perforin) and IFNγ. [e] Activated γδ T cells inhibit osteoclast generation through IFNγ.
The present study has provided a new insight into understanding the crosstalk of γδ T cells with osteoclasts that can be extrapolated to patients with bone metastasis such as multiple myeloma, breast and prostate cancer. Through a vicious cycle, metastasized tumor cells increase osteoclast generation, activity and survival by releasing cytokines such as IL6, PTHrP, TNFα and prostaglandin E2. These tumor cells recruit immune cells at bone microenvironment by releasing IL7, IL8 and parathyroid hormone related protein (PTHrP). Memory T cells have been detected in bone microenvironment but their proliferation and function are inhibited by increased levels of TGFβ, released upon bone resorption by osteoclasts [36]. Blocking of TGFβ at metastatic sites activates local antitumor immune responses by these T cells [37]. Thus, in case of bone metastasis, metastasized tumor cells exacerbate the situation by enhancing osteoclastogenesis and compromising the immune system.
Anti-IL6 or anti-RANKL therapies have shown effective results in control of bone metastasis. Zoledronate, a third generation amino bisphosphonate, is a most potent antiresorptive drug, with antitumor activity [38]. It has high affinity for bone minerals and thus gets incorporated into bone and is slowly released in the bone microenvironment by resorbing osteoclasts. Zoledronate inhibits fernesyl pyrophosphate synthase (FPPS), a key enzyme in mevalonate pathway/ cholesterol pathway, which causes osteoclast and tumor cell apoptosis. Inhibition of FPPS causes accumulation of IPP, which in turn activates γδ T cells. αβ T cells remain unaffected upon amino bisphosphonate treatment [38,39].
In this scenario, Zoledronate treatment to breast cancer patients provides a favorable environment for the consistent activation of the γδ T cells in bone microenvironment. Activation of γδ T cells through amino bisphosphonates could exert a potent inhibitory effect on osteoclasts and tumor cells. Activated γδ T cells mediate their cytotoxic effects through release of perforin, granzyme and cytokines (IFNγ and TNFα). IFNγ alone has an ability to up regulate expression of MHC I and II molecules and promote activation of CD4+, CD8+ T cells , B cells, dendritic cells and monocyte-macrophage precursor cells and thus increase antigen presentation by these cells [40] . Activated CD4+ T cells secrete pro-osteoclastogenic cytokines like IL17, TNFα, IL1β and IL6 which support and enhance osteoclastogenesis. Unlike αβ T cells, γδ T cells are Th1 cells and predominantly produce copious amount of IFNγ upon activation. IFNγ has multiple antitumor effects like direct inhibition of tumor growth, inhibition of angiogenesis and macrophage stimulation. It has also been reported that, metastatic breast cancer cells produce factors which promote survival of osteoclasts and block the apoptotic effects of bisphosphonates [41]. Aminobisphosphonates can activate γδ T cells that are capable of exhibiting antitumor effects. This action of amino bisphosphonates may counteract the inhibitory effects of tumor derived factors.
A growing body of evidence points towards the role of γδ T cells as an anticancer immunotherapeutic treatment modality. Bisphosphonates are known to activate γδ T cells and therefore, their use in cancer therapy warrants further investigation. Bisphosphonates can be used as bone targeting anticancer agent that have direct effect on tumor cell proliferation, invasion and bone metastasis. The interesting aspect is that cytokines (IFNγ) released by bisphosphonate activated γδ T cells has anti-osteoclastogenic effect. Fine tuning the activation status and cytokine dynamics of γδ T cells may pave way for development of future immunotherapeutic modalities for patients with primary breast, prostate cancer and multiple myeloma and bone metastasis.
References
- Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7: 292-304.
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, et al. (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408: 600-605.
- Drake MT (2013) Osteoporosis and cancer. Curr Osteoporos Rep 11: 163-170.
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337-342.
- Lee SK, Lorenzo J (2006) Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol 18: 411-418.
- Rifas L, Weitzmann MN (2009) A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum 60: 3324-3335.
- Weitzmann MN, Cenci S, Rifas L, Haug J, Dipersio J, et al. (2001) T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand-dependent and -independent mechanisms. J Bone Miner Res 16: 328-337.
- Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS (2008) The cell biology of bone metabolism. J Clin Pathol 61: 577-587.
- Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, et al. (2005) IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol 35: 3353-3363.
- Born WK, Kemal Aydintug M, O'Brien RL (2013) Diversity of gammadelta T-cell antigens. Cell Mol Immunol 10: 13-20.
- Rizzoli R (2010) Zoledronic Acid for the treatment and prevention of primary and secondary osteoporosis. Ther Adv Musculoskelet Dis 2: 3-16.
- Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83: 1032-1045.
- Caccamo N, Todaro M, Sireci G, Meraviglia S, Stassi G, et al. (2013) Mechanisms underlying lineage commitment and plasticity of human gammadelta T cells. Cell Mol Immunol 10: 30-34.
- Konigshofer Y, Chien YH (2006) Gammadelta T cells - innate immune lymphocytes? Curr Opin Immunol 18: 527-533.
- Kabelitz D, Kalyan S, Oberg HH, Wesch D (2013) Human Vdelta2 versus non-Vdelta2 gammadelta T cells in antitumor immunity. Oncoimmunology 2: e23304.
- Sharp LL, Jameson JM, Cauvi G, Havran WL (2005) Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol 6: 73-79.
- Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL (2005) Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med 201: 1269-1279.
- Havran WL, Jameson JM, Witherden DA (2005) Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol 289: G627-630.
- Pollinger B, Junt T, Metzler B, Walker UA, Tyndall A, et al. (2011) Th17 cells, not IL-17+ gammadelta T cells, drive arthritic bone destruction in mice and humans. J Immunol 186: 2602-2612.
- Cui Q, Shibata H, Oda A, Amou H, Nakano A, et al. (2011) Targeting myeloma-osteoclast interaction with Vgamma9Vdelta2 T cells. Int J Hematol 94: 63-70.
- Meliconi R, Pitzalis C, Kingsley GH, Panayi GS (1991) Gamma/delta T cells and their subpopulations in blood and synovial fluid from rheumatoid arthritis and spondyloarthritis. Clin Immunol Immunopathol 59: 165-172.
- Bank I, Cohen L, Mouallem M, Farfel Z, Grossman E, et al. (2002) gammadelta T cell subsets in patients with arthritis and chronic neutropenia. Ann Rheum Dis 61: 438-443.
- Bodman-Smith MD, Anand A, Durand V, Youinou PY, Lydyard PM (2000) Decreased expression of FcgammaRIII (CD16) by gammadelta T cells in patients with rheumatoid arthritis. Immunology 99: 498-503.
- Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, et al. (2016) IL-17-producing gammadelta T cells enhance bone regeneration. Nat Commun 7: 10928.
- Colburn NT, Zaal KJ, Wang F, Tuan RS (2009) A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum 60: 1694-1703.
- Phalke SP  (2015) Activation status of γδ T cells dictates their effect on osteoclast generation and bone resorption. Bone Reports 1: 8.
- Devlin RD, Reddy SV, Savino R, Ciliberto G, Roodman GD (1998) IL-6 mediates the effects of IL-1 or TNF, but not PTHrP or 1,25(OH)2D3, on osteoclast-like cell formation in normal human bone marrow cultures. J Bone Miner Res 13: 393-399.
- Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, et al. (2003) Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32: 1-7.
- Kim N, Kadono Y, Takami M, Lee J, Lee SH, et al. (2005) Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 202: 589-595.
- Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, et al. (2000) Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 106: 1229-1237.
- Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y (2001) Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem 276: 563-568.
- Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, et al. (1999) Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 25: 255-259.
- Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116: 1186-1194.
- Kurokouchi K, Kambe F, Yasukawa K, Izumi R, Ishiguro N, et al. (1998) TNF-alpha increases expression of IL-6 and ICAM-1 genes through activation of NF-kappaB in osteoblast-like ROS17/2.8 cells. J Bone Miner Res 13: 1290-1299.
- Kamolmatyakul S, Chen W, Li YP (2001) Interferon-gamma down-regulates gene expression of cathepsin K in osteoclasts and inhibits osteoclast formation. J Dent Res 80: 351-355.
- D'Amico L, Roato I (2012) Cross-talk between T cells and osteoclasts in bone resorption. Bonekey Rep 1: 82.
- Wrzesinski SH, Wan YY, Flavell RA (2007) Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 13: 5262-5270.
- Dhar S, Chiplunkar SV (2010) Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vgamma9Vdelta2 T cells. Cancer Immun 10: 10.
- Fournier PG, Chirgwin JM, Guise TA (2006) New insights into the role of T cells in the vicious cycle of bone metastases. Curr Opin Rheumatol 18: 396-404.
- Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75: 163-189.
- Hussein O, Tiedemann K, Komarova SV (2011) Breast cancer cells inhibit spontaneous and bisphosphonate-induced osteoclast apoptosis. Bone 48: 202-211.
Citation: Phalke S, Chiplunkar SV (2016) Cytokine Dynamics of γδ T Cells: A Double Edged Sword in Osteoclastogenesis. J Cytokine Biol 1:111. DOI: 10.4172/2576-3881.1000111
Copyright: © 2016 Phalke S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4791
- [From(publication date): 0-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3854
- PDF downloads: 937