Cystoscopic Management of a Ureteral Orifice Obstructive Clot Early after Renal Transplantation
Received: 24-May-2018 / Accepted Date: 13-Sep-2018 / Published Date: 21-Sep-2018 DOI: 10.4172/2475-7640.1000120
Keywords: Kidney transplantation; Bleeding; Obstruction; Cystoscopy
Introduction
Renal transplantation is an established method for improving both quality of life and patient survival for those with end stage renal disease [1]. Early allograft function may be compromised by several factors including vascular thrombosis, rejection, infection, as well as obstruction. Ureteral obstruction complicates approximately 10% of renal transplants [2], most often related to ischemic strictures at the ureteroneocystostomy [3]. Early post-operative obstruction, usually diagnosed after worsening allograft function and concordant hydronephrosis seen on imaging, is most commonly treated with percutaneous nephrostomy. In the context of ureteral stent placement, limited literature exists regarding methods for identifying the source of obstructive uropathy early in the post-transplantation setting. Here we describe our experience with a renal transplant patient with declining graft function and evidence of hydronephrosis despite an appropriately positioned ureteral stent.
Case Report
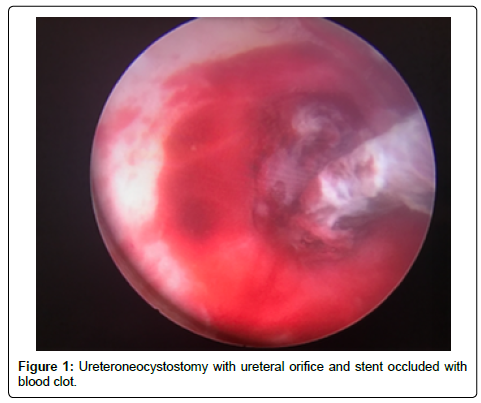
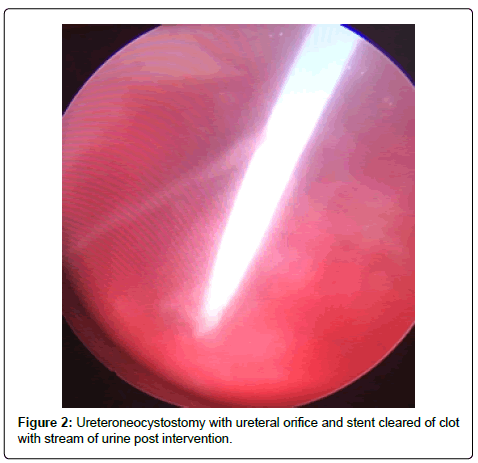
A 56-year-old Hispanic male with end-stage renal disease, secondary to type 2 diabetes mellitus and hypertension, presented after 2 active years on the kidney transplant waiting-list for a deceased donor renal transplantation. Pertinent recipient history includes severe aortoiliac calcification bilaterally and coronary artery disease with recent drug-eluting stent placement on dual anti-platelet therapy including aspirin and clopidigrel. The recipient had zero PRA with the donor and recipient both being blood type B. The patient received a five Human Leukocyte Antigen (HLA) antigen mismatch 35-year-old right kidney from a female donation after brain death donor with a history significant only for substance abuse and BMI of 51 kg/m2. Donor initial and terminal creatinine was 1.18 mg/dL and 1.13 mg/dL, respectively. The donor had two renal arteries without plaque separated by 1.5 cm on a carrel patch and one right renal vein. The right renal vein was elongated by TA stapler using a vena cava extension. The carrel patch was minimized by excising the distance between renal arteries with the two ends brought together using a running 6-0 prolene suture to limit the extent of diseased vessel arteriotomy. A recipient midline incision was used given the extent of circumferential bilateral iliac calcification and the left proximal external iliac was deemed the softest area to clamp upon dissection of the vessels. The kidney was reperfused with 6.5 hours of cold ischemic time. The ureteroneocystostomy was performed using the Lich-Gregoir technique over a 6 french 12 cm stent without incident, however the bladder was of relatively small capacity. An abdominal drain was placed close to the bladder in the pelvis. There was minimal urine output initially after reperfusion however output increased dramatically within the first 24 hours with over 2,800 ml of blood tinged urine produced. Of note, the patient remained on dual anti-platelet therapy given his drug-eluting stents. Post-operative duplex demonstrated no hydronephrosis and patent artery and vein with arterial resistive index of 0.7 with the creatinine down trending from 6.1 mg/dL to 4.5 mg/dL over the first 48 hours. The foley catheter was removed on the third post-operative day per protocol with variable urine output recorded given his visual impairment and difficulty capturing all the output. The following day, creatinine increased to 6.1 mg/dL prompting repeat duplex ultrasonography which demonstrated a normal bladder and new mild to moderate hydronephrosis with resistive indices of 1.0 throughout the kidney, documenting patency of the renal artery and vein. Non-contrast computed tomography was performed demonstrating mild to moderate hydronephrosis and a large organized hematoma within the bladder with ureteral stent in appropriate position. A 20-french foley catheter was placed as a urethral stricture would not allow larger bore foley. Bladder irrigation was performed immediately until clear drainage after a significant amount of blood clots were evacuated; however, only 30 cc of urine was produced over next 12 hours with further rise of creatinine to 7.0 mg/dL Repeat duplex ultrasonography was performed after irrigation of the bladder with no significant change in hydronephrosis or resistance. The urology service was consulted for cystoscopy and clot evacuation as placing early percutaneous nephrostomy in a fresh renal transplant on dual antiplatelet therapy was thought to be high risk. Upon cystoscopy the large clot seen on CT scan had been drained with the bladder irrigation prior, but the ureteral orifice and stent remained obstructed by adherent clot (Figure 1). The ureteral orifice was cleared of clot by grasper and manipulation of the stent with immediate return of urine (Figure 2). Post procedure abdominal drain fluid was sent for fluid creatinine to evaluate for urine leak which was 5.8 mg/dL with concordant serum creatinine of 6.1 mg/dL. The urine output over the next 24 hours was over 4 L and creatinine improved subsequently to 4.9 mg/dL prior to discharge on post-operative day 7.
Discussion
Ureteral complications leading to obstructive uropathy following renal transplant increase the risk for graft failure [4]. Percutaneous nephrostomy is the gold standard to relieve obstruction, rapidly diverting urine externally. Studies have shown that this intervention improves graft survival, allowing for delayed relief of downstream obstruction [5]; however, percutaneous nephrostomy is not without complication. While effective in relieving obstruction, percutaneous nephrostomy has risks including dislodgment, bleeding and bowel injury [6,7]. This patient was on dual anti-platelet therapy following his drug-eluting cardiac stents, causing a high degree of concern for bleeding with percutaneous nephrostomy. There was additional concern however with cystoscopy as the necessary bladder filling could put higher tension on the fresh ureteroneocystostomy. Currently, cystoscopy is occasionally employed as a diagnostic modality in the workup of obstructive hydronephrosis in renal transplantation but usually only after the patient has been diverted with percutaneous nephrostomy. Here, we propose that in carefully selected patients, early cystoscopy may both address obstruction and obviate the need for nephrostomy tube placement.
We strongly suspected that a distal obstruction remained post bladder irrigation and despite appropriate positioning of the stent on imaging. Given that our patient had significant risk factors for bleeding including receiving aspirin and clopidogrel, we felt it reasonable to pursue evaluation of the bladder, ureteral orifice, and stent as the cause of obstruction prior to urinary diversion with nephrostomy. Cystoscopy revealed clot burden blocking both the ureteral stent and the transplant ureteral orifice.
Cystoscopy has long been used in the evaluation of the urethra and bladder; however, it has thus far had a limited role in the workup of renal transplant graft dysfunction. Although, cystoscopy does have its own set of risks including ascending infection, secondary to the introduction of bacteria from the urinary tract, as well as bleeding, it remains considerably non-invasive compared to percutaneous nephrostomy. The risk of urinary tract infection after cystoscopy is approximately 5% and likely would be treated by the required prophylactic antibiotics after transplant (Table 1) [8]. Additionally, as cystoscopy in this situation is not for cancer surveillance and does not employ biopsy, the risk of bleeding is low compared to percutaneous nephrostomy. The results of cystoscopic intervention in our patient suggests that cystoscopy may be a better first line therapy to diagnose and relieve specific obstructions in certain high risk renal transplant populations.
| Post-Transplant Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Hemoglobin g/dL | 10.1 | 8.4 | 8.1 | 8.2 | 7.7 | 6.9 | 8.9* | 8.8 |
| Creatinine mg/dL | 6.1 | 5.3 | 4.5 | 6.1 | 6.3 | 7 | 6.1** | 4.9 |
Table 1: Post-Transplant lab trend. *Transfused; **Post-cystoscopy.
While the distinct improvement in graft function after cystoscopy in our patient appears to strongly correlate to the intervention, it is not known if clot formation over the ureteral stent and orifice occurs in other patients without clinical significance. Additionally, it is unclear if removal of the clot by cystoscopy was necessary to improve graft function or if intrinsic thrombolytic enzymes such as urokinase would have eventually caused clot resolution. It is known that the ureter produces both urokinase and tissue-type plasminogen activator, which both function to dissolve blood clot [9-11]; however, allowing the clot to dissolve by natural means might have contributed to delayed graft function in an already debilitated recipient. Given the minimally invasive nature of cystoscopy, we believe its consideration is warranted in patients with risk factors for clot formation who develop acute postrenal transplant obstruction with graft dysfunction.
References
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K (1993) Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 270: 1339-1343.
- Kumar S, Ameli-Renani S, Hakim A, Jeon JH, Shrivastava S, et al. (2014) Ureteral obstruction following renal transplantation: Causes, diagnosis and management. Br J Radiol 87: 20140169.
- Wilson CH, Bhatti AA, Rix DA, Manas DM (2005) Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 19: CD004925
- Rigg KM, Proud G, Ross Taylor RM (1994) Urological complications following renal transplantation. Transpl Int. 7: 120-126.
- Kaskarelis I, Koukoulaki M, Georgantas T, Bairamidis E, Kokkinos C, et al (2008) Ureteral Complications in Renal Transplant Recipients Successfully Treated With Interventional Radiology. Transplant Proc. 40: 3170-3172.
- Patel U, Jeon JH, Kumar S (2015) Thirty-day outcomes after percutaneous nephrostomy of renal transplant kidneys: 19-year experience and comparison with existing practice parameters. AJR Am J Roentgenol 205: 1326-1331.
- Radecka E, Magnusson A (2004) Complications associated with percutaneous nephrostomies. a retrospective study. Acta Radiol. 45: 184-188.
- Almallah, Y.z (2000) “Urinary tract infection and patient satisfaction after flexible cystoscopy and urodynamic evaluation.†Urology 56: 37-39.
- Ljungner H, Astedt B, Bergqvist D, Pandolfi M (1984) Human ureter, a source of tissue plasminogen activator. Thromb Res. 34: 217-224.
- Kumar S, Singh S, Parmar KM, Garg N (2014) Recurrent clot anuria following laparoscopic pyeloplasty in a solitary functioning kidney: Managing with double guide wire technique. BMJ Case Rep
- Doehn C, Matthew AT, Minford EJ, Kumar A, Forsythe JL (1997) Thrombotic occlusion of ureteric stent: An unusual cause of anuria after kidney transplantation. Nephrol Dial Transplant 12: 1267-1268.
Citation: Zhang L, Bodzin AS (2018) Cystoscopic Management of a Ureteral Orifice Obstructive Clot Early After Renal Transplantation. J Clin Exp Transplant 3: 120. DOI: 10.4172/2475-7640.1000120
Copyright: © 2018 Zhang L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4064
- [From(publication date): 0-2018 - Apr 17, 2025]
- Breakdown by view type
- HTML page views: 3293
- PDF downloads: 771