Case Report Open Access
Cutaneous Acanthamoebiasis with CNS Involvement Post-Transplantation: Implication for Differential Diagnosis of Skin Lesions in Immunocompromised Patients
Andrea D’Auria1,* Jamie Lin2 P. Jan Geiseler2 Yvonne Qvarnstrom4 Rebecca Bandea4 Sharon Roy5 Rama Sriram5 Christopher Paddock6 Sherif Zaki6 Gene Kim3,# and Govinda S. Visvesvara5,#
1Department of Pathology and Laboratory Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA 90089, USA
2Department of Infectious Disease, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
3Department of Dermatology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
4Division of Parasitic Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA 30333, USA
5Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA 30333, USA
6Division of Viral and Rickettsial Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA 30333, USA
#These authors contributed equally to this manuscript.
- *Corresponding Author:
- Andrea D’Auria
Department of Pathology and Laboratory
Medicine, Keck School of Medicine, University
of Southern California, Los Angeles, CA 90089, USA
E-mail: andrea.dauria@gmail.com
Received Date: 9 August 2012 Revised Date: 14 September 2012 Accepted Date: 21 September 2012
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
We report a 62-year-old male who presented status post lung transplantation with subcutaneous nodules. One week later, he showed signs of altered mental status; brain imaging demonstrated mass effect in the cerebellum and meningoencephalitis. In spite of treatment with a broad range of antimicrobials, he died. A punch biopsy of the skin lesions showed a superficial and deep mixed inflammatory infiltrate admixed with large mononuclear cells. A diagnosis of cutaneous amoebiasis was made and the amoebae were identified as Acanthamoeba spp. based on immunofluorescent stains and PCR assays. Cutaneous Acanthamoebiasis is a rare infection in immunocompromised patients, particularly organ recipients. It is important that this entity is included in the differential diagnosis of immunocompromised patients who have cutaneous infections that are not responding to antibiotics. An early diagnosis is crucial since cutaneous Acanthamoebiasis can disseminate to the central nervous system and cause granulomatous amoebic encephalitis (GAE), which is usually fatal.
We report a 62-year-old male who presented status post lung transplantation with subcutaneous nodules. One week later, he showed signs of altered mental status; brain imaging demonstrated mass effect in the cerebellum and meningoencephalitis. In spite of treatment with a broad range of antimicrobials, he died. A punch biopsy of the skin lesions showed a superficial and deep mixed inflammatory infiltrate admixed with large mononuclear cells. A diagnosis of Cutaneous amoebiasis was made and the amoebae were identified as Acanthamoeba spp. based on immunofluorescent stains and PCR assays. Cutaneous Acanthamoebiasis is a rare infection in immunocompromised patients, particularly organ recipients. It is important that this entity is included in the differential diagnosis of immunocompromised patients who have Cutaneous infections that are not responding to antibiotics. An early diagnosis is crucial since Cutaneous Acanthamoebiasis can disseminate to the central nervous system and cause granulomatous amoebic encephalitis (GAE), which is usually fatal.
Keywords
Cutaneous amoebiasis; Acanthamoeba; transplant; granulomatous amebic encephalitis
Introduction
Among the many genera of free-living amoebae present in the environment, only a few (several species of Acanthamoeba, the only described species of Balamuthia, B. mandrillaris, and only one species of Naegleria, N. fowleri) are known to cause fatal infections in humans and other animals [9,18,21,23,33,40]. Acanthamoeba spp. and N. fowleri are ubiquitous amebae found world-wide in soil, water, and other environmental sources. Acanthamoeba spp. and B. mandrillaris cause a rare but frequently fatal chronic infection of the central nervous system (CNS) called granulomatous amebic encephalitis (GAE) whereas N. fowleri causes an acute, fulminating, and almost always fatal CNS infection called primary amebic meningoencephalitis (PAM), most commonly among children and young adults with recent fresh water exposure [9,18,21,23,33,40]. GAE caused by Acanthamoeba spp. and B. mandrillaris is often associated with infections of the skin, lungs, and sinuses, which may be the primary source of infection that later disseminates hematogenously [9,18,21,23,33, 40]. GAE due to Acanthamoeba spp. occurs primarily in immunocompromised patients whereas Balamuthia GAE occurs in both immunocompetent and immunosuppressed individuals. Acanthamoeba spp. has also been associated with sight-threatening keratitis in contact lens wearers. In addition to these three amoebae, another free-living ameba Sappinia pedata (formerly identified as S. diploidea) has been identified to have caused a single case of meningoencephalitis in an immunocompetent man who ultimately recovered from this infection [15,30].
Case report
62-year-old Latino male with history of idiopathic pulmonary fibrosis received a double lung transplant in October 2009. The month following transplant he was diagnosed with acute rejection and received a 1.5 gram solumedrol burst over 3 days. His immunosuppressive regimen included tacrolimus, mycophenolate mofetil, and prednisone. He was also on antibiotic prophylaxis with valganciclovir, voriconazole, and trimethoprinsulfamethoxazole.
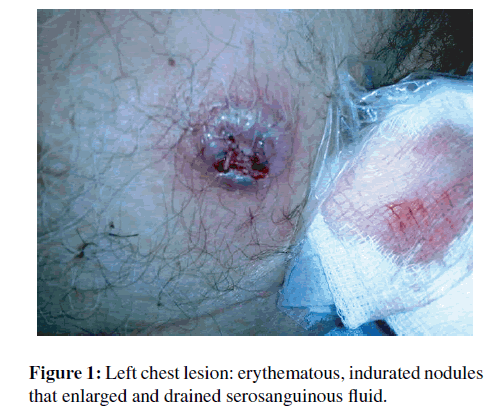
In mid-December 2009, he began developing skin abscesses. The first abscess appeared on his right chest wall (Figure 1), the second on his left chest wall, and the third on his posterior right lower extremity. The lesions began as firm erythematous nodules that grew in size and went on to become painful with drainage of sero-purulent fluid. At the end of January, he was admitted to the hospital complaining of right lower extremity pain and shortness of breath. A bronchoscopy with biopsy and bronchoalveolar lavage was performed. The bronchoalveolar lavage cultures grew >10,000 colony forming units of Pseudomonas aeruginosa and the biopsy was consistent with acute cellular rejection, type A1-2. He received a 3 gram solumedrol burst over three days, cefepime, and inhaled tobramycin for the Pseudomonas infection, and vancomycin for the skin lesions.
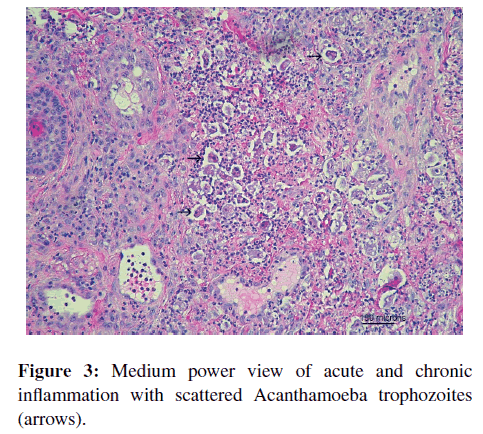
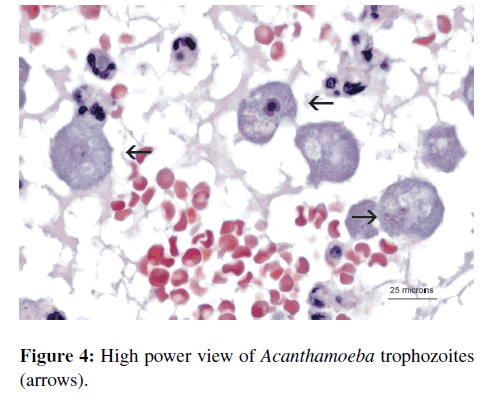
A punch biopsy of the right chest lesion showed a diffuse superficial and deep dermal infiltrate of mixed inflammatory cells with scattered large mononuclear cells (Figures 2 and 3). These mononuclear cells were large with a single prominent round nucleus, a central nucleolus surrounded by a rigid halo, and abundant cytoplasm with evidence of phagocytized red blood cells and neutrophils (Figure 4). Special stains showed negativity for CD68, acid fast bacilli (AFB), Gomori methenamine silver (GMS), and periodic acid-schiff (PAS). The mononuclear cells were suspicious for amoeba, so samples of the punch biopsy were sent to the Centers for Disease Control and Prevention (CDC) for confirmation of amebic infection.
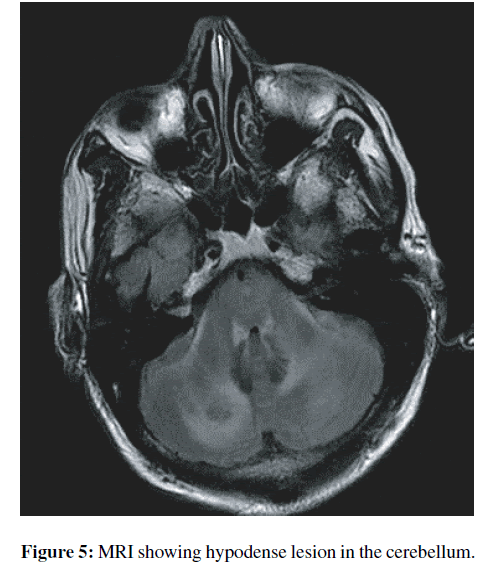
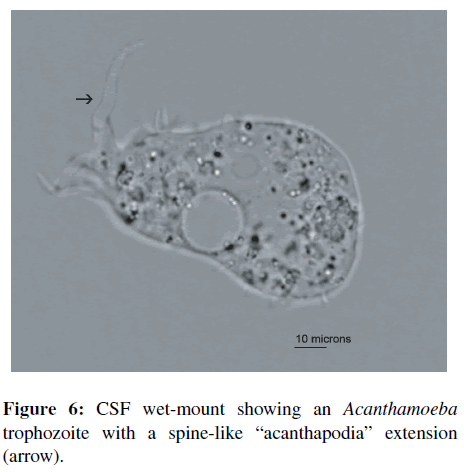
One week into his hospitalization the patient became progressively altered and agitated. An emergent computed tomography (CT) scan of his head revealed patchy areas of hypodensity with associated mass effect in the cerebellum, effacement of the fourth ventricle and perimedullary cistern (Figure 5). Subsequent magnetic resonance imaging of the brain revealed diffuse leptomeningeal enhancement involving the cerebellum, basilar cisterns, and middle cranial fossa consistent with meningoencephalitis. Because of mass effect and developing hydrocephalus, an emergent ventriculostomy was placed by neurosurgery. The cerebrospinal fluid was spun and wet mount examination performed showed motile amoebic trophozoites (Figure 6). At this point, the primary concern was for granulomatous amebic encephalitis (GAE) caused either by Balamuthia or Acanthamoeba spp. The patient was started on a broad range of antimicrobials including fluconazole, trimethoprimsulfamethoxazole, flucytosine, pentamidine, intrathecal amphotericin B, and amikacin. Additionally, the medical team attempted to obtain Miltefosine from overseas, which did not arrive in time. The patient developed progressive hydrocephalus and brain herniation and was declared brain-dead.
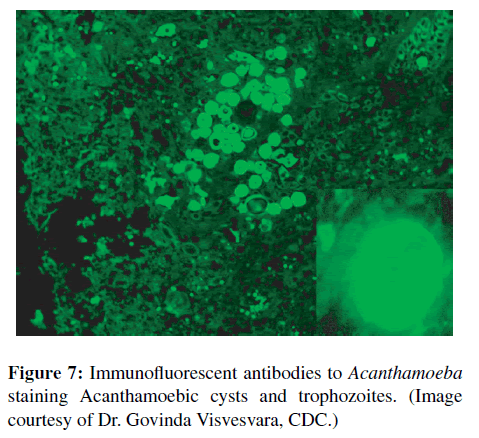
Samples of the skin biopsy, CSF and brain tissue were forwarded to CDC and processed for culture, immunofluorescent (IFA) staining [9,18,21,23,33,40] and real-time PCR [31]. IFA staining of the punch biopsy was positive for Acanthamoeba spp. (Figure 7). The skin biopsy specimen was inoculated onto agar plates coated with Escherichia coli and yielded a culture of large Acanthamoeba trophozoites measuring 35 μm to 45μm. The trophozoites differentiated within a week into cysts measuring 25 μm to 30μm. The cysts possessed a nearly round, gently rippled ectocyst and a stellate endocyst with 5 to 8 rays that contacted the inside of the ectocyst. Based on these characteristics, the isolate was identified as belonging to Acanthamoeba Group 1. DNA extracted from all of the tissue samples tested positive for Acanthamoeba in a real-time PCR assay that simultaneously detects Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri [31]. Genotyping of the Acanthamoeba isolate was performed by amplifying a 1,456 base pair fragment of the small subunit ribosomal RNA gene with PCR primers JDP1and B [14,32]. Pair-wise DNA sequence comparisons showed the highest similarity (around 95%) to Acanthamoeba strains belonging to the newly described T17 genotype [26] and unambiguously placed the amoeba isolate among the other group 1 species: A. tubiashi, A. astronyxis, and A. comandoni.
Discussion
Acanthamoeba is a ubiquitous free-living organism found world-wide and lives in diverse environmental niches including soil, waste dumps, cooling towers of air conditioning systems, humidifiers, aquaria, dialysis machines, dental equipment, as well as bottled tap, and sea waters. Additionally, they have been isolated from the nasal secretions of healthy individuals, including children, students, and military recruits indicating that subclinical infections might be common [9,18,21,23,33,40].
The life cycle of Acanthamoeba includes an active trophozoite stage in which it feeds on bacteria, and a dormant cyst which forms when environmental conditions become harsh. The cyst has a protective double wall that is resistant to chemicals and therapeutic drugs [21].
Acanthamoeba causes two significant clinical infections: amoebic keratitis in immunocompetent contact lens wearers and granulomatous amoebic encephalitis (GAE) in immune suppressed people [10,38]. Amoebic keratitis is a painful eye infection in which the amoebic organisms directly penetrate the ocular tissues and has been associated with soft contact lenses [9,10,18,21,23,33,38,40]. Acanthamoebic GAE is a progressive disease of the central nervous system and occurs principally in immunocompromised patients. The route into the central nervous system appears to be from the skin (Cutaneous Acanthamoebiasis), naropharynx or lungs after which it disseminates hematogenously.
GAE has been an infection of increasing importance in the literature, particularly in organ transplant patients as well as patients with AIDS or those taking immunosuppressant medications. It is thought that the use of immunosuppressant medications predisposes patients to infection with Acanthamoeba spp. [21]. We report a case of a doublelung transplant recipient who presented with Cutaneous Acanthamoebiasis followed by GAE. The first case of Cutaneous Acanthamoebiasis preceding GAE in a transplant patient was reported in 1982 by A. J. Martinez [22]. Since then, the reported cases of Acanthamoeba spp. infections in transplant patients have been increasing in the literature. To date, there have been 20 cases of Acanthamoeba spp. infections reported in organ transplant patients (Table 1); five kidney transplants [22,24,25,35,36], seven bone marrow/stem cell transplants [1,3,4,7,12,17,28], five lung transplants [11,27,37,39,41], one stomach, duodenum and small intestine transplant [16], one heart transplant [5], and two liver transplants [13,43]. Eight of the Acanthamoeba spp. infections reported in recipients of organ transplants presented with skin lesions before the onset of systemic symptoms [4,5,11,24,27,28,35,37], and one case had skin lesions which appeared after the onset of systemic symptoms [22]. The skin lesions were described as nodules [4,5, 11,22,27,28,35], ulcers [24,37], and cellulitis [16,27,37]. Most of the patients that have presented with skin findings have firm papulonodules that eventually drain purulent fluid and develop into non-healing ulcers. Typically, wound cultures and blood cultures in Acanthamoeba spp. infections are negative for bacteria and fungi. Four other organ transplant patients with Acanthamoeba spp. infections presented with sinusitis [1,4,28,39] and two had lung nodules [7,22] prior to the development of GAE.
| Year [ref] | Age/sex | Organ transplanted | Clinical manifestations | Histology of skin biopsy | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1982[22] | 38/M | Kidney | Skin nodules,nodular lunglesions | Fat necrosis, acuteinflammation | Broad spectrum antibiotics,corticosteroids | Died after18 days |
| 1994[4] | Unk | HSCT | Subcutaneous nodulesthat ulcerated, sinusitis | Unknown | Broad spectrum antibiotics | Died(unknownduration) |
| 1994[35] | 31/M | Kidney | Skin nodules | Nonspecific inflammationand granulomatous changessuggestive of pyodermagangrenosum, scatteredround-to-ovoid amebicforms, measuring up to20 µm in diameter | Pentamidine isethionate(IV), topical therapy withchlorhexidine gluconate,followed by 2 percentketoconazole cream andoutpatient itraconazole | Survived |
| 1999[27] | 39/F | Lung | Skin nodule withcellulitis | Abscess formation withacute inflammation andtrophozoites | Pentamidine, 5-fluorocytosine,itraconazole, topicalchlorhexidinegluconate/ketoconazole cream | Survived |
| 2001[7] | 38/M | Lung (double) | Right foot ulcer withcellulitis | Trophozoite and cyst forms | Pentamidine, itraconazole,topical ketoconazole,chlorhexidine | Died after6 months |
| 2006[11] | 60/M | Lung (double) | Skin nodules | Granulomatousinflammation,coagulative necrosis ofthe dermis and subcutiswith rare trophozoites | Broad spectum antibiotics(diagnosis made post mortem) | Died after17 days |
| 2006[24] | 51/M | Kidney | Skin ulcerations onlegs, left facialtwitching and left armand leg numbness | Unknown | Broad-spectrum antibiotics, IVvancomycin, trimethoprim-sulfamethoxazole, fluconazole,meropenem, liposomalamphotericin B, pentamidine,rifampin, azithromycin,flucytosine, sulfadiazine, and metronidazole | Died afterthree weeks |
| 2007 [5] | 39/M | Heart | Trunk and leg abscesses |
Cysts and trophozoites (30 µm diameter) in the dermal-hypodermal junction with polymorphous inflammatory granulomas associated with ischemic necrosis |
Pentamidine, 5-fluorocytosine, and itraconazole |
Died after 23 days |
| 2009Our case | 62/M | Lung (double) | Trunk and legabscesses | Cysts and trophozoites withacute inflammation andnecrosis, no granulomatousinflammation | Fluconazole, trimethoprim-sulfamethoxazole,flucytosine, pentamidine,intrathecal amphotericin Band amikacin | Died afterthree weeks |
M; male, F; female, HSCT; hematopoietic stem cell transplant, Unk; unknown, IV; intravenous.
Table 1: Acanthamoeba spp. infections in transplant patients who presented with skin manifestations.
Etiological diagnosis of Cutaneous Acanthamoebiasis or GAE can be facilitated by polymerase chain reaction (PCR) of biopsy specimens or body fluids containing amoebic organisms such as cerebrospinal fluid (CSF) [31]. Currently, there is no distinct clinical or laboratory feature that differentiates Acanthamoeba spp. from B. mandrillaris infections [9,18,21,23,31,33,40].
Histopathologically, the skin lesions show acute and chronic inflammation with granulomatous inflammation [5,7,11,13,35] or without granulomatous inflammation [5,22,27,28]. The variation in the presence of granulomatous inflammation is thought to be due to the state of the patient’s immune system; some organ transplant patients are more immune suppressed than others and therefore cannot mount a normal immune reaction to the amoebae. A presumptive diagnosis is often established when trophozoites and/or cysts are seen using hematoxylin and eosin (H and E) stains. The amoebic trophozoites are large cells (10–60 micrometers in diameter) with a single round nucleus and a centralized prominent nucleolus forming a halo. They have abundant cytoplasm, which may contain phagocytized red blood cells or neutrophils (Figure 4). Acanthamoeba spp. cysts are typically smaller (15–30 micrometers) and have a double-wall. The outer wall is thicker than the inner wall, which is characteristically scalloped. The nucleus in the cyst is similar to that of the trophozoite, round with a centrally placed prominent nucleolus (Figure 8) [21]. In some of the previously reported cases, Gomori methenamine silver (GMS) and periodic acid-schiff (PAS) stains were used in addition to H and E stains of the tissue sections. However, the GMS and PAS stains were negative in two of the reported cases [35], PAS was only weakly positive in one case [39], and one case had a positive PAS and GMS staining in cysts [13]. A PAS stain was positive in the amoebic cysts in our case (Figure 8). Therefore, a positive PAS and/or GMS stain is not necessary for confirmation of the diagnosis.
Acanthamoeba spp. trophozoites can resemble other organisms, macrophages and/or necrotic epithelial cells in H and E stained preparations. Negative CD68 and pancytokeratin immunohistochemical stains exclude macrophages and necrotic keratinocytes as possibilities. Other organisms with similar appearance include Entamoeba histolytica. However, E. histolytica often has more than one nucleus and typically has smaller nucleoli. Balamuthia species are also free-living amoeba that can cause similar nonhealing ulcers in immunocompromised patients and have the same morphological appearance especially in tissue sections. Therefore, polymerase chain reaction or immunohistochemical studies are necessary to confirm that the amoebae are indeed Acanthamoeba spp.
It is uncommon to see the organisms on wet mount, but rare cases have been described in addition to our case [6, 8,19,20,29,34]. Typically, the trophozoites are large motile organisms with spine-like projections termed “acanthapodia” and nuclear features similar to those previously described (Figure 5). It is recommended that the diagnosis of GAE should be subsequently confirmed with PCR or immunofluorescence tests on the CSF since host cells can be mistaken for Acanthamoeba spp. Also, PCR can be used to confirm the presence of amoebae in the CSF without direct visual identification of trophozoites and/or cysts. However, in practice this is rare. Most cases are diagnosed at autopsy. CT scan of the brain may show single or multiple ring enhancing lesions (Figure 4). Microscopically, the brain lesions associated with GAE have been described as necrotic with mixed inflammation and trophozoites and/or cysts with or without granulomatous inflammation [12,22,28].
The time from initial presentation of skin lesions to dissemination is described as weeks to months. This time period is critical because once the infection disseminates to become GAE, the survival rate is poor. Clinically the signs and symptoms of GAE are headaches, dizziness, confusion, and behavioral changes, as well as focal neurological deficits such as aphasia, hemiparesis, and eventual coma [23]. To date, two reported cases of transplant patients with Cutaneous lesions [27,35] and one case of sinusitis [39] were successfully treated before any signs or symptoms of dissemination. Once the infection has disseminated to GAE, the majority of organ transplant patients did not survive. There are two reported cases of organ transplant patients who survived brain abscesses associated with Acanthamoeba spp. [13,27].
Currently, there are no standardized treatment recommendations for Cutaneous Acanthamoebiasis or GAE. Organ transplant patients who survived infections with Acanthamoeba spp. have been treated with pentamidine, itraconazole, topical cleansing with chlorhexidine [27, 35], 5-fluorocytosine [27], rifampin, and cotrimoxazole [13]. There have been cases of other immunosuppressed patients with successfully treated infections with topical Miltefosine as well as oral Miltefosine [2,42]. It is thought that decreasing the dose of the immunosuppressant medications is helpful for the patient to mount an immune response against the amoebae.
Cutaneous Acanthamoebiasis can be a precursor to disseminated GAE and requires a high index of suspicion for diagnosis. Non-healing skin ulcerations or abscesses in immunocompromised patients should be cultured and biopsied for proper evaluation. Cutaneous Acanthamoebiasis can be diagnosed by the identification of cysts and/or trophozoites on H and E stains. Once the infection disseminates to GAE, the survival rate is low and currently there is no standardized treatment for disseminated disease.
Acknowledgments
The authors thank Melanie Moser and Dr. Norman J. Pieniazek for helpful suggestions.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Department of Health and Human Services or the Centers for Disease Control and Prevention.
References
- H. Abd, A. Saeed, S. Jalal, A. N. Bekassy, and G. Sandstr¨om, Ante mortem diagnosis of amoebic encephalitis in a haematopoietic stem cell transplanted patient, Scand J Infect Dis, 41 (2009), 619–622.
- A. C. Aichelburg, J. Walochnik, O. Assadian, H. Prosch, A. Steuer, G. Perneczky, et al., Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine, Emerg Infect Dis, 14 (2008), 1743–1746.
- G. Akpek, A. Uslu, T. Huebner, A. Taner, A. P. Rapoport, I. Gojo, et al., Granulomatous amebic encephalitis: an underrecognized cause of infectious mortality after hematopoietic stem cell transplantation, Transpl Infect Dis, 13 (2011), 366–373.
- P. Anderlini, D. Przepiorka, M. Luna, L. Langford, M. Andreeff, D. Claxton, et al., Acanthamoeba meningoencephalitis after bone marrow transplantation, Bone Marrow Transplant, 14 (1994), 459–461.
- S. Barete, A. Combes, J. F. de Jonckheere, A. Datry, S. Varnous, V. Martinez, et al., Fatal disseminated Acanthamoeba lenticulata acanthamebiasis in a heart transplant patient, Emerg Infect Dis, 13 (2007), 736–738.
- J. H. Callicott Jr., Amebic meningoencephalitis due to free-living amebas of the Hartmannella (Acanthamoeba)-Naegleria group, Am J Clin Pathol, 49 (1968), 84–91.
- A. Castellano-Sanchez, A. C. Popp, F. S. Nolte, G. S. Visvesvara, M. Thigpen, I. Redei, et al., Acanthamoeba castellani encephalitis following partially mismatched related donor peripheral stem cell transplantation, Transpl Infect Dis, 5 (2003), 191–194.
- P. G. Cleland, R. V. Lawande, G. Onyemelukwe, and H. C. Whittle, Chronic amebic meningoencephalitis, Arch Neurol, 39 (1982), 56–57.
- B. da Rocha-Azevedo, H. B. Tanowitz, and F. Marciano- Cabral, Diagnosis of infections caused by pathogenic free-living amoebae, Interdiscip Perspect Infect Dis, 2009 (2009), Article ID 251406.
- J. K. Dart, V. P. Saw, and S. Kilvington, Acanthamoeba keratitis: diagnosis and treatment update 2009, Am J Ophthalmol, 148 (2009), 487–499.e2.
- A. G. Duarte, F. Sattar, B. Granwehr, J. F. Aronson, Z.Wang, and S. Lick, Disseminated Acanthamoebiasis after lung transplantation, J Heart Lung Transplant, 25 (2006), 237–240.
- J. M. Feingold, J. Abraham, S. Bilgrami, N. Ngo, G. S. Visvesara, R. L. Edwards, et al., Acanthamoeba meningoencephalitis following autologous peripheral stem cell transplantation, Bone Marrow Transplant, 22 (1998), 297–300.
- K. T. Fung, A. P. Dhillon, J. E. McLaughlin, S. B. Lucas, B. Davidson, K. Rolles, et al., Cure of Acanthamoeba cerebral abscess in a liver transplant patient, Liver Transpl, 14 (2008), 308–312.
- R. J. Gast, Development of an Acanthamoeba-specific reverse dot-blot and the discovery of a new ribotype, J Eukaryot Microbiol, 48 (2001), 609–615.
- B. B. Gelman, S. J. Rauf, R. Nader, V. Popov, J. Borkowski, G. Chaljub, et al., Amoebic encephalitis due to Sappinia diploidea, JAMA, 285 (2001), 2450–2451.
- A. H. Gene, P. A. Gardner, and M. E. Couce Matovelle, Rapidly expanding brain mass, Transpl Infect Dis, 9 (2007), 211–213.
- D. R. Kaul, L. Lowe, G. S. Visvesvara, S. Farmen, Y. A. Khaled, and G. A. Yanik, Acanthamoeba infection in a patient with chronic graft-versus-host disease occurring during treatment with voriconazole, Transpl Infect Dis, 10 (2008), 437–441.
- N. A. Khan, Acanthamoeba spp., in Emerging Protozoan Pathogens, N. A. Khan, ed., Taylor and Francis, New York, 2008, 3–69.
- M. K. Lalitha, V. Anandi, A. Srivastava, K. Thomas, A. M. Cherian, and S. M. Chandi, Isolation of Acanthamoeba culbertsoni from a patient with meningitis, J Clin Microbiol, 21 (1985), 666–667.
- R. C. MacLean, N. Hafez, S. Tripathi, C. G. Childress, N. R. Ghatak, and F. Marciano-Cabral, Identification of Acanthamoeba sp. in paraffin-embedded CNS tissue from an HIV+ individual by PCR, Diagn Microbiol Infect Dis, 57 (2007), 289–294.
- F. Marciano-Cabral and G. Cabral, Acanthamoeba spp. as agents of disease in humans, Clin Microbiol Rev, 16 (2003), 273–307.
- A. J. Martinez, Acanthamoebiasis and immunosuppression. Case report, J Neuropathol Exp Neurol, 41 (1982), 548–557.
- A. J. Martinez and G. S. Visvesvara, Free-living, amphizoic and opportunistic amebas, Brain Pathol, 7 (1997), 583–598.
- M. S. McKellar, L. R. Mehta, J. E. Greenlee, D. C. Hale, G. C. Booton, D. J. Kelly, et al., Fatal granulomatous Acanthamoeba encephalitis mimicking a stroke, diagnosed by correlation of results of sequential magnetic resonance imaging, biopsy, in vitro culture, immunofluorescence analysis, and molecular analysis, J Clin Microbiol, 44 (2006), 4265–4269.
- D. Mutreja, Y. Jalpota, R. Madan, and V. Tewari, Disseminated Acanthamoeba infection in a renal transplant recipient: a case report, Indian J Pathol Microbiol, 50 (2007), 346–348.
- W. Nuprasert, C. Putaporntip, L. Pariyakanok, and S. Jongwutiwes, Identification of a novel t17 genotype of Acanthamoeba from environmental isolates and t10 genotype causing keratitis in Thailand, J Clin Microbiol, 48 (2010), 4636–4640.
- S. Oliva, M. Jantz, R. Tiernan, D. L. Cook, and M. A. Judson, Successful treatment of widely disseminated Acanthamoebiasis, South Med J, 92 (1999), 55–57.
- J. Pem´an, I. Jarque, J. Frasquet,C.Alberola, M. Salavert, J. Sanz, et al., Unexpected postmortem diagnosis of Acanthamoeba meningoencephalitis following allogeneic peripheral blood stem cell transplantation, Am J Transplant, 8 (2008), 1562–1566.
- F. Petry, M. Torzewski, J. Bohl, T. Wilhelm-Schwenkmezger, P. Scheid, J. Walochnik, et al., Early diagnosis of Acanthamoeba infection during routine cytological examination of cerebrospinal fluid, J Clin Microbiol, 44 (2006), 1903–1904.
- Y. Qvarnstrom, A. J. da Silva, F. L. Schuster, B. B. Gelman, and G. S. Visvesvara, Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis, J Infect Dis, 199 (2009), 1139–1142.
- Y. Qvarnstrom, G. S. Visvesvara, R. Sriram, and A. J. da Silva, Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri, J Clin Microbiol, 44 (2006), 3589–3595.
- J. M. Schroeder, G. C. Booton, J. Hay, I. A. Niszl, D. V. Seal, M. B. Markus, et al., Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge, J Clin Microbiol, 39 (2001), 1903–1911.
- F. L. Schuster and G. S. Visvesvara, Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals, Int J Parasitol, 34 (2004), 1001–1027.
- T. Singhal, A. Bajpai, V. Kalra, S. K. Kabra, J. C. Samantaray, G. Satpathy, et al., Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials, Pediatr Infect Dis J, 20 (2001), 623–627.
- C. A. Slater, J. Z. Sickel, G. S. Visvesvara, R. C. Pabico, and A. A. Gaspari, Brief report: successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient, N Engl J Med, 331 (1994), 85–87.
- J. P. Steinberg, R. L. Galindo, E. S. Kraus, and K. G. Ghanem, Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and Cutaneous lesions: case report and literature review, Clin Infect Dis, 35 (2002), e43–e49.
- C. Van Hamme, M. Dumont, M. Delos, and J. M. Lachapelle, Cutaneous Acanthamoebiasis in a lung transplant patient, Ann Dermatol Venereol, 128 (2001), 1237–1240.
- J. R. Verani, S. A. Lorick, J. S. Yoder, M. J. Beach, C. R. Braden, J. M. Roberts, et al., National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States, Emerg Infect Dis, 15 (2009), 1236–1242.
- S. E. Vernon, B. C. Acar, S. M. Pham, and D. Fertel, Acanthamoeba infection in lung transplantation: report of a case and review of the literature, Transpl Infect Dis, 7 (2005), 154– 157.
- G. S. Visvesvara, H. Moura, and F. L. Schuster, Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea, FEMS Immunol Med Microbiol, 50 (2007), 1–26.
- R. Walia, J. G. Montoya, G. S. Visvesvera, G. C. Booton, and R. L. Doyle, A case of successful treatment of Cutaneous Acanthamoeba infection in a lung transplant recipient, Transpl Infect Dis, 9 (2007), 51–54.
- J. Walochnik, A. Aichelburg, O. Assadian, A. Steuer, G. Visvesvara, N. Vetter, et al., Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient, J Clin Microbiol, 46 (2008), 338–340.
- A. L. Young, N. R. Leboeuf, S. J. Tsiouris, S. Husain, and M. E. Grossman, Fatal disseminated Acanthamoeba infection in a liver transplant recipient immunocompromised by combination therapies for graft-versus-host disease, Transpl Infect Dis, 12 (2010), 529–537.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 15330
- [From(publication date):
December-2012 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10755
- PDF downloads : 4575