Review Article Open Access
Current Status of Endoscopic Treatment of Advanced Hilar Cholangiocarcinoma
Varayu Prachayakul*
Department of Internal Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
- *Corresponding Author:
- Varayu Prachayakul
Siriraj GI Endoscopy Center
Division of Gastroenterology
Department of Internal Medicine
Faculty of Medicine
Siriraj Hospital, Mahidol University, Bangkok
Thailand 10700
Tel: +66818654646
Fax: +6624121088
E-mail: kaiyjr@gmail.com
Received date: January 03, 2014; Accepted date: January 27, 2014; Published date: February 06, 2014
Citation: Prachayakul V (2014) Current Status of Endoscopic Treatment of Advanced Hilar Cholangiocarcinoma. J Gastroint Dig Syst 4:168. doi:10.4172/2161-069X.1000168
Copyright: © 2014 Prachayakul V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Cholangiocarcinoma is a high-mortality primary hepatic malignancy. A higher incidence of cholangiocarcinoma was reported in Asia, especially Southeast Asia, than in Western countries. Hilar cholangiocarcinoma is a specific type of extrahepatic cholangiocarcinoma that involves the hepatic hilum and has a worse prognosis. More than half of the patients with jaundice are inoperable at the time of first diagnosis. Therefore, biliary drainage is the mainstay of palliative treatment in these patients. Endoscopic biliary drainage via endoscopic retrograde cholangiopancreatography, the modality of choice, for the advanced hilar type is more difficult and complex than those in distal cholangiocarcinoma. Endoscopists should consider many factors before selecting the most appropriate treatment for each patient. Here we discuss the factors systematically. In cases of transpapillary approach failure, other therapeutic modalities should be considered. Percutaneous transhepatic biliary drainage is the most popular method in such cases. At the present, endoscopic ultrasound-guided biliary drainage, especially hepaticogastrostomy, is an alternative procedure with the same efficacy and low complications when it was carried out in the expert hands. Furthermore, recent locoregional therapies for tumor control including trans-luminal photodynamic therapy and radiofrequency ablation also benefit these patients.
Keywords
Unresectable hilar cholangiocarcinoma; Inoperable hilar cholangiocarcinoma; Endoscopic treatment; Biliary stenting; Photodynamic therapy; Radiofrequency ablation; RFA; PDT
Introduction
Cholangiocarcinoma (CCA) is a fatal malignant tumor of the hepatobiliary system with a higher incidence in Eastern countries. The highest incidence of CCA was found in Southeast Asia, especially Thailand, which was 71.3:100,000 in men and 31.6:100,000 in women [1], followed by China and some other countries in Southeast Asia [2], whereas data from the USA, Europe, and Australia showed overall incidences of 0.82:100,000, 0.9–5.5:100,000, and 0.8–1.0:100,000, respectively [3-5]. This discrepancy in disease incidence can be explained according to the pathogenesis of CCA, although cases of CCA were sporadic.
Strong risk factors for CCA include older age, male gender, chronic hepatobiliary tract inflammation such as primary sclerosing cholangitis, chronic hepatolithiasis, choledochal cyst, and chronic parasitic infestations such as Opisthorchis viverrini and Chlonorchis sinensis [6-8]. Other recently reported possible risk factors include chronic viral hepatitis infections such as hepatitis B and C, obesity, non-alcoholic steatohepatitis, and metabolic syndrome [9-13]. In the highest incidence area of CCA, O. viverrini plays a very important role in the tumorgenicity of CCA [14].
Understanding the pathogenesis of CCA could be very helpful for tumor screening and providing targeted treatment of CCA in the future. However, CCA currently has a very high mortality rate worldwide. Regarding the clinical characteristics of this silent and slow-growing disease, the lack of a standardized protocol for screening for early-stage disease and the limitations of using CA19-9 as a cancer marker delay the diagnosis in some patients. Even in this era of high-quality imaging studies and improved endoscopic techniques, the ability to achieve a definite cytopathological or histopathological diagnosis in patients with suspected CCA remains at 26–80% [15-17].
Once the diagnosis of CCA is made, R0 resection is the only treatment that provides a potentially curable disease, whereas R1 resection leads to unacceptably low 5-year survival rates and a very high tumor recurrence rate within 2 years [18-20]. Despite the fact that the prognosis of patients with advanced or unresectable cholangiocarcinoma is poor with a median survival time of <6 months [21], it is necessary to ensure a good quality of life and limit the tumor invasion. In this article, we review the endoscopic treatments and techniques in terms of endoscopic biliary drainage and intraluminal procedures for locoregional tumor control that are beneficial for patients with CCA.
Unresectable Cholangiocarcinoma
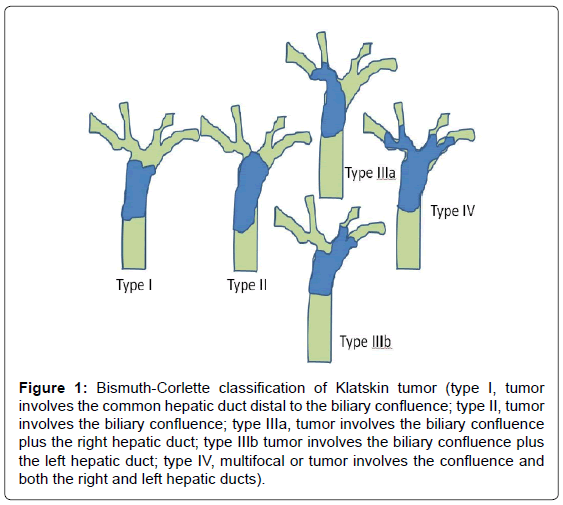
CCA is classified into extrahepatic and intrahepatic types, the ratios of which vary 0.2–187:1 [2,21,22]. Hilar CCA, an extrahepatic type, was classified by Bismuth and Corlette [23] in 1975 into four subtypes (Figure 1). According to Memorial Sloan Kettering Cancer Center Criteria [20], unresectable CCA is characterized by the presence of at least one of the following: peritoneal or noncontiguous intrahepatic metastasis; periduodenal, retropancreatic, common hepatic, or celiac node involvement; main portal vein involvement or bilateral involvement of the secondary biliary radicles; or unilateral tumor extension to the secondary biliary radicles with contralateral lobar atrophy or portal vein involvement. All of these criteria were applied based on the result of imaging studies performed prior to surgery.
Figure 1: Bismuth-Corlette classification of Klatskin tumor (type I, tumor involves the common hepatic duct distal to the biliary confluence; type II, tumor involves the biliary confluence; type IIIa, tumor involves the biliary confluence plus the right hepatic duct; type IIIb tumor involves the biliary confluence plus the left hepatic duct; type IV, multifocal or tumor involves the confluence and both the right and left hepatic ducts).
Studies have shown that 15–29.5% of patients with CCA were deemed unresectable at the time of diagnosis [24-26]. In addition, in some institutes, patients with potentially resectable CCA would undergo laparoscopic evaluation prior to laparotomy. Barlow et al. [27] reported that as many as 45% of patients had inoperable disease and that another 35% of patients who underwent laparotomy for attempted R0 resection had inoperable disease. This study provided the overall laparoscopy yield of 45% and accuracy of 71%. Therefore, approximately two thirds of patients with CCA are in the advanced stage of the disease at the time of first diagnosis.
Biliary drainage is the main palliative strategy for patients with advanced CCA. The advantages and disadvantages of endoscopic biliary drainage compared to percutaneous drainage and surgical biliary bypass procedures such as hepaticojejunostomy have been reported and debated [28-30]. Endoscopic procedures are currently the preferred palliative treatment options for patients with advanced CCA worldwide.
Endoscopic Biliary Drainage
Endoscopic biliary drainage of hilar CCA is more difficult compared to that of distal CCA. To achieve the best therapeutic outcome, endoscopists need to answer the following questions: (a) How many segments of the liver should be drained; (b) Should unilateral, multisegmented unilateral, or bilateral stenting be used; (c) Should plastic or self- expandable metal stents (SEMS) be used; and (d) Which technique is the best? This article discusses these issues as shown below.
How many segments of the liver should be drained and (b) Should unilateral, multi-segmented unilateral, or bilateral stenting be used?
The Asia Pacific consensus [31] recommendation in 2013 stated that the goal for palliative drainage of hilar cholangiocarcinoma is to drain ≥ 50% of the liver volume, although 25% drainage might be enough to relieve jaundice. In terms of estimated hepatic volume, the right (anterior and posterior segment), left, and caudate lobes account for 55–60% (35% and 30%), 30–35%, and 10%, respectively, so draining this according to reference volume alone might not be the “real” liver volume.
To ensure effective biliary drainage, “targeted stent placement” based on pre-procedural imaging studies was found to be cost-effective [32]. Vienne et al. [33] reported cross-sectional imaging studies in which effective drainage consisted of >50% of the liver volume while the factors associated with long survival was >50% drainage (119 days vs. 59 days, p=0.005) and chemotherapy. Therefore, pre-procedural evaluations with cross-sectional imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) are essential.
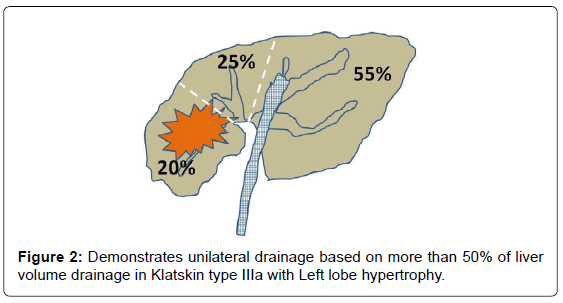
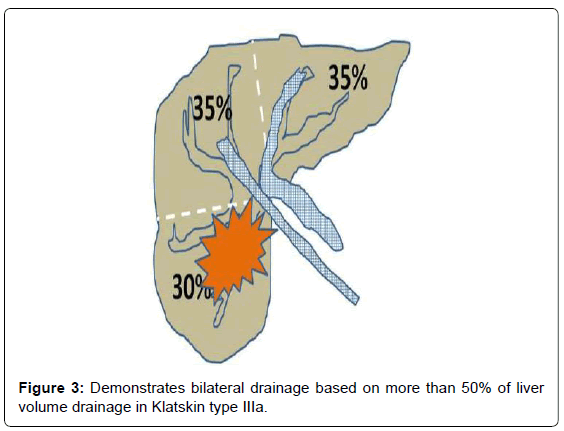
Liver volume evaluations can be divided according to the usual portal system distribution into three sectors: left (segments II and III), right posterior (segments VI and VII), and right anterior (segments V and VIII). Segment IV was assigned to the left or right posterior segment depending on each patient’s anatomy. The relative area of each sector is calculated as a fraction on each side, while the relative volume of each sector is inferred from the pool surface analysis of all sides. For example, in a patient with Bismuth type IIIa with right lobe atrophy and left lobe hypertrophy as shown in Figure 2, would benefit from leftsided stent placement (unilateral drainage). In contrast, another patient with Bismuth type IIIa required drainage of at least two segments of the intra-hepatic bile ducts (Figure 3). Therefore, from our perspective, there is no “routine” or “best” approach in terms of unilateral versus bilateral drainage for hilar CCA. However, volume-based drainage is still limited to cases in which no cholangitis occurred.
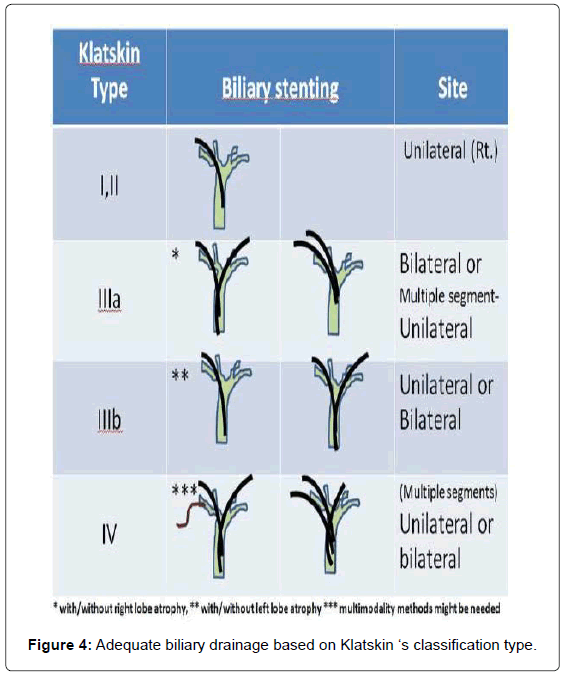
For patients with cholangitis, drainage of all suspected infected intra-hepatic segmental branches should be performed. Thus, in some cases, multimodality biliary drainage such as transpapillary drainage in combination with percutaneous transhepatic biliary drainage should be offered. Given that three-dimensional CT or MRI might not be possible in all institutes, here we propose an algorithm for adequate biliary drainage based on Klatskin’s classification (Figure 4).
For type I hilar lesions, which are connected to the right and left systems, the placement of only one stent in any functioning liver lobe would be appropriate. Type II lesions, which are also connected to both biliary systems, can also be treated with unilateral drainage, a right-system approach is the first priority as stent insertion is easier because of less angulation. However, for type III hilar tumors, adequate drainage requires at least two stents, bilateral for type IIIb and bilateral or multisegmented– unilateral stenting into the right system for type IIIa hilar tumors. For type IV lesions, stents should be inserted into at least two different intra-hepatic segments. The percutaneous approach might also benefit in cases where drainage by the transpapillary route has failed. Adequate cholangiography for complete evaluation of the entire biliary system is also essential.
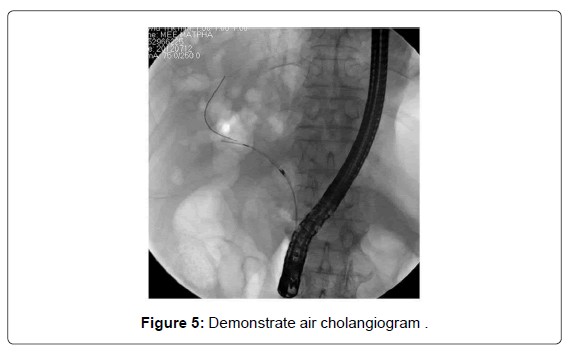
The use of complete cholangiography in a conventional contrast study in patients with Klatskin type IV might be associated with a higher risk of post-procedural cholangitis. However, there have been few studies on the benefit of using air or carbon dioxide inflation instead of contrast agent. An air cholangiogram is shown in Figure 5. The usefulness of air cholangiography was first reported by Sud et al. [34] in 2010. Zhang et al. [35] recently demonstrated a significantly lower rate of post-ERCP cholangitis in patients who underwent CO2 cholangiography compared to a conventional contrast study (5.6% vs. 33.3%, p=0.04). Thus, we recommend the use of air or CO2 cholangiography for the treatment of type IV hilar lesions.
Should plastic stents or self-expandable metal stents (SEMS) be selected?
To answer this question, endoscopists who perform the procedures must know the advantages and limitations of both stent types. Plastic stents are less expensive and technically easier to insert, remove, and exchange if stent malfunction or occlusion occurs. SEMS, which are composed of either stainless steel or nickel shape-retaining titanium (Nitinol), are designed and produced in many different styles and have advantages such as better stent patency than plastic stents as well as the ability to drain the side branches through the mesh. However, SEMS are much more expensive than plastic stents.
Prior to selecting a stent, endoscopists should consider patient life expectancy, cost effectiveness, stent patency, and the need for stent revision. The patency of SEMS and plastic stents in hilar cholangiocarcinoma were previously reported in many studies as 3.4– 5.5 months and 1.2–1.86 months, respectively [21,36]. A meta-analysis by Hong et al. [37,38] demonstrated that SEMS had a higher successful drainage rate with an odds ratio (OR) of 0.26 and 95% confidence interval (CI) of 0.16–0.42, lower early complication OR of 2.92 (95% CI, 1.65–5.17), longer stent patency with a hazard ratio (HR) of 0.43 (95% CI, 0.30–0.61), and longer patient survival HR of 0.73 (95% CI, 0.56–0.96).
Sangchan et al. [36] reported a model based on a cost utility analysis and demonstrated that SEMS is more cost effective than plastic stents, while the median survival time of SEMS and plastic stents in this study was 129 and 49 days, respectively. Therefore, SEMS, rather than plastic stents, should be considered for patients with a life expectancy of >3 months. Other factors worthy of consideration include procedure cost compared to stent cost (plastic stents might require more revisions than SEMS), the possibility of complications during follow-up (such as acute cholangitis due to stent dysfunction), and patient ability to undergo a second procedure. Thus, stent type is an individual consideration.
Considering both factors (stent types and bilateral or unilateral drainage), Liberato and Canena [39] reported a retrospective study of 480 patients with CCA who underwent bilateral and unilateral biliary stenting and found a higher cumulative stent patency for bilateral stenting, either SEMS or plastic. Naitoh et al. [40] revealed a higher cumulative stent patency rate in the bilateral biliary stenting group. However, few studies from Japan [41,42] failed to demonstrate the clinical benefits of SEMS over plastic stents. Although data were inconclusive, we believed that bilateral stenting with or without SEMS was preferable for type III and IV CCA.
Which stenting technique is better?
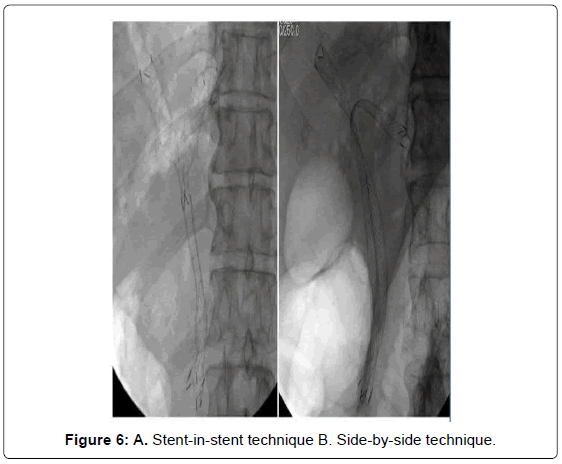
The endoscopic technique for both methods can be described as shown in Figures 6A and 6B. With the stent in stent technique (Y stenting), the stricture site would first be negotiated with the guidewire after successful selective cannulation into the desired intra-hepatic segment (most commonly the left system). The first stent is inserted and deployed, after which the second guidewire is passed through the middle part of the first stent (through the mesh) into the second desired biliary segment. The mesh is then dilated using a balloon followed by second stent insertion and deployment. Using the Y-stent technique, some studies have shown an 86.7% technical success rate and a 100% functional success rate regardless of stent type [43].
Using the side-by-side technique, given the difficulty of the stentin- stent technique using the guidewire to cannulate the contralateral duct, more time is needed to create the fenestration of the first stent mesh including the challenge of dilatation of the tight stent mesh. This technique requires the placement of two guidewires into two desired ducts in side-by-side fashion (subsequently or in parallel). Lee et al. [44] reported a study of the feasibility of using the side-by-side stenting technique in hilar CCA and showed a 91% technical success rate and a 100% functional success rate. This study also used small-caliber introducer (7 Fr introducer, 8-mm diameter) stents for the parallel deployment technique. Interestingly, the data showed no statistically significant difference between stent patency and median survival of the 8-mm and 10-mm groups.
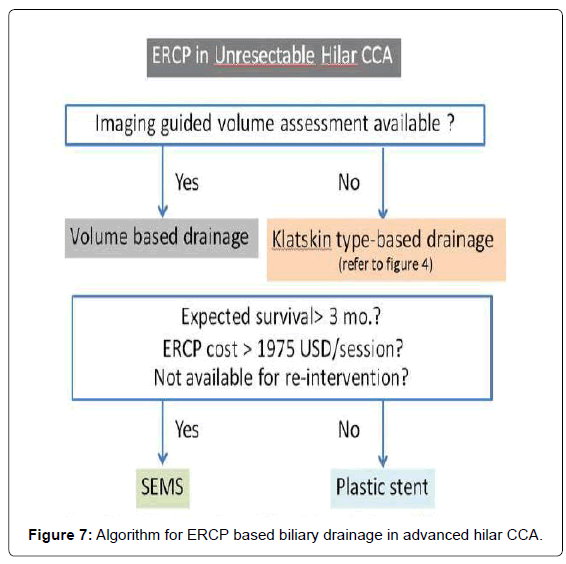
Law and Baron [45] reported the use of a small (6-mm) introducer SEMS for bilateral biliary stenting for both techniques which found no difference in terms of technical success, procedural time, rate of stent revision, and revision success rate. This might refer to the benefit of using SEMS with a smaller introducer. Therefore, the choice of stent insertion technique was according to endoscopist preference. However, the stent type that should be selected in these procedures is summarized in Table 1. With regard to the endoscopic techniques for hilar CCA, we postulate an algorithm for endoscopic biliary drainage based on ERCP approach as shown in Figure 7.
| Stent-in-stent | Side-by-side | |
|---|---|---|
| Technical success | + + + | + + + |
| Clinical success | + + + | + + + |
| Ease of stent revision | + + | + + + |
| Technical tips | - Need dilation of the first stent’s mesh using balloon or dilators - First stent insertion into more difficult side - Using large-open cell type stent will be easier for second stent insertion and stent revision in case of first stent occlusion | - Distal ends of both stents should be in the same level and outside the ampulla - First stent insertion into more difficult side |
| Preferable stent types | - First stent should be large-open cell type mesh or special designed stent for Y- configuration - Second stent should be large-open cell type mesh - Second stent should be small introducer | - Both stents should be small introducer( ≤ 7Fr.) and small diameter |
Table 1: Technical tips and preferable stent types to be considered for stent-in-stent and side-by-side technique.
Re-intervention for Biliary Drainage
Successful endoscopic biliary drainage with SEMS carries the chance of stent dysfunction either through tumor in-growth, tumor overgrowth, or stent migration. The reported rate of SEMS dysfunction following hilar CCA biliary drainage was 45–57%. The success rate of endoscopic SEMS revision was 75–93.2% [44]. Ridtitid et al. [46] reported longer median stent patency of second SEMS than plastic stents (150 days vs. 60 days, p<0.05). Thus, SEMS is the preferable option for re-intervention in cases of SEMS dysfunction.
Drainage Options for Failed ERCP
Since failed ERCP or incomplete drainage after ERCP accounted for 5–10% of all procedures, multi-modality drainage should be considered. The combination of ERCP and percutaneous drainage was acceptable. The use of endoscopic ultrasound-guided biliary drainage (EUS-BD) was also feasible for a left system drainage procedure in patients with advanced cholangiocarcinoma who failed transpapillary drainage [47,48]. EUS-BD was a novel endoscopic-based approach for patients in whom conventional biliary drainage failed. For hilar CCA, the procedure of choice is EUS-guided hepaticogastrostomy, which allows left system access only.
Prachayakul and Aswakul [49] reported a case series of EUS-BD by using non-cauterization and a non-balloon dilatation technique that showed a lower complication rate of 6% compared to other techniques that reported 11–35% with comparable technical and clinical success rates. However, with regard to EUS working group recommendations [50], the performance of EUS-guided biliary drainage is limited to skillful endosonographers and is not yet a standard approach. In cases of failure of all interventional options, surgical bypass should be considered the last rescue procedure.
Locoregional Tumor Control
Photodynamic therapy (PDT)
PDT is a relatively new therapeutic approach for local tumor control. Based on the mechanism that the photosensitizing agent concentrates in the lesion, the photosensitizer is activated by non-thermal laser light of an appropriate wavelength, which leads to subsequent damage of the neoplastic tissue by generated free oxygen radicals [51]. Four photosensitizing agents are currently used for CCA. The most commonly used include hematoporphyrin derivatives (Photofrin and Photosan), ∂-aminolevulinic acid, and meso-tretra(hydroxyphenyl) chlorin. However, the use of some photosensitive substances such as photofrin had considerable disadvantage, including strong phototoxic skin reactions that can persist for weeks. On the other hands, ∂-aminolevulinic acid which is a second generation photosensitizer had advantage over the first -generation photosensitizers such as photofrin, including a lack of prolonged photosensitization and laser light exposure. Nevertheless, the data which demonstrate the efficacy of this new agent in hilar cholangiocarcinoma was still limited. The first case report of successful PDT was published in 1991 of a patient who underwent seven PDT sessions in the span of 4 years [52].
The endoscopic PDT technique involves intravenous 48-hour administration of the photosensitizing agent prior to the laser light illumination. Commonly used agents are shown in Table 2. These agents are cleared from normal tissue in 48–72 hours but retained for longer intervals within the skin and tumor tissues. The laser light sources that deliver the appropriate wavelengths have been used for intraluminal illumination. A power density of 300–400 mW/cm and a power energy of 180–200 J/cm (of the diffuser length) is delivered through the fiber, while the irradiation time is 400–600 s [51]. This leads to 2–6- mm tumor necrosis depth. After selective cannulation in the desired intrahepatic duct, the catheter will be placed over the guidewire, which is then retrieved and replaced by the light laser fiber across the stricture site. However, this light laser fiber was stiff and prone to breakage, which can occur in up to one third of the procedures, making the procedure a bit more cumbersome and affecting treatment cost.
| Agents | Dosage (mg/ Kg Body weight) | Time before activation | Light dose (J/cm2) |
|---|---|---|---|
| Photofrin-2 | 2 | 48 hrs | 150-250 |
| Photosan-3 | 2 | 48 hrs | 200 |
| б-ALA60 | 60 | 48 hrs | 200 |
| mTHPC | 0.15 | 48 hrs | 10-50 |
Table 2: Photosensitizing agents which commonly used.
A meta-analysis of 24 studies by Gao et al. [53] showed that PDT prolonged survival (493 vs. 98 days) (p<0.0001) and improved biliary drainage and quality of life. Complications were reported in 0.3–27% of cases and cholangitis was the most common complication (27.5%). Another systematic review conducted by Tomizawa and Tian [54] confirmed that the benefit of PDT for survival (630 vs. 210 days for PDT and endoprosthesis alone, respectively, p<0.01). A study by Lee et al. in 2012 [55] reported a cohort study of 18 patients who underwent PDT using intravenous Photofrin II 2 mg/kg (Axcan Pharma, Quebec, Canada) showed longer patient survival in the PDT group (median survival time, 356 ± 213 days vs. 230 ± 73 days, p=0.006). Therefore, PDT is a preferable standard of therapy for unresectable hilar CCA.
Radiofrequency ablation (RFA)
Percutaneous image-guided RFA has received increasing attention as a promising technique for the treatment of liver cancer. An RFA catheter that is suitable for endoscopic delivery into the biliary tree over a 0.035-inch guidewire was recently produced. The Habib Endo HPB® (EMcision Ltd., London, UK) consists of a 2.6-mm catheter with a 180- cm working length. The distal end has a 5-mm leading tip proximal to two circumferentially placed, 8-mm-wide stainless steel electrodes. The distance between the proximal and distal electrodes was 8 mm, which allows for a coagulative effect of approximately 2.5 cm between the distal and proximal electrode margins [56]. The catheter is a bipolar device and enables connection to the power source. Tissue damage, both depth and length, after PFA treatment occurs according to the power setting and treatment duration.
The endoscopic technique for RFA consists of the following: After selective intra-hepatic duct cannulation, the 0.035-inch guidewire is placed across the stricture point. Full cholangiography is then performed to delineate the target lesion. A sphincterotomy is performed and the RFA catheter is advanced into the bile duct. RFA is applied at 7 W in 120-s bursts with a pause of 60 s before catheter movement. RFA application should be performed from the upstream stricture margin to the downstream stricture margin to cover the entire area of tumor invasion. Once the RFA catheter is removed, a balloon sweep should be performed to remove all the coagulated tissue from the biliary system. The stent is then subsequently inserted according to regular technique [57].
Only a handful of data exists regarding intraductal RFA for hilar CCA. Monga et al. [58] reported a clinical case report of successful therapy of intraductal CCA, while Steel et al. [59] reported the feasibility of using intraductal therapy for malignancy, of which only six of 21 cases were diagnosed as CCA. Therefore, more information regarded the efficacy and safety of this particular therapy is still needed; however, it was considered a potential “new tool” for the endobiliary treatment of hilar CCA.
Conclusion
Endoscopic therapy for advanced hilar CCA results in better quality of life and longer survival. Adequate biliary drainage should be the most important factor, and a good plan should be prepared for each patient to ensure the best clinical outcome. Photodynamic therapy is accepted as an effective local therapy option, while newer modalities such as endobiliary RFA treatment awaits further supportive data.
References
- Sripa B, Pairojkul C (2008) Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 24: 349-356.
- Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, et al. (2010) Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev 11: 1159-1166.
- Njei B (2013) The Changing Pattern of Epidemiology in Intrahepatic Cholangiocarcinoma. Hepatology.
- Floreani A, Lisiero M, Baldovin T, Baldo V (2013) Epidemiological aspects of biliary tree tumors in a region of northern Italy: emerging trends and sex-based differences. Eur J Gastroenterol Hepatol 25: 1347-1351.
- Pinter M, Hucke F, Zielonke N, Waldhör T, Trauner M, et al. (2013) Incidence and mortality trends for biliary tract cancers in Austria. Liver Int.
- Songserm N, Promthet S, Sithithaworn P, Pientong C, Ekalaksananan T, et al. (2012) Risk factors for cholangiocarcinoma in high-risk area of Thailand: role of lifestyle, diet and methylenetetrahydrofolate reductase polymorphisms. Cancer Epidemiol 36: e89-94.
- Chan-On W, Nairismägi ML, Ong CK, Lim WK, Dima S, et al. (2013) Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 45: 1474-1478.
- Sriraj P, Aukkanimart R, Boonmars T, Juasook A, Sudsarn P, et al. (2013) Does a combination of opisthorchiasis and ethyl alcohol consumption enhance early cholangiofibrosis, the risk of cholangiocarcinoma? Parasitol Res 112: 2971-2981.
- Srivatanakul P, Honjo S, Kittiwatanachot P, Jedpiyawongse A, Khuhaprema T, et al. (2010) Hepatitis viruses and risk of cholangiocarcinoma in northeast Thailand. Asian Pac J Cancer Prev 11: 985-988.
- Rizvi S, Gores GJ (2013) Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 145: 1215-1229.
- Wu Q, He XD, Yu L, Liu W, Tao LY (2012) The metabolic syndrome and risk factors for biliary tract cancer: a case-control study in China. Asian Pac J Cancer Prev 13: 1963-1969.
- Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, et al. (2011) Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 54: 463-471.
- Montella M, Polesel J, Talamini R, Crispo A, Giudice A, et al. (2011) Metabolic syndrome is also a risk factor for primary liver cancer in patients younger than 65 years of age? Hepatology 54: 2277-2278.
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, et al. (2012) The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol 28: 395-407.
- Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA (2013) Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 17: S0016-5107(13)02400-0.
- Zhimin G, Noor H, Jian-Bo Z, Lin W, Jha RK (2013) Advances in diagnosis and treatment of hilar cholangiocarcinoma -- a review. Med Sci Monit 19: 648-656.
- Tamada K, Ushio J, Sugano K (2011) Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J Clin Oncol 2: 203-216.
- Tan JW, Hu BS, Chu YJ, Tan YC, Ji X, et al. (2013) One-stage resection for Bismuth type IV hilar cholangiocarcinoma with high hilar resection and parenchyma-preserving strategies: a cohort study. World J Surg 37: 614-621.
- Schiffman SC, Reuter NP, McMasters KM, Scoggins CR, Martin RC (2012) Overall survival peri-hilar cholangiocarcinoma: R1 resection with curative intent compared to primary endoscopic therapy. J Surg Oncol 105: 91-96.
- Matsuo K, Rocha FG, Ito K, D'Angelica MI, Allen PJ, et al. (2012) The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 215: 343-355.
- Prachayakul V, Chaisayan S, Aswakul P, Deesomsak M (2013) Clinical characteristics and treatment outcomes of patients with unresectable cholangiocarcinoma in Thailand: are there differences dependent on stent type? Asian Pac J Cancer Prev 14: 529-532.
- Butthongkomvong K, Sirachainan E, Jhankumpha S, Kumdang S, Sukhontharot OU (2013) Treatment outcome of palliative chemotherapy in inoperable cholangiocarcinoma in Thailand. Asian Pac J Cancer Prev 14: 3565-3568.
- Bismuth H, Corlette MB (1975) Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 140: 170-178.
- Cho MS, Kim SH, Park SW, Lim JH, Choi GH, et al. (2012) Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 16: 1672-1679.
- Matull WR, Dhar DK, Ayaru L, Sandanayake NS, Chapman MH, et al. (2011) R0 but not R1/R2 resection is associated with better survival than palliative photodynamic therapy in biliary tract cancer. Liver Int 31: 99-107.
- Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, et al. (2013) Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 258: 129-140.
- Barlow AD, Garcea G, Berry DP, Rajesh A, Patel R, et al. (2013) Staging laparoscopy for hilar cholangiocarcinoma in 100 patients. Langenbecks Arch Surg 398: 983-988.
- Anderson JE, Hemming AW, Chang DC, Talamini MA, Mekeel KL (2012) Surgical management trends for cholangiocarcinoma in the USA 1998-2009. J Gastrointest Surg 16: 2225-2232.
- Choi J, Ryu JK, Lee SH, Ahn DW, Hwang JH, et al. (2012) Biliary drainage for obstructive jaundice caused by unresectable hepatocellular carcinoma: the endoscopic versus percutaneous approach. Hepatobiliary Pancreat Dis Int 11: 636-642.
- Castaño R, Lopes TL, Alvarez O, Calvo V, Luz LP, et al. (2010) Nitinol biliary stent versus surgery for palliation of distal malignant biliary obstruction. Surg Endosc 24: 2092-2098.
- Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, Khor CJ, Ponnudurai R, et al. (2013) Asia-Pacific Working Group on Hepatobiliary Cancers (2013) Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol 28: 593-607.
- Harewood GC, Baron TH (2002) Cost analysis of magnetic resonance cholangiography in the management of inoperable hilar biliary obstruction. Am J Gastroenterol 97: 1152-1158.
- Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, et al. (2010) Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc 72: 728-735.
- Sud R, Puri R, Hussain S, Kumar M, Thawrani A (2010) Air cholangiogram: a new technique for biliary imaging during ERCP. Gastrointest Endosc 72: 204-208.
- Zhang R, Zhao L, Liu Z, Wang B, Hui N, et al. (2013) Effect of CO2 cholangiography on post-ERCP cholangitis in patients with unresectable malignant hilar obstruction - a prospective, randomized controlled study. Scand J Gastroenterol 48: 758-763.
- Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P (2012) Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc 76: 93-99.
- Hong WD, Chen XW, Wu WZ, Zhu QH, Chen XR (2013) Metal versus plastic stents for malignant biliary obstruction: an update meta-analysis. Clin Res Hepatol Gastroenterol 37: 496-500.
- Hong W, Sun X, Zhu Q (2013) Endoscopic stenting for malignant hilar biliary obstruction: should it be metal or plastic and unilateral or bilateral? Eur J Gastroenterol Hepatol 25: 1105-1112.
- Liberato MJ, Canena JM (2012) Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol 12: 103.
- Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, et al. (2009) Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol 24: 552-557.
- Yasuda I, Mukai T, Moriwaki H (2013) Unilateral versus bilateral endoscopic biliary stenting for malignant hilar biliary strictures. Dig Endosc 25 Suppl 2: 81-85.
- Iwano H, Ryozawa S, Ishigaki N, Taba K, Senyo M, et al. (2011) Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Dig Endosc 23: 43-48.
- Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, et al. (2011) Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scand J Gastroenterol 46: 326-332.
- Lee TH, Park D H, Lee SS, Choi HJ, Lee JK, et al. (2013) Technical feasibility and revision efficacy of the sequential deployment of endoscopic bilateral side-by-side metal stents for malignant hilar biliary strictures: a multicenter prospective study. Dig Dis Sci 58: 547-555.
- Law R, Baron TH (2013) Bilateral metal stents for hilar biliary obstruction using a 6Fr delivery system: outcomes following bilateral and side-by-side stent deployment. Dig Dis Sci 58: 2667-2672.
- Ridtitid W, Rerknimitr R, Janchai A, Kongkam P, Treeprasertsuk S, et al. (2010) Outcome of second interventions for occluded metallic stents in patients with malignant biliary obstruction. Surg Endosc 24: 2216-2220.
- Panpimanmas S, Ratanachu-ek T (2011) Endoscopic ultrasound-guided hepaticogastrostomy for hilar cholangiocarcinoma: the first trial in Thailand. J Med Assoc Thai 94 Suppl 2: S129-134.
- Prachayakul V, Aswakul P (2012) Successful endoscopic treatment of iatrogenic biloma as a complication of endosonography-guided hepaticogastrostomy: The first case report. J Interv Gastroenterol 2: 202-204.
- Prachayakul V, Aswakul P (2013) A novel technique for endoscopic ultrasound-guided biliary drainage. World J Gastroenterol 19: 4758-4763.
- Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, et al. (2013) Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol 19: 1372-1379.
- Talreja JP, Kahaleh M (2010) Photodynamic therapy for cholangiocarcinoma. Gut Liver 4 Suppl 1: S62-66.
- McCaughan JS Jr, Mertens BF, Cho C, Barabash RD, Payton HW (1991) Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch Surg 126: 111-113.
- Gao F, Bai Y, Ma SR, Liu F, Li ZS (2010) Systematic review: photodynamic therapy for unresectable cholangiocarcinoma. J Hepatobiliary Pancreat Sci 17: 125-131.
- Tomizawa Y, Tian J (2012) Photodynamic therapy for unresectable cholangiocarcinoma. Dig Dis Sci 57: 274-283.
- Lee TY, Cheon YK, Shim CS, Cho YD (2012) Photodynamic therapy prolongs metal stent patency in patients with unresectable hilar cholangiocarcinoma. World J Gastroenterol 18: 5589-5594.
- Yokoyama T, Egami K, Miyamoto M, Watanabe H, Hasegawa H, et al. (2003) Percutaneous and laparoscopic approaches of radiofrequency ablation treatment for liver cancer. J Hepatobiliary Pancreat Surg 10: 425-427.
- Wadsworth CA, Westaby D, Khan SA (2013) Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr Opin Gastroenterol 29: 305-311.
- Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, et al. (2011) Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc 74: 935-937.
- Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, et al. (2011) Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 73: 149-153.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 19899
- [From(publication date):
February-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 15111
- PDF downloads : 4788