Current status of Diagnostic Techniques for detecting Neurocysticercosis
Received: 22-Dec-2021 / Manuscript No. jceni-21-50390 / Editor assigned: 24-Dec-2021 / PreQC No. jceni-21-50390(PQ) / Reviewed: 07-Jan-2022 / QC No. jceni-21-50390 / Revised: 17-Jan-2022 / Manuscript No. jceni-21-50390(R) / Published Date: 24-Jan-2022
Abstract
Neurocysticercosis, caused by Cysticercus cellulosae larva of Taenia solium, is considered a neglected tropical disease. Though neglected, the disease cannot be forgotten because of its high endemicity in poor countries and increasing cases in well-developed countries of the world due to increased migration and travel. One of the major reasons for its emergence is the lack of absolute diagnosis of the disease. Recent diagnostic criteria by Del Brutto, illustrate that the definitive diagnosis cannot be done without neuroimaging evidence. This becomes a challenge for the diagnosis of the most vulnerable population residing in endemic areas with deficit resources. Hence, the review highlights the need of enhancing the research in the field of Neurocysticercosis diagnosis. Various diagnostic techniques which are being employed in the Neurocysticercosis diagnosis have been explored, the highlights and challenges are discussed which would help in filling the gaps in the knowledge of absolute diagnosis of this disease.
Keywords
Neurocysticercosis; Taenia solium; Diagnosis; Tropical disease; Diagnostic assay; Endemic population.
Introduction
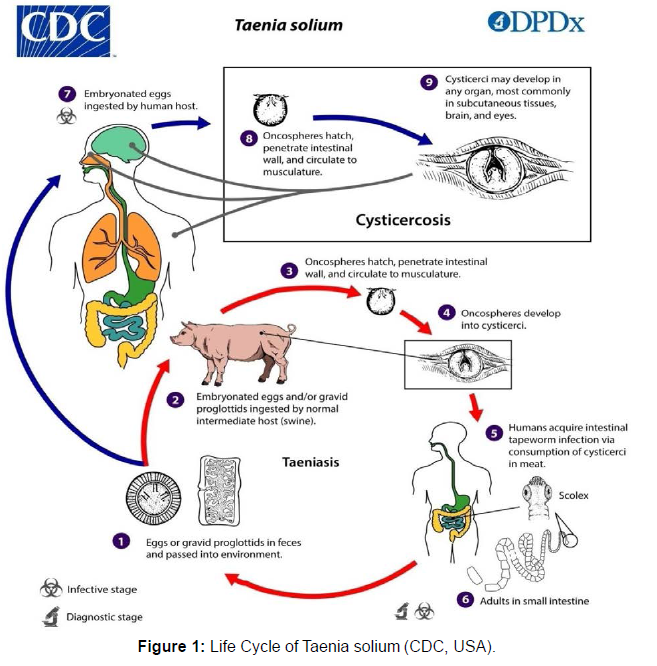
Neurocysticercosis, the infection of the central nervous system is caused by the larva Cysticercus cellulosae of the cestode parasite Taenia solium. It is a significant cause of seizures and many other neurological disorders in many regions of the world. According to the WHO report, globally there are 2.56-8.30 million cases of NCC [1]. This disease is endemic in most of the developing countries of the world such as America, Africa, and Asia (Table 1). Due to increased human migration, it is also emerging in non-endemic areas. It is reported that the larvae of T.solium cannot live for more than 7 years in their host's body [2]. NCC although a single disease has a broad spectrum. The different forms of NCC depend upon pathogenesis, location, and optical management. The most common form of NCC, that is observed in India is Single enhancing lesions. Many combinations of modern diagnostic tests, advanced anti-inflammatory treatments, minimally invasive neurosurgery, and improved drugs have better outcomes in NCC patients. Despite this, NCC is one of the most common neurological disorders and public threats in many portions of the world [3]. Despite the cosmopolitan impact of NCC, uncertainties about the disease still exists and the present review would summarize the various diagnostic methods and the way forward to improve the methods, especially for low costs laboratory tests (Figure 1).
Detection and Diagnostic Techniques: Neurocysticercosis diagnosis is based on the combination of radiological, clinical, immunological, and epidemiological findings. The diagnostic methods have been progressively improved in recent years. In the early 1950s, the radiological techniques available were quite aggressive [2]. The very first immunological assay developed was the complement fixation test. The CT scan was developed in 1970s and MRI was introduced in 1980s. Both these techniques had completely changed the scenario of diagnosis of neurocysticercosis. Summarizing all the recent advances that have been made for the diagnosis of neurocysticercosis, a proposal for diagnostic criteria has recently been validated for the first time (Table 1).
| 1. Parenchymal neurocysticercosis Includes: |
| 1. Parenchymal cyst with diagnosis (32) related to pathology |
| 2. Single or multiple active parenchymal cysts, with at least one cyst with scolex as identified by CT or MRI |
| 3. Multiple parenchymal vesicles without scolex associated with either |
| a. Seizures: focal or generalized tonic‐clonic or |
| b. Positive serum or CSF ELISA, EITB test |
| 4. Combination of the parenchymal cysticercus in any different evolutive stages: vesicular either with or without scolex degenerative (colloidal or nodular) and calcified |
| 2.Probable parenchymal neurocysticercosis includes |
| 1. Single parenchymal calcification or vesicle without scolex or degenerating cyst(s) establishing differential diagnosis w etiologies which are associated with at least two of the following: |
| a. Seizures: focal or generalized |
| b. Subcutaneous or muscle cysts location as confirmed by biopsy |
| c. Positive serum or CSF by ELISA or EITB test |
| d. Plain X‐ray showing “cigar shaped” calcifications |
| e. Individuals who live or have lived in or have traveled frequently to endemic countries |
| 2. Multiple parenchymal calcifications in individuals who live or have lived in or have traveled frequently to endemic co in whom clinical state excludes other etiologies of calcifications |
| 3. Extra parenchymal neurocysticercosis (intraventricular/basal subarachnoid) Includes: |
| 1. Extra parenchymal cyst with diagnosis related to pathology |
| 2. One or more extra parenchymal cysts with scolex in at least one of them as revealed by MRI |
| 3. One or more extra parenchymal cysts without scolex associated with at least two of the following as revealed by MRI: |
| a. Hydrocephalus |
| b. Inflammatory CSF |
| c. Positive CSF by ELISA, EITB test |
| d. Presence of single / multiple calcifications or parenchymal vesicular / degenerative cyst |
| 4. Definitive parenchymal and extra parenchymal neurocysticercosis |
| Combination of the above definitive parenchymal and definitive extra parenchymal criteria |
Table 1: Del Brutto diagnostic criteria.
Biopsy: As there is no single test that can definitively diagnose NCC, except by brain biopsy, inclusion of this in diagnostic criteria is necessary. The technique is very rarely used Now a day. It was preferred for extraneural cysticercosis. However, the invasive and painful procedure of this technique is its major drawback, therefore leading to its decreased use.
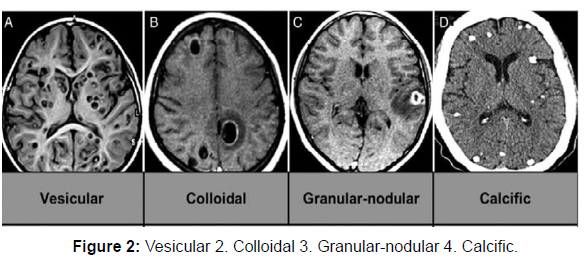
Neuroimaging techniques: These techniques are vital as they provide evidence about the number, location, size, presence, and stage of the cyst. Imaging studies reveal a bright nodule, seen in the interior giving an appearance of 'hole with a dot' where the dot represents scolex (Figure 2).
Myelography: This technique is used for the diagnosis of spinal leptomeningeal or extramedullary cysticercosis. The unique feature of myelography is that it can record the mobility of the cyst [3]. The limitation inaccurate diagnosis is that there is a possibility that the cyst had moved during the laminectomy instead of the myelographic stud. It is still considered for spinal NCC, because it is capable of showing various filling defects which correspond to cyst in a contrast column.
Computerized tomography Scan (CT): The CT scan for diagnosis of NCC cyst shows specificity and sensitivity of about 95%. CT scan is more readily available and is cheaper as compared to other imaging techniques. Around 50% of patients suffer from calcified NCC and a CT scan is considered the most accurate for detecting calcifications. The CT scan is considered less efficient for ventricular, cisternal NCC and for Small Single Enhancing Lesions (SSEL). It is very difficult to differentiate NCC cyst from small tuberculoma and authors themselves believe that they cannot distinguish it on a CT scan basis alone.
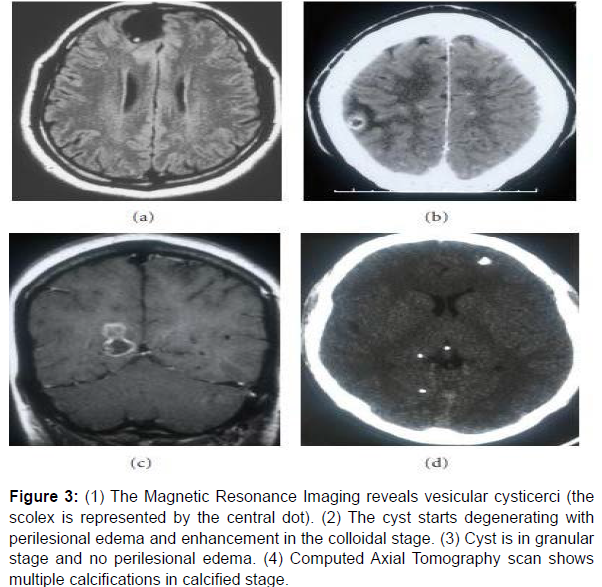
Magnetic Resonance Imaging Scan (MRI): The MRI technique having excellent tissue contrast depiction quality and multiplanar capability, is considered as one of the most important techniques of non-invasive diagnosis of NCC [4]. The limitation of this technique is observed when severe forms of NCC must be diagnosed. It is considered that for severe NCC forms, 3D MR sequences are better for diagnosis. Another main limitation of MRI is limited availability and high cost (Figure 3).
Figure 3: (1) The Magnetic Resonance Imaging reveals vesicular cysticerci (the
scolex is represented by the central dot). (2) The cyst starts degenerating with
perilesional edema and enhancement in the colloidal stage. (3) Cyst is in granular
stage and no perilesional edema. (4) Computed Axial Tomography scan shows
multiple calcifications in calcified stage.
3D Spoiled Gradient Recalled Echo Sequences (SPGR): This technique is considered for intraventricular NCC diagnosis. It only provides T1 contrast information. Enhanced SPGR is preferred for the detection of degeneration of cyst in intraventricular NCC. The limitation is, it is less sensitive for the detection of scolex as compared to the FIESTA but still can be considered in MRI diagnosis of intraventricular NCC.
Fast Imaging Employing Steady-state Acquisitio (FIESTA) or Constructive interference in steady-state (CISS): It is considered as the most sensitive diagnostic technique for subarachnoid spaces (SAB) because it can detect the difference in the signal intensity of CSF and parasite content and can also recognize the structure of the parasite [5]. Hence, this technique should be a part of routine diagnosis in the endemic areas where the cases of SAB are high [18,19]. It is also reviewed as an efficient technique for the diagnosis of intraventricular NCC. By this technique, we can also visualize the ventricular cavities as sometimes the cyst can reach the cavities. This phenomenon can be best visualized by FLAIR MRI. Other recent MRI sequences are susceptibility-weighted imaging (SWI) and Arterial Spin Labeling (ASL).
Stool microscopy: It is a very traditional method for assessing the T.solium eggs. The method is considered to be highly specific, provided that the examiner should be an expert.
The sensitivity of the test is low, as to be visible under a microscope certain threshold of eggs is required. Only 10-15% of neurocysticercosis patient have taeniasis. Stool microscopy cannot be considered as a species-specific detection method as the eggs of Taenia species are morphologically similar.
Immunological assays
Antigen Detection Assays
Co-agglutination test (Co-A): It is a basic slide agglutination test [6]. A study conducted using the Co-A test in the urine samples reported a sensitivity of 55.5% for NCC cases diagnosed clinically and 62.5% for radio logically diagnosed cases.
Enzyme-Linked Immunosorbent Assay (ELISA): The Ag-ELISA is performed using monoclonal antibody HP10. A study conducted on the CSF samples showed the sensitivity and specificity of 86% and 96% respectively. This assay has also been employed for the detection of T. solium antigen in urine. It shows a sensitivity of 92% while in the case of a single cyst it decreases to as low as 62.5%. The recent guidelines of IDSA/ASTMH suggested the restricted use of ELISA due to high false positive and negative results.
Dipstick ELISA: The dipstick immunoassay is more user- friendly as compared to ELISA and EITB, yet there is very little research done on its development and standardization [7]. Although, the sensitivity detected by the serum was low as compared to the conventional micro plate Ag- ELISA.
Antibody Detection Assays-
Complement Fixation: The test was described in 1909 by Weinberg. Primarily, the test was used for the diagnosis in serum. Further, when it was applied to CSF samples, it showed better results. The drawback of this assay is that it cannot detect CSF abnormalities. The test is also found to be less sensitive for ventricular CSF analysis as compared to the subarachnoid CSF analysis.
Latex Agglutination: It is a simple latex-based method. The test for the determination of antibodies in the CSF sample reported a sensitivity of 89.5% and a specificity of 75%. The limitation of the test reported in the study was cross-reactivity with tubercular meningitis in both CSF and serum samples [8]. Otherwise, the test is an ideal assay for rural setup as it is rapid, can give results in minutes, economical, and requires minimal setup.
Hemagglutination Assay: The hemagglutination assay uses red blood cells treated with formalin and tannin. The HA assay is simple to execute, bear less cost, and can be easily established in less complex laboratories. It showed specificity and sensitivity of 94.4% and 81.7% using Cc antigen. The major limitation of the test is difficulty in obtaining homogenous and stable lots of RBC.
Immunoperoxidase Test: The IP test was used for CSF samples. The test challenged to overcome the difficulties of obtaining the homologous antigens required in immunoenzymatic and immunofluroscence methods [9]. The advantage of the test is, results can be read using a common light microscope. The disadvantage of the test is low reactivity and it requires approximately 10 fold higher amount of conjugates as compared to ELISA.
Antibody detection ELISAs: In this assay, semi purified cyst fluid antigens or somatic parasites are used. They are considered to have suboptimal sensitivity and a high rate of cross- reaction with some of the common cestodes. Some of the good results are found by using recombinant or synthetic peptides.
Dot-ELISA: A modified ELISA showed a sensitivity of 95.1% and specificity of 90.6% in CSF and sensitivity of 56.25% and a specificity of 92% in serum [10]. Even with moderate sensitivity, the assay is believed to have the potential to be utilized in field studies and poorly equipped laboratories as the procedure is simple, rapid and the result can be easily visualized terminating the use of an ELISA reader. In Dot- ELISA, it is possible to test around 100 samples by only one individual in about 3 hrs.
Quick ELISA: The assay generally uses native LLGP proteins. It showed a sensitivity of 96% and specificity of 99% for multiple cysts in the serum sample. The sensitivity and specificity values are comparable with the EITB assay. The major advantages of this assay are, it can be performed manually as well as automated, gives quick results as 10 serum samples can be tested within 60 minutes [11].
Avidin-Biotin Peroxidase Complex ELISA (ABC-ELSIA) and Protein A-ELISA: These modifications of the ELISA technique have proved to be more sensitive technique as compared to the conventional ELISA. One of the significant advantages reported in the Lee et al, 1993 study was that in ABC-ELISA, only 1-2 microliter of sample is sufficient to detect the antibodies. In a study, the sensitivity of ABCELISA and Protein-A ELISA using serum samples was reported as 86.1% and 82.6% respectively. The specificity reported was 93.8% and 93.3% respectively.
2D Gel Electrophoresis: The method was developed in 1970s and become the first method for the resolution and simultaneous display of many proteins. The technique is assumed to be sensitive as it allows early differentiation of Taenia solium- specific IgG antibodies passed through the mother and antibodies produced by newborn child [12]. The study conducted on Clinically and radiologically suggestive NCC patients from North India found 100% sensitivity. When results were compared with ELISA, 60% of patients were found to be seronegative by ELISA. The study was also challenged to have higher sensitivity of 10–30 kDa 2D-PAGE EITB test as compared to the gold standard LLGP- EITB technique. Lower molecular mass antigenic fractions, used in the assay, are 100% specific and can be used for the diagnostic purpose to obviate cross-reactions.
Dot-Immuno gold-Silver Staining assay (Dot-IGSS): The assay was described to detect serum antibodies. The major disadvantage of the assay was the cross-reactivity with the sera hydatidosis patients. A conducted showed the assay having false-positive results of around 2% in 50 healthy patients. Therefore the assay had limited utility and can be applied only in the regions where hydatid disease is not prevalent.
Electro immuno transfer Blot (EITB): The EITB assay is recognized as a "gold standard" technique for serological detection of neurocysticercosis (Pan American Health Organization. 1995). The LLGP-EITB is the primarily recommended assay for the detection of antibodies [13]. For multiple lesions, it shows the specificity of about 100% and sensitivity varies from 94 to 98 % while the sensitivity in case of single viable NCC or degenerating cyst is illustrated to be low 60 to 70%. The drawback of the test arises in the case of single intracranial cysticerci where it shows frequent false-negative results [49]. Another limitation is that it shows positive results in the case of taeniasis. This hinders the accuracy of an assay when applied for determining the prevalence in endemic areas. Some of the technical difficulties are standardization and complex purification of extracted antigens.
Magnetic immuno chromatography assay (MICT): The MICT assay is easy to perform, portable, gives numerical output, and takes. Only 40 minutes to complete the test. The unique feature is that the magnetic particles which are conjugated do not bleach over time as seen in the case of gold and fluorescent reporters [14]. In the case of neurocysticercosis containing more than two viable brain cysts, the sensitivity was 93.9% and the specificity was 98.9%. The two major disadvantages reported in this study were the use of liquid conjugate and dependence on bench- top magnetic assay reader.
Line Immunoassay or Multi Antigen Printing Immunoassay (MAPIA): The unique feature of the assay is that it can detect several unrelated antigens within a single assay. Recombinant rT24H antigen is the best suited for use. The major limitation of the assay is the lack of specificity. The suggested use is in the study of map cases in epidemiology and to ascertain the seroprevalence of cysticercosis [15].
Rapid lateral flow test: The lateral flow assay is popular for several decades for its use in veterinary and clinical diagnosis. The test recognizes the cysticidal antibodies present in the body and showed the clinical specificity and sensitivity of 98% and 96% respectively in case of multiple cysts. The settings used in the assay comprised of minimal infrastructure, hence suitable for shipping worldwide and storage at an appropriate temperature.
Lymphocyte transformation test: It is a recent technique established for the detection of NCC. It has shown a sensitivity of 93.7% and a specificity of 96.2%. In fact, in a single cyst case, it showed a sensitivity of 87.5% which is much higher as compared to ELISA or EITB. The major drawback of the test is the high cost and utilization of 3H-TdR which requires the need to handle radioactive isotopes.
DNA-based methods / Molecular methods:
Polymerase chain reaction (PCR): The PCR assay had reported high specificity and sensitivity. In endemic areas where the antibodybased test can show highly false-positive results, PCR technique can help in diagnosis. The very first study by Goyal et al, which performed PCR assay on both blood and urine samples [16] , showed a sensitivity of 64% in the urine sample and 57% in blood sample whereas the specificity depicted was quite high as of 87% in urine samples and 94% in blood samples. These results showed the encouraging future for urine PCR as the procedure can be done with the limited resources and is noninvasive.
Real-time quantitative polymerase chain reaction (qPCR): This PCR is an interesting assay for biomarker and diagnostic advancement. Studies showed 100% specificity for the assay. The detection rate was seen as 15/18 (83.3%) in CSF samples, 13/14 (92.9%) in CSF samples from patients with definitive NCC, and 0/9 (0%) in serum samples. Another study conducted to describe and developed a TsolR13 qPCR assay based on novel repeats for Taenia solium demonstrated 100% specificity in the case of subarachnoid or ventricular NCC. But the overall sensitivity is reduced to 76.5% in the case of plasma samples.
LAMP (Loop-Mediated Isothermal Amplification): It has been recently developed as a DNA- based detection assay. In T. solium infections the assay is developed to targets mainly the cox 1 gene and cathepsin L-like cysteine peptidase (clp). The assay targeting the cox 1 gene can detect parasite DNA in 86.0% of samples and the clp gene can detect 13 (30.2%) samples. It has the advantage that it requires low equipment and can easily differentiate between different Taenia species [17]. The assay gains worldwide popularity because of its simple setup as it does not require any special instrument for maintaining temperature. Another interesting benefit of the Assay is the visual endpoint judgment by turbidity, but there is also a possibility of yielding false-positive results. The advancement in LAMP known as Real-time LAMP assay is based on florescence detection which is more accurate and avoid false positive results. One of the recent studies on the diagnosis of cysticercal DNA targeting cox 1 gene in the blood of patients having neurocysticercosis had shown the sensitivity ranged from 66.7% to 78.2%. The sensitivity of calcified cysts is around 73% which is better as compared to the EITB assay, however, the sensitivity is less in the case of multiple cysts. The specificity of the assay is significantly high and is around 90%. Another advantage of the assay is that it is much cheaper as compared to the EITB (US Dollar 10-14/- per test versus 200/- per test). The faster performance of this assay can also be considered as an add-on advantage as the reaction takes only 90 minutes to complete. Hence the assay is suitable for its application in field surveys (Table 2).
| Method of Diagnosis | Population studied | Result |
| Next generation sequencing | China,1296 CSF samples | T. soliumDNA sequences detected 7 patients [18] |
| Quantitative Polymerase Chain Reaction Assay | Maryland,46 plasma and 36 CSF samples. |
TsolR13-97.3% sensitive,100% analytic specific. [19] |
| Enzyme-linked immuno electro transfer blot (EITB) assay | India, 256 serum samples | 15 kDa band had significant association with NCC. Sensitivity- 91.5% Specificity - 91.6%.[20] |
| Enzyme-linked immuno electro transfer blot (EITB) assay | Mexico, 58 serum samples | Glycoprotien .band 39-42, InDRE - 72.4% CDC- 55.4% Glycoprotien band 23-26 and 6-8 LDBio - 69.4%, 61.2% respectively. [21] |
| Real Time Loop Mediated Isothermal Amplification Assay | India , 100 blood samples | Taenia solium cox1 gene detected in 74% samples. Sensitivity - 74%. [22] |
Table 2: Some recent publications in molecular diagnosis of Neurocysticercosis.
Discussion
➢ Imaging techniques for the diagnosis of neurocysticercosis is most efficient and accurate. However, high cost and requirement of technical skills become its major lacunae
➢ Vast number of antibody detection test has been implied for the diagnosis till date. The Electro immuno blotting (EITB) is gold standard for the diagnosis whereas there is other serological technique as well which matches the assay sensitivity and specificity. The scope of serological techniques in an accurate diagnosis is low because of its high rate of cross reaction with other antigen and inability to differentiate active from past infections.
➢ Antigen detection tests as well as other molecular tests to detect DNA had a better future for improving the diagnosis. Molecular techniques such as Polymerase Chain Reaction (PCR) and Loop Mediated Isothermal Amplification (LAMP) are very sensitive and can detect even a low parasite load, and hence have potential to strengthen the future of neurocysticercosis diagnosis.
Conclusion
Neurocysticercosis was under recognized, infrequently diagnosed, and essentially untreatable before 1970. But now it is a commonly recognized disease that is a leading cause of seizures in endemic regions. Yet, there are huge gaps in determining the global severity of disease and infection, in understanding disease pathophysiology and genesis of epilepsy. Neurocysticercosis can be prevented and eradicated with proper strategies. International and national health agencies are helping to control cysticercosis and therefore would prevent millions of cases of epilepsy too. Eradication programs should be effective for all control purposes, especially for humans carrying adult tapeworms, pigs and infected eggs in the environment. Because of various advances, the diagnosis of Neurocysticercosis has improved significantly in specialized hospital setting but the need of improvement is still required in low equipped laboratory areas. Improved diagnosis is necessary to eradicate the disease, as the disease mainly affect the most vulnerable sector of population.
References
- White AC, Jr. Coyle CM, Rajshekhar V, Singh G, Hauser WA, et al.( 2018) Diagnosis and Treatment of Neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 66: e49-e75.
- Cuetter AC, Andrews RJ (2002) literature. Neurosurg focus 12:1-7.
- Del Brutto OH, Rajshekhar V, White Jr A, Tsang V, Nash T, et al. (2001) Proposed diagnostic criteria for neurocysticercosis. Neurol. 57:177-183.
- Garcia HH, Del Brutto OH (2003) Imaging findings in neurocysticercosis. Acta tropica 87:71-78.
- Hawk MW, Shahlaie K, Kim KD, Theis J (2005) Neurocysticercosis: a review. Surg. Neurol 63:123-132.
- Santín G, Jorge VS (1966) Roentgen study of cysticercosis of central nervous system .Radiol 86:520-528.
- Zee C, Segall HD, Miller C, Tsai F, Teal J, et al. (1980)Unusual neuroradiological features of intracranial cysticercosis. Radiol 137:397-407.
- Wadia R, Makhale C, Kelkar A, Grant K (1987) Focal epilepsy in India with special reference to lesions showing ring or disc-like enhancement on contrast computed tomography. J Neurol Neurosurg Psychiatry 50:1298-301.
- Sinha S, Sharma B (2009) Neurocysticercosis: a review of status and management. J Clin Neurosci 16:867-876.
- Singh S, Gibikote S, Shyamkumar N (2003) Isolated fourth ventricular cysticercus cyst: MR imaging in 4 cases with short literature review. Neurol India 51:394-398
- Govindappa SS, Narayanan JP, Krishnamoorthy VM, Shastry CHS, Balasubramaniam A, et al. (2000)Improved detection of intraventricular cysticercal cysts with the use of three- dimensional constructive interference in steady state MR sequences . AJNR Am J Neuroradiol 21:679-684.
- Robbani I, Razdan S, Pandita KK (2004) Diagnosis of intraventricular cysticercosis by magnetic resonance imaging: improved detection with three‐dimensional spoiled gradient recalled echo sequences. Australas Radiol 48:237-239.
- Hingwala D, Chatterjee S, Chandrasekharan Kesavadas BT, Kapilamoorthy TR (2011) Applications of 3D CISS sequence for problem solving in neuroimaging. Indian J Radiol Imaging 21:90-93
- Martinez HR, Rangel-Guerra R, Elizondo G, Gonzalez J, Todd LE, et al. (1989) MR imaging in neurocysticercosis: a study of 56 cases AJNR Am J Neuroradiol 10:1011-1019.
- Gilman RH, Gonzalez AE, Llanos-Zavalaga F, Tsang VC, Garcia HH, et al. (2012)Prevention and control of Taenia solium taeniasis/cysticercosis in Peru. Pathog Glob Health 106:312-318.
- Parija M, Biswas R, Harish B, Parija S (2004) Detection of specific cysticercus antigen in the urine for diagnosis of neurocysticercosis. Acta tropica 92:253-260.
- Tefera G (2008) Development of a dipstick ELISA for the detection of circulating antigens of Taenia saginata /Taenia solium cysticercosis. Trop Med Int
- Fei X, Li C, Zhang Y, Zhang H, Liu X, Ji X, et al.(2020) Next-generation sequencing of cerebrospinal fluid for the diagnosis of neurocysticercosis. Clin Neurol Neurosurg 193:105752.
- O’Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB (2020) A novel, highly sensitive quantitative polymerase chain reaction assay for the diagnosis of subarachnoid and ventricular neurocysticercosis and for assessing responses to treatment. Arch Clin Infect Dis 70:1875-1881.
- Arora N, Kaur R, Rawat SS, Kumar A, Singh AK, Tripathi S, et al. (2020) Evaluation of Taenia solium cyst fluid-based enzyme linked immuno electro transfer blot for Neurocysticercosis diagnosis in urban and highly endemic rural population of North India. Clin Chim Acta 508:16-21.
- Romo ML, Hernández M, A studillo O-G, Diego G, De-la-Rosa-Arana JL, Meza-Lucas A, et al. (2020) Diagnostic value of glycoprotein band patterns of three serologic enzyme-linked immuno electro transfer blot assays for neurocysticercosis. Parasitol Res 119:2521-2529.
- Goyal G, Phukan AC, Hussain M, Lal V, Modi M, Goyal MK, et al .(2020) Sorting out difficulties in immunological diagnosis of neurocysticercosis: Development and assessment . J Neurol Sci 408:116544.
Indexed at Google ScholarCrossref
Indexed at Google Scholar, Crossref
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Mehta Y, Kaura T, Kaur U, Sehgal R (2022) Current Status of Diagnostic Techniques for Detecting Neurocysticercosis. J Clin Exp Neuroimmunol, 7: 140.
Copyright: © 2022 Sehgal R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4898
- [From(publication date): 0-2022 - Nov 26, 2025]
- Breakdown by view type
- HTML page views: 4136
- PDF downloads: 762