Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Commentary Open Access
Current Perspective on Hydrogen Sulfide: Stinky Outside Precious Inside
| Sangita Singh* | |
| DuPont, DuPont Knowledge Center, Hyderabad, India | |
| Corresponding Author : | Sangita Singh DuPont, DuPont Knowledge Center Hyderabad, India E-mail: Sangita.Singh@dupont.com; singh1.sangita@gmail.com |
| Received: July 25, 2015; Accepted: August 11, 2015; Published: August 18, 2015 | |
| Citation: Singh S (2015) Current Perspective on Hydrogen Sulfide: Stinky Outside Precious Inside. Biochem Physiol 4:175. doi:10.4172/2168-9652.1000175 | |
| Copyright: © 2015 Singh S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Hydrogen sulfide is the newest member of gasotransmitter family. Endogenous production of hydrogen sulfide in brain was first reported in 1996. Since then a great deal of interest has been paid to understand various physiological roles of this molecule, still there are a lot of gaps that need to be addressed. This article provides author’s perspective on research accomplishments in this area briefly, gaps and future opportunities, which may transform the area of medicine.
| Keywords |
| Hydrogen sulfide; Cardiovascular; Cystathionine betasynthase; Cystathionine-gamma-lyase; Gasotransmitter |
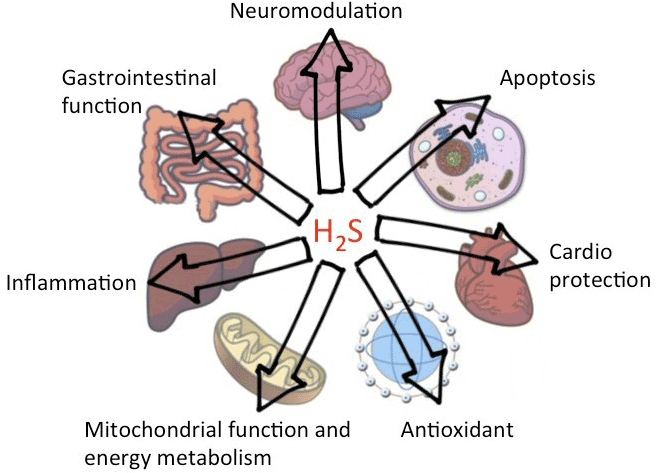
| Many of us experienced foul rotten egg like smell of hydrogen sulfide (H2S) in chemistry laboratories during school days. Did you know back then that it is actually produced in our body? H2S is the third and newest member of gasotransmitter family, along with nitric oxide (NO) and carbon monoxide (CO). Since the first report on physiological role of endogenously produced H2S in mammals [1], there has been an explosion of research articles on its varied biological roles. Availability of grants from American Health Association (AHA), United States Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH) and other government agencies over two decades enabled deep dive research on the role of H2S in cardiovascular health and other diseases. For example, H2S induced vasorelaxation in cardiovascular system via its interaction with KATP channels [2,3], role of H2S in protein S-sulfhydration [4,5], redox balance [6,7], and regulation of levels of second messenger like free calcium, cGMP, cAMP [8-10], were discovered. Abnormal endogenous metabolism of H2S was found to have direct relationship with disorders in cardiovascular, gastrointestinal, nervous system and other diseases [Figure 1; details are reviewed in [11,12]. Even human genetic diseases were linked to H2S-generating enzymes. |
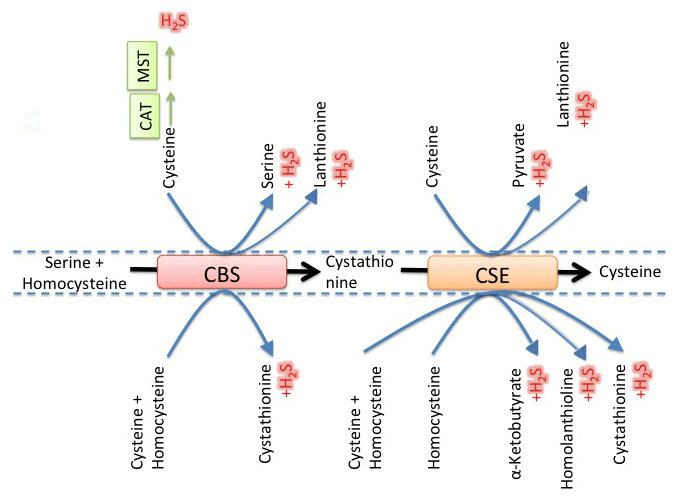
| H2S is a weak acid with pKa1 and pKa2 values 6.9 and >12, respectively. At physiological pH (~7.4), the ratio of HS− to H2S is around 3:1. It is unclear which one among hydrosulfide ion (HS−) or H2S is the dominant mediator of various physiological effects. Above 20 μM, H2S inhibits cytochrome c oxidase in intact cells and is known to mess up electron transfer chain. Three major enzymes have been identified in mammalian cells that catalyze endogenous production of H2S (Figure 2). These are cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS) and mercaptopyruvate sulfurtransferase (MST). CSE and CBS are PLP dependent and utilize various substrates including cysteine and homocysteine. MST does it from 3-mercaptopyruvate [13- 15]. While CBS and CSE are located in cytosol, MST is partly localized in cytosol and partly in mitochondria [16,17]. CSE produces H2S in peripheral tissues like cardiovascular and liver. CSE knockout mice were shown to have decreased level of H2S level in serum [18,19]. CBS is mostly responsible for H2S production in brain and kidney [20]. MST is believed to be responsible for H2S production in brain and in vascular endothelial tissues [21,22]. Mutations in human CSE gene could cause hereditary cystathioninuria and hypercystathioninaemia [23,24]. Similarly, inborn errors in CBS gene were shown to be associated with human hereditary diseases like hyperhomocysteinaemia and homocystinuria. These were directly related to long-term problems like systemic inflammation, cardiovascular complications and damage of organs [25-27]. Based on in vitro data, kinetic simulations predicted that under normal condition and assuming equal concentrations of CBS and CSE, CBS is capable of contributing from 25 to 70% of total H2S production depending on its extent of allosteric activation by S-adenosylmethionine. However, these simulations predicted that total H2S formation and relative contribution of CBS in H2S genesis could decrease under hyperhomocysteinemic conditions where CSE produced 74%, while CBS contributed only 26% of total H2S in cell. |
| Extensive investigation on H2S brought to light that lanthionine and homolanthionine were formed as by products during H2S biogenesis [13-14]. Presence of lanthionine in mammalian brain is well known for long time, but recent studies have identified neuroprotective and anti-inflammatory properties of a related metabolite, called lanthionine ketamine [28,29]. Researchers are interested to explore mammalian LanC1-like proteins, which are homologous to bacterial enzymes responsible for prokaryotic lantibiotic synthesis [30,31]. Although none of these LanC-like proteins (LanCL) have been shown to catalyze lanthionine formation, there is evidence that LanCL1 directly binds and inhibits CBS [32]. Lanthionine and lanthionine ketamine molecules could be biomarkers for pathologies associated with altered metabolism of H2S in cell [33,34]. The discovery that CBS is the only source of enzymatic production of lanthionine in cells till date could be useful for treatment of metabolic dysfunctions and neurodegenerative diseases by controlling its metabolism. |
| So much is done, still there is plenty of blue sky in the horizon. Better understanding of physiological role of H2S and mechanism of metabolism over last decade led to the discovery of new drug candidates / inhibitors that either aimed to improve or to suppress its endogenous production. These molecules have different mode of actions and they are at different stages in their development pipelines or clinical trials [35,36]. So H2S based therapeutics is yet several years away from reality or commercialization. Progress in H2S field has been also hampered by the lack of availability of selective inhibitors of various enzymes that contribute to biosynthesis of this gasotransmitter [37]. Compounds like aminooxyacetic acid (AOAA), trifluoroalanine and hydroxylamine (HA) inhibit both CBS and CSE with different IC50 values. There are claims that propargylglycine (PAG), β-cyano-L-alanine (BCA), L-aminoethoxyvinylglycine (AVG) could be selective inhibitors of CSE but the hunt for specific inhibitors of CBS still continues. Companies need to invest more on R&D and resources to discover more and drive some of those key candidates forward. Clear understanding of signaling mechanism will bring new targets and help to develop drug candidates with new mode of actions. Health data compiled from more than 190 countries shows that heart disease remains the number one global cause of death with 17.3 million deaths each year. These numbers are high, so H2S based therapeutics can be an enormous opportunity for business, if any of the trial candidates become a break through success. |
| This field also needs attention from fundamental science and technology professionals from diverse background for novel insights. For example nanotechnology or nanomedicine can contribute to the development of organelle specific targeted delivery devices with controlled released properties. It is equally important to be able to accurately measure levels of H2S in tissues. This area will require synergy between chemistry and biology for development of new sensors, which will glow up in presence of trace amount of H2S. Several reaction-based fluorescent probes were already developed which offered advantages like high sensitivity, while maintaining selectivity for H2S over other RSONs and free thiols [38-41]. Only a few were used successfully in live liveanimal imaging and more such probes need to be developed [42,43]. In addition to that development of high end imaging techniques, better contrast to clearly understand H2S biogenesis in various animal models will enable monitoring the effect of drugs on enhancement or inhibition of H2S synthesis in live cells. The exact ratio of ionic forms of H2S inside cell is unknown, but based on its two pKa values; it is expected to be a mixture of HS- and H2S under physiological pH, although they are in equilibrium. In such cases, knowledge about the reaction mechanism of a dye and reasons for its selectivity towards H2S over other reactants inside cell need to be clearly understood as it will help researchers to come up with even better solutions. Nonetheless, studies by Qian et al. [42] and Matthew et al. [43] are quite promising for researchers as well as clinical studies. Not only H2S, sensitive tools are required to detect and estimate quantities of other known metabolites like lanthionine and lanthionine ketamine in live-cells. Among other areas omics will play crucial role in future. For example, targeted or untargeted proteomics and metabolomics using advanced mass spectrometry based detection technologies will help to profile proteins or metabolites related to H2S metabolism that are either up or down regulated under pathological conditions. These advancements will have power to solve the toughest problems in H2S research and, in turn, transform cardiovascular and other fields of medicine. Solutions to these are at the interfaces of technologies, so collaboration will continue to remain as critical element for successful, high-impact research. |
| As we all know, taking pills is a reactive action; an alternate way to enjoy good health is through proper diet planning. Methionine cannot be synthesized by our body and must be supplied through diet. Cysteine, which is synthesized by our body, requires a steady supply of dietary sulfur in order to do so [44]. These amino acids are directly or indirectly utilized in catalytic production of H2S in cell. Recently polysulfides and similar classes of compounds were identified as H2S donors. Garlic, onion, Chinese chive, shallot and many other vegetables are rich sources of organosulfides like acyclic disulfide, trisulfide, tetrasulfide and cyclic polysulfide [45,46]. Regular dietary supplementations of polysulfides or organosulfides were found to be beneficial for prevention of cardiovascular diseases [47,48]. Along with that improved H2S signaling helped sustain reduced blood pressure, promote growth of new arterioles, reduce bad cholesterol and triglyceride levels. However it should be kept in mind that excess of H2S is toxic. So a controlled consumption of rich natural sources of sulfides through good food practices could be an inexpensive and sustainable way to healthy heart and life. |
| Footnotes |
| Sangita Singh worked on H2S biogenesis during her Ph.D. at the University of Nebraska, Lincoln and University of Michigan, Ann Arbor under the tutelage of Professor Ruma Banerjee |
References
|
--
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Post your comment
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13824
- [From(publication date):
September-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9455
- PDF downloads : 4369
