Case Report Open Access
Curcumin Modulates Molecular Chaperones and Autophagy-Lysosomal Pathways In Vitro after Exposure to A�?²42
Panchanan Maiti1-3*, Julien Rossignol1,2,4 and Gary L Dunbar1-3*1Department of Psychology and Neuroscience Program, Central Michigan University, MI, USA
2Department of Biology, Saginaw Valley State University, Saginaw, MI, USA
3Field Neurosciences Institute, St. Mary’s of Michigan, USA
4College of Medicine, Central Michigan University, Mt. Pleasant, MI 48859, USA
- Corresponding Authors:
- Panchanan Maiti
Department of Psychology and Neuroscience Program
Central Michigan University, MI, USA
Tel: 989-497-3026
E-mail: maiti1p@cmich.edu - Gary L Dunbar
Department of Psychology and Neuroscience Program
Central Michigan University, MI, USA
Tel: 989-497-3026
E-mail: dunba1g@cmich.edu
Received date: December 13, 2016; Accepted date: January 09, 2017; Published date: January 16, 2017
Citation: Maiti P, Rossignol J, Dunbar GL (2017) Curcumin Modulates Molecular Chaperones and Autophagy-Lysosomal Pathways In Vitro after Exposure to Aβ42. J Alzheimers Dis Parkinsonism 7:299. doi:10.4172/2161-0460.1000299
Copyright: © 2017 Maiti P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Accumulation of misfolded amyloid beta proteins (Aβ) and hyper phosphorylated-tau are the key players in the pathologenesis of Alzheimer’s disease (AD). Molecular chaperones (heat shock proteins, HSPs), autophagylysosomal pathways (ALPs), and chaperone-mediated autophagy (CMA) are actively involved in degradation of these aggregates. Dysregulation of these systems has been observed in the AD brain, which demands the maintenance or restoration of these systems. In this context, as an anti-amyloid, curcumin (Cur) has potential role for AD therapy. Becuase of its solubility and bioavailability issues, recently we have used solid lipid curucmin particles (SLCP), which showed great permeability and neuroprotection. In this context, the present study was designed to investigate the role of Cur on HSPs and ALPs, In vitro, after exposure to Aβ42. Human cortical neurons (SH-SY5Y) and mouse neuroblastoma (N2a) cells were exposed with Aβ42 (10 μM) for 24 h and incubated with or without different concentrations of dietary Cur and or SLCP and several markers for HSPs and ALPs were investigated. We found that the most HSPs (HSP90, HSP70, HSP60, HSP40) were downregulated after exposure to Aβ42 and Cur treatment restored their levels. Similarly, markers for CMA, such as HSC70, LAMP2A, CHIP were downregulated by the Aβ42 exposure and Cur and or SLCP treatment restored their levels. In contrast, macroautophagy markers, such as LC3A/B-II and beclin-1 were upregulated after exposure to Aβ42, while Cur and or SLCP treatment further increased their levels. Therefore, maintenance or restoration of HSPs and regulation of ALPs by Cur may provide a promising strategy to degrade Aβ-aggregates from neurons in the AD brain.
Keywords
Alzheimer’s disease; Molecular chaperones; Chaperonemediated autophagy; Autophagy lysosomal pathway; Curcumin; Neurodegeneration
Abbreviations
Cur: Curcumin; SLCP: Solid Lipid Curcumin Particles; Aβ: Amyloid Beta Protein; AD: Alzheimer’s Disease; APPsw: Amyloid Precursor Protein Swedish; ANOVA: One Way Analysis of Variance; AU: Arbitrary Unit; HSD: Honestly Significant Difference; CMA: Chaperone-Mediated Autophagy; DMEM: Dulbecco Modified Eagle’s Medium; EDTA: Ethylene-Di-Amino-Tetra-Acetic-Acid; EGTA: Ethylene Glycol Tetra Acetic Acid; FBS: Fetal Bovine Serum; HSP: Heat Shock Protein; ALP: Autophagy Lysosmal Pathway; CMA: Chaperone Mediated Autophagy; OD: Optical Density; SLN: Solid Lipid Nanoparticle; SDS: Sodium Dodecyl Sulphate; TBS: Tris Buffer Saline; RIPA: Radio Immune Precipitation Assay
Introduction
Gradual accumulation of misfolded amyloid beta proteins (Aβ) and phosphorylated tau (p-tau) are the key pathological hallmarks of Alzheimer’s disease (AD) [1-3]. Excess accumulation of these misfolded protein aggregates cause neurodegeneration and impairs synaptic communication [4]. However, several protein degradation pathways are involved in disposing these misfolded protein debris, directly or indirectly, in the cell. Failure of these systems severely affects the disposal of these toxic materials as observed in different neurological diseases, including AD [5-7].Therefore, it is vital to keep these protein degradation pathways active in order to avoid the build-up of misfolded proteins in the cell. However, among all these protein degradation systems, the molecular chaperones and autophagy-lysosomal pathway (ALP), play vital role in degradation of misfolded protein aggregates [8,9].
The molecular chaperones are basically the heat shock proteins (HSPs), which are involved in protein quality control and help the proteasome system in the final degradation of misfolded protein debris [10,11]. These chaperones have been co-localized with Aβ and p-tau in AD brain and play a significant role in degradation of protein debris [12]. Similarly, ALPs, which include macroauophagy, microautophagy and chaperone-mediated autophagy (CMA), are also associated with degradation of misfolded protein debris in the lysosomal lumen [5,8,13]. Indeed, macroautophagy and microautophagy are responsible for degradation of relatively larger aggregates, and CMA is a more precise mechanism involved in the degradation of relatively small soluble protein debris [14,15]. This CMA is a multi-step process, which involves substrate recognition by a special protein, called heat shock cognate 70 (HSC70). This substrate protein binds with lysosomal membrane protein, called lysosome associated membrane protein 2A (LAMP2A), and promotes their unfolding and translocation into the lysosomal lumen, leading to substrate degradation by lysozymes [16].
Curcumin is a natural polyphenol, derived from the root of the herb, Curcuma longa, a traditional dietary spice in Indian and South Asian countries [17]. Because of its pleiotropic actions, including antiamyloid [18,19], anti-oxidant [20] and anti-inflammatory properties [21], Cur has been targeted for AD therapy [22-28]. Unfortunately, the poor solubility, instability in physiological fluids and low bioavailability of Cur are the major obstacles for achieving its optimum theranostic value [29,30]. One of the major metabolites of curcumin is Tetrahydrocurcumin (THC), which is more stable in biological fluids and has similar potency as curcumin [31]. However, recently, a solid lipid Cur particles (SLCP) formulation has been shown to increase its solubility, stability and bioavailability [22,26,32,33].This SLCP formula (also known as Longvida) is considered one of the most promising strategies for restoration and up regulation of several synaptic markers and maintenance of protein degradation pathways in transgenic mouse models of AD [11,22,26,28,33]. This formulation can penetrate 65 times greater into the brain tissue and can bind to Aβ plaques more effectively than dietary Cur, a finding that may lead to mitigating the deleterious effects of Aβ and p-tau aggregates in AD [22,26,33]. Given this, the present study was designed to investigate whether dietary Cur, THC and/or SLCP can affect molecular chaperones, ALPs, on neuronal cell lines which have been exposed to Aβ42. Our results suggest that dietary Cur and or SLCP can be used to ameliorate the dysregulation of HSPs and ALPs, in neuronal cell lines after exposure to Aβ2.
Materials and Methods
Chemicals
Aβ peptide, Cur (~80% pure), tetrahydrocurcumin (~99% pure), HFIP (1,1,1,3,3,3-hexafluoroisopropanol and other accessory chemicals were purchased from Sigma (St. Louis, MO). The solid lipid Cur particles (SLCPTM) or Longvida® or Longvida® Optimized Curcumin contain 26% of Cur, was a kind gift from Verdure Science (Noblesville, IN). This SLCP consists of high-purity, long-chain phospholipid bilayer and a long-chain fatty acid solid lipid core, which coats the Cur. The SLCP have been well characterized by Maiti et al. [34] and by Cole and Frautschy laboratory, at the University of California, Los Angeles in collaboration with Verdure Science [26,35-37]. Details sources of all the antibodies used in this study are summarized in Table 1.
| Antibodies | Source | Type | Company | Catalog no. | Address | |
|---|---|---|---|---|---|---|
| HSP90 | Rabbit | Monoclonal | Cell signaling Technology | 4877 | Danvers, MA | |
| HSP70 | Rabbit | Polyclonal, | Cell signaling Technology | 4872 | Danvers, MA | |
| HSP60 | Rabbit | Monoclonal | Cell signaling Technology | 12165 | Danvers, MA | |
| HSP40 | Rabbit | Monoclonal | Cell signaling Technology | 4871 | Danvers, MA | |
| HSC70 | Mouse | Monoclonal | Santa Cruz Biotech | sc-7298 | Santa Cruz, CA | |
| LAMP-2A | Rat | Monoclonal | Santa Cruz Biotech | sc-20004 | Santa Cruz, CA | |
| LC3A/B | Rabbit | Polyclonal | Cell signaling Technology | 4108 | Danvers, MA | |
| Beclin-1 | Rabbit | Polyclonal | Cell Signaling Technology | 3738 | Danvers, MA | |
| CHIP | Mouse | Monoclonal | Santa Cruz Biotech | sc-133066 | Santa Cruz, CA | |
| CDC37 | Mouse | Monoclonal | Santa Cruz Biotech | Sc-135862 | Santa Cruz, CA | |
| P23 | Mouse | Monoclonal | Abcam | Ab2814 | Cambridge, MA | |
| β-tubulin | Rabbit | Monoclonal | Cell signaling Technology | 2128 | Danvers, MA | |
Table 1: Sources of different antibodies used in this study.
Peptide preparation and treatment+
The Aβ peptide preparation for the study of neurotoxicity was described previously [38]. Briefly, synthesized Aβ42 was first dissolved in cold HFIP (1,1,1,3,3,3-hexafluoroisopropanol) to get disassembledpreformed aggregates. After being dissolved, the peptide solution was sonicated for 1 min and allowed to sit for additional 30 min at room temperature. Then, the peptide solution was aliquoted into small Eppendorf tubes to achieve the desired concentration, and allowed to dry overnight at room temperature under the fume-hood. The tubes were covered with Kimwipe disposable wipers to protect them from dust. On the next day, the tubes were completely dried, using nitrogen flow and then stored at -20ºC until subsequent use. Prior to experimentation, the peptide was dissolved in a 60 mM NaOH solution and sonicated for 1 min at room temperature. Following this, the peptide was diluted with fresh cell culture medium to achieve the desired concentration and then added to the cell culture dishes [38]. The final NaOH concentration was 6 mM, which was also added to the control cells.
Cell culture
Human cortical neuronal cells (SH-SY5Y) and mouse neuroblastoma cells (N2a) (ATCC, Manassas, VA) were used for this study. The SH-SY5Y cells were used to investigate the changes of HSPs, using different concentrations of Cur and after exposure to Aβ42, and rest of the experiments were done with N2a cells. Briefly, the SH-SY5Y and N2a cells were grown in Dulbecco modified Eagle’s medium and F12K (DMEM/F12K, 1:1) and minimum essential medium (MEM, GIBCO, Grand Island, NY), respectively, with both cultures containing 10% heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin (1 μg/mL). The cultures were maintained at 37°C in a humidified atmosphere at 5% CO2. Prior to the experiment, the cells were grown either in 60 mm Petri dishes or glass-coverslips with fresh DMEM or MEM, lacking growth factors, depending on the experimental setup.
Curcumin, tetrahydrocurcumin (THC) and SLCP treatment
The Cur, THC and SLCP dissolve more readily in methanol than other solvents (e.g. 10 mM NaOH, DMSO, and PBS) due to their hydrophobic nature [34]. Therefore, the stock Cur, THC and SLCP were dissolved in methanol, and then diluted in the DMEM or MEM (methanol was <1%), before being added to the petri-dish containing the cells. Initially, different doses of Cur have been studied with HSPs in SH-SY5Y cells and, based on the experimental results; we found that 1 μM is more potent than any other concentration. Therefore we used to this dose of Cur in rest of the experiments.
Immunocytochemistry
Prior to the experiments, the media was replaced with fresh MEM, containing 0.1% Pen/Strep solution, but without growth factors. The cells were exposed to Aβ42 (10 μM) and then treated with Cur and or SLCP (1 μM), dissolved in methanol and diluted with fresh MEM (final methanol concentration was <1%) for 24 h. Immunocytochemistry was performed to identify different protein markers for HSPs, ALP and CMA, using different antibodies along with appropriate secondary antibodies tagged with fluorescent dyes. Briefly, after the stipulated period of treatment, the cover slips containing the cells were washed in PBS, twice, and then fixed with 4% paraformaldehyde for 15 min. Before starting immunocytochemistry, the coverslips were washed with PBS thrice, 10 min each. Then the cells were incubated with 0.5% Triton-X 100 (Fisher Scientific, Pittsburgh, PA), along with 0.3 M glycine, 1% BSA and 10% normal goat serum (Santa Cruz Biotech, CA) for 1 h at room temperature. Then the cover slips were incubated with primary antibodies (1:100; Sigma), which were dissolved in PBS, along with 10% goat serum, and placed on a shaker at low speed at 4ºC, overnight. On the next day, the cells were thoroughly washed with PBS, three times, for 10 min each. Then the cells were incubated with appropriate secondary antibodies (1:200), tagged with either FITC or Alexa-594 (Molecular Probes, OR), for 30 min at room temperature. Then the sections were washed thoroughly with distilled water, dehydrated, cleared and mounted on slides using anti-fading Fluoro-mount aqueous mounting media (Sigma) and visualized using a fluorescence microscope (Leica, Mode-CTR6500HS, Germany) with appropriate excitation and emission filters. The images and immunofluorescent intensity is the representative of three independent experiments and the fluorescent intensity of each marker was measured with at least 200 cells from three independent experiments, using Image-J software (https://imagej.nih.gov/ij/).
Western blot
After the stipulated period in each experiment, the media was removed from the culture dish and washed thrice with sterile PBS (pH 7.4). After that, the cells were scrapped and lysed with cold radio-immuno precipitation assay (RIPA) buffer (10 mM Tris-Cl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1% Triton-X 100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, pH 7.4) with protease and phosphatase inhibitors (Sigma) and sonicated (Fisher scientific) for 1 min in ice-cold conditions. Then the lysate was centrifuged at 16,000x g for 15 min at 4°C. The supernatant was collected and aliquoted in PCR tubes and stored at -80°C until use. Total protein concentrations for individual samples were determined using the Pierce BCA protein assay (ThermoScientific, Rockford, IL). Samples were added with equal amount of 2x SDS-sample buffer (125 mM Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol and 10% 2-mercaptoethanol) and boiled for 2 min. Approximately, 50 μg of protein per lane was loaded and electrophoresed on 10% Tris-glycine gel and transferred to PVDF membrane (Millipore, Bedford, MA). After probing with respective primary and secondary antibodies, the blots were developed with ImmobilonTM Western Chemiluminescent HRP-substrate (Millipore, Billeria, MA). The relative optical density was measured using Image-J software (https://imagej.nih.gov/ij/). To ensure equal protein loading in each lane, the blots were stripped and re-probed for β-actin (in case of SH-SH5Y cells) and β-tubulin (in case of N2a cells). The images are the representative of three independent experiments.
Statistical analysis
The data were expressed as mean ± SEM. Data were analyzed using one way analysis of variance (ANOVA), with post-hoc Tukey HSD (honestly significant difference) tests used when appropriate. Probability below or equal to 0.05 was considered as statistically significant.
Results
Cur and THC ameliorated molecular chaperones (HSPs) after exposure to Aβ42
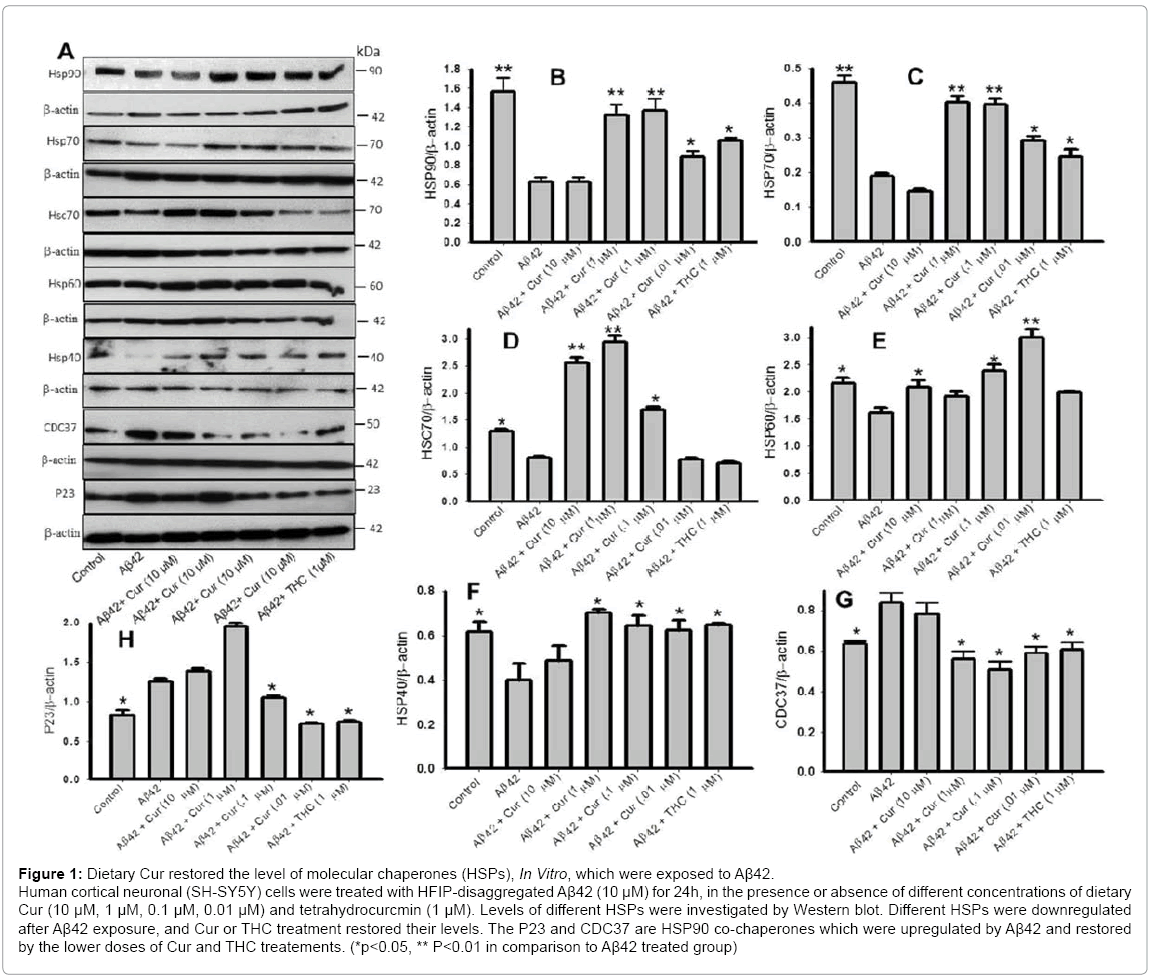
Human cortical neurons (SH-SY5Y) were exposed with Aβ42 (10 μM) for 24 h in absence and presence of different concentration of dietary Cur (in μM: 10, 1, 0.1 and 0.01) and also THC (1 μM). Different HSPs, such as HSP90, HSP70, HSC70, HSP60, HSP40 and HSP90 cochaperons, such as CDC37 and P23 were investigated by Western blot analysis. We observed that HSP90, HSP70, HSC70, HSP60 and HSP40, all were significantly downregulated (p<0.01) after exposure to Aβ42 and different concentrations of Cur and THC (1 μM) treatment restored their levels (Figure 1). We also observed that HSP90 co-chaperones CDC37 and P23 were significantly increased after Aβ42 exposure, and only lower doses of Cur (e.g. 0.1 μM and 0.01 μM) and THC (1 μM) were able to reduce these two co-chaperones (Figure 1).
Figure 1: Dietary Cur restored the level of molecular chaperones (HSPs), In Vitro, which were exposed to Aβ42. Human cortical neuronal (SH-SY5Y) cells were treated with HFIP-disaggregated Aβ42 (10 μM) for 24h, in the presence or absence of different concentrations of dietary Cur (10 μM, 1 μM, 0.1 μM, 0.01 μM) and tetrahydrocurcmin (1 μM). Levels of different HSPs were investigated by Western blot. Different HSPs were downregulated after Aβ42 exposure, and Cur or THC treatment restored their levels. The P23 and CDC37 are HSP90 co-chaperones which were upregulated by Aβ42 and restored by the lower doses of Cur and THC treatements. (*p<0.05, ** P<0.01 in comparison to Aβ42 treated group)
Cur and THC increased HSP90 and HSP70 levels in SH-SY5Y cells
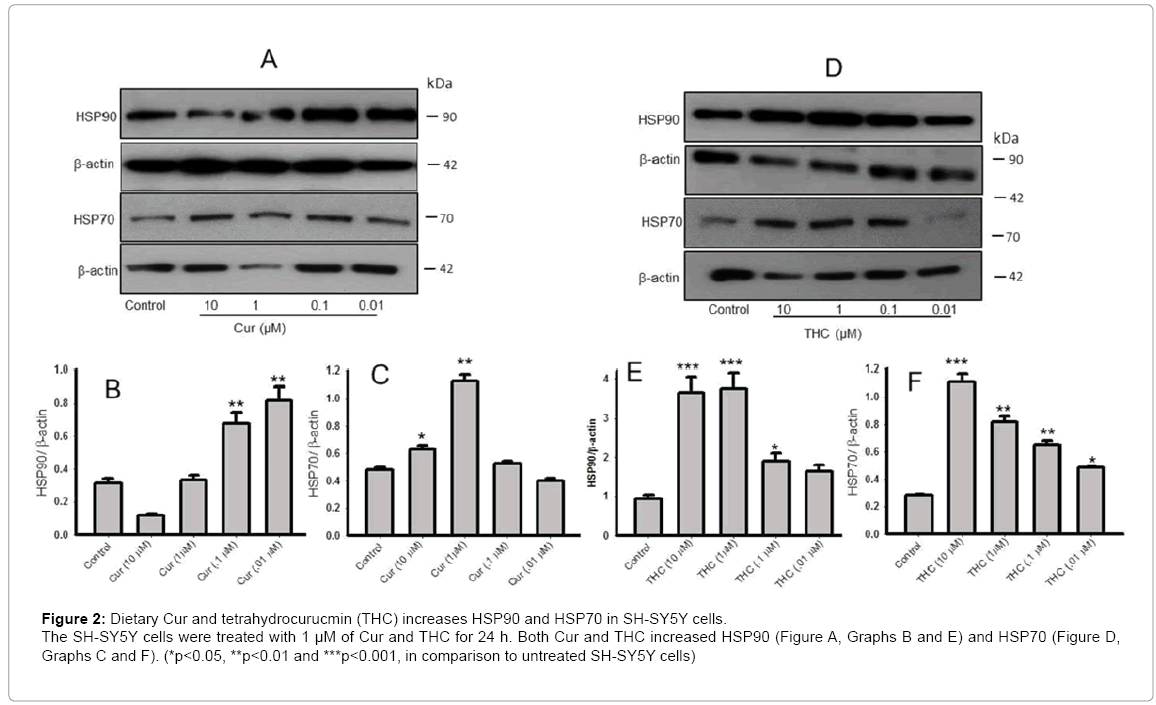
We also challenged SH-SY5Y cells with different doses of Cur (In μM: 10, 1, 0.1 and 0.01) and THC for 24 h and observed that both Cur and THC increased HSP90 and HSP70 levels in SH-SY5Y cells significantly (Figure 2).
Figure 2: Dietary Cur and tetrahydrocurucmin (THC) increases HSP90 and HSP70 in SH-SY5Y cells. The SH-SY5Y cells were treated with 1 μM of Cur and THC for 24 h. Both Cur and THC increased HSP90 (Figure A, Graphs B and E) and HSP70 (Figure D, Graphs C and F). (*p<0.05, **p<0.01 and ***p<0.001, in comparison to untreated SH-SY5Y cells)
Comparison of heat shock response in N2a cells after exposure to Aβ42 and Cur and SLCP treatment
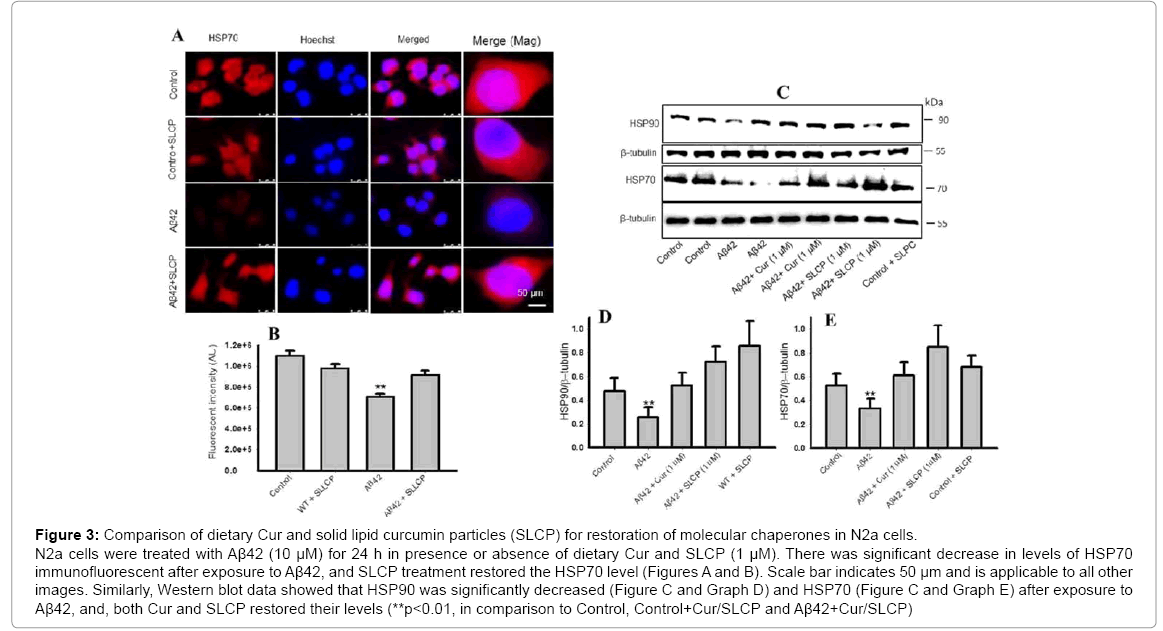
The SLCP has greater cellular permeability and neuro protection as described previously [26,33,34]. Therefore, to compare the HSPs response, we treated the N2a cells with 1 μM of Cur and or SLCP for 24 h after exposed with Aβ42 (10 μM). Our Western blot data showed that both HSP90 and HSP70 levels were significantly decreased (p<0.01) after Aβ42 exposure, as observed in SH-SY5Y cells, and both Cur and SLCP ameliorated their effects (Figures 3C-3E). In addition, immune fluorescent intensity analysis showed that HSP70 level was significantly less (p<0.01) in Aβ42 treated cells, and SLCP treatment restored this protein level (Figure 3A and Graph B). A similar finding was also observed in the case of Cur-treated cells (data not shown).
Figure 3: Comparison of dietary Cur and solid lipid curcumin particles (SLCP) for restoration of molecular chaperones in N2a cells. N2a cells were treated with Aβ42 (10 μM) for 24 h in presence or absence of dietary Cur and SLCP (1 μM). There was significant decrease in levels of HSP70 immunofluorescent after exposure to Aβ42, and SLCP treatment restored the HSP70 level (Figures A and B). Scale bar indicates 50 μm and is applicable to all other images. Similarly, Western blot data showed that HSP90 was significantly decreased (Figure C and Graph D) and HSP70 (Figure C and Graph E) after exposure to Aβ42, and, both Cur and SLCP restored their levels (**p<0.01, in comparison to Control, Control+Cur/SLCP and Aβ42+Cur/SLCP)
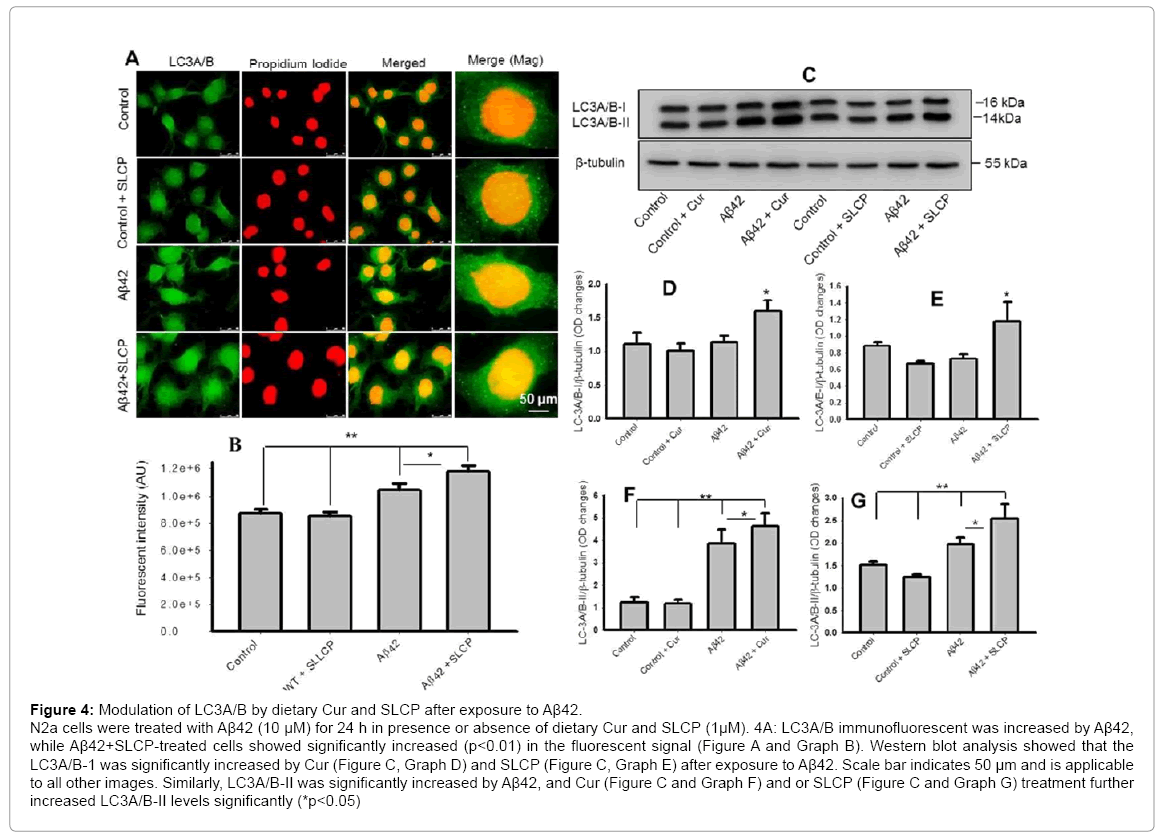
LC3A/B in neurons was upregulated after exposure to Aβ42 and further increased following treatments with Cur and/or SLCP.
An increased level of LC3A/B-II indicates formation of auto phagosomes, which are considered a gold standard for autophagic mechanisms. In the present study, we observed that LC3A/B immunofluorescent signal was significantly increased when N2a cells were exposed with Aβ42 and that this signal was further increased after Cur and or SLCP treatment (Figure 4A and Graph B). Similarly, in our Western blot experiment, we found that it was significantly increased after Aβ42 exposure (p<0.01) and Cur and SLCP treatment further increased their levels (p<0.05) (Figure 4C and Graphs F and G). In addition, LC3A/B-I was significantly increased in the Aβ-exposed cells after treatment with Cur or SLCP (Figures 4C-4E).
Figure 4: Modulation of LC3A/B by dietary Cur and SLCP after exposure to Aβ42. N2a cells were treated with Aβ42 (10 μM) for 24 h in presence or absence of dietary Cur and SLCP (1μM). 4A: LC3A/B immunofluorescent was increased by Aβ42, while Aβ42+SLCP-treated cells showed significantly increased (p<0.01) in the fluorescent signal (Figure A and Graph B). Western blot analysis showed that the LC3A/B-1 was significantly increased by Cur (Figure C, Graph D) and SLCP (Figure C, Graph E) after exposure to Aβ42. Scale bar indicates 50 μm and is applicable to all other images. Similarly, LC3A/B-II was significantly increased by Aβ42, and Cur (Figure C and Graph F) and or SLCP (Figure C and Graph G) treatment further increased LC3A/B-II levels significantly (*p<0.05)
Beclin-1 level after exposure to Aβ42 and treatment with cur and or SLCP
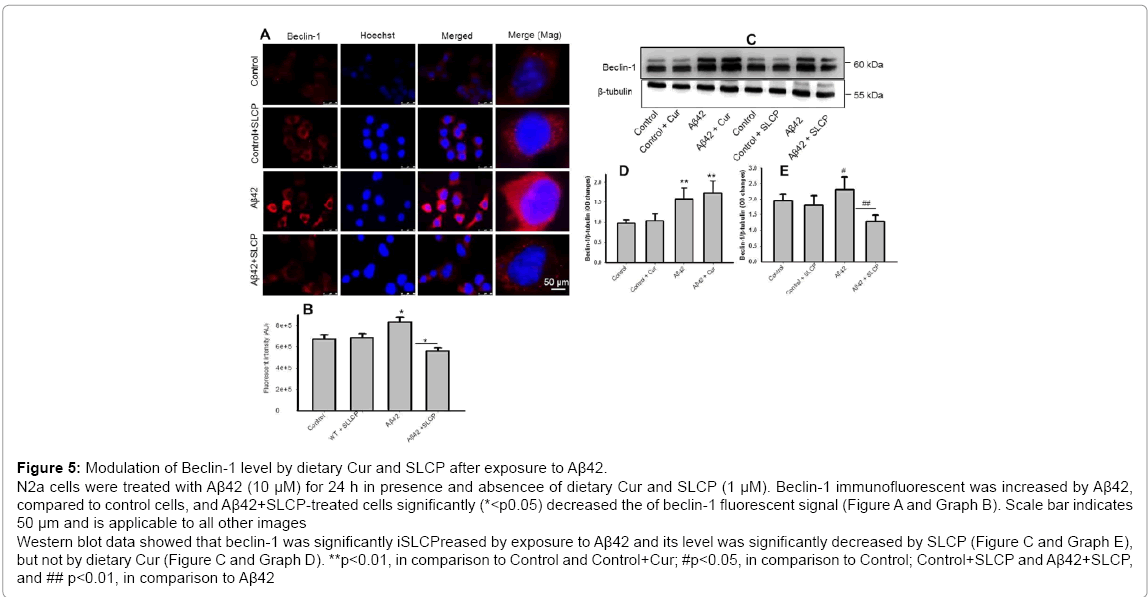
Beclin-1 is involved in autophagic pathways, including formation of autophagosome or autophagosome and endosome maturation. In our immunofluorescent study, we observed that the beclin-1 level was increased after Aβ treatment, while SLCP treatment decreased its level (Figure 5A and Graph B). Similarly, our Western blot showed that beclin-1 level was significantly increased (p<0.01) after exposure to Aβ42, and Cur and or SLCP treatments significantly decreased its level (Figure 5C and Graph E), but Cur was unable to reduce this level (Figure 5C and Graph D).
Figure 5: Modulation of Beclin-1 level by dietary Cur and SLCP after exposure to Aβ42. N2a cells were treated with Aβ42 (10 μM) for 24 h in presence and absencee of dietary Cur and SLCP (1 μM). Beclin-1 immunofluorescent was increased by Aβ42, compared to control cells, and Aβ42+SLCP-treated cells significantly (*<p0.05) decreased the of beclin-1 fluorescent signal (Figure A and Graph B). Scale bar indicates 50 μm and is applicable to all other images Western blot data showed that beclin-1 was significantly iSLCPreased by exposure to Aβ42 and its level was significantly decreased by SLCP (Figure C and Graph E), but not by dietary Cur (Figure C and Graph D). **p<0.01, in comparison to Control and Control+Cur; #p<0.05, in comparison to Control; Control+SLCP and Aβ42+SLCP, and ## p<0.01, in comparison to Aβ42
HSC70 level was downregulated after Aβ42 exposure and it but restored by Cur and/or SLCP treatments
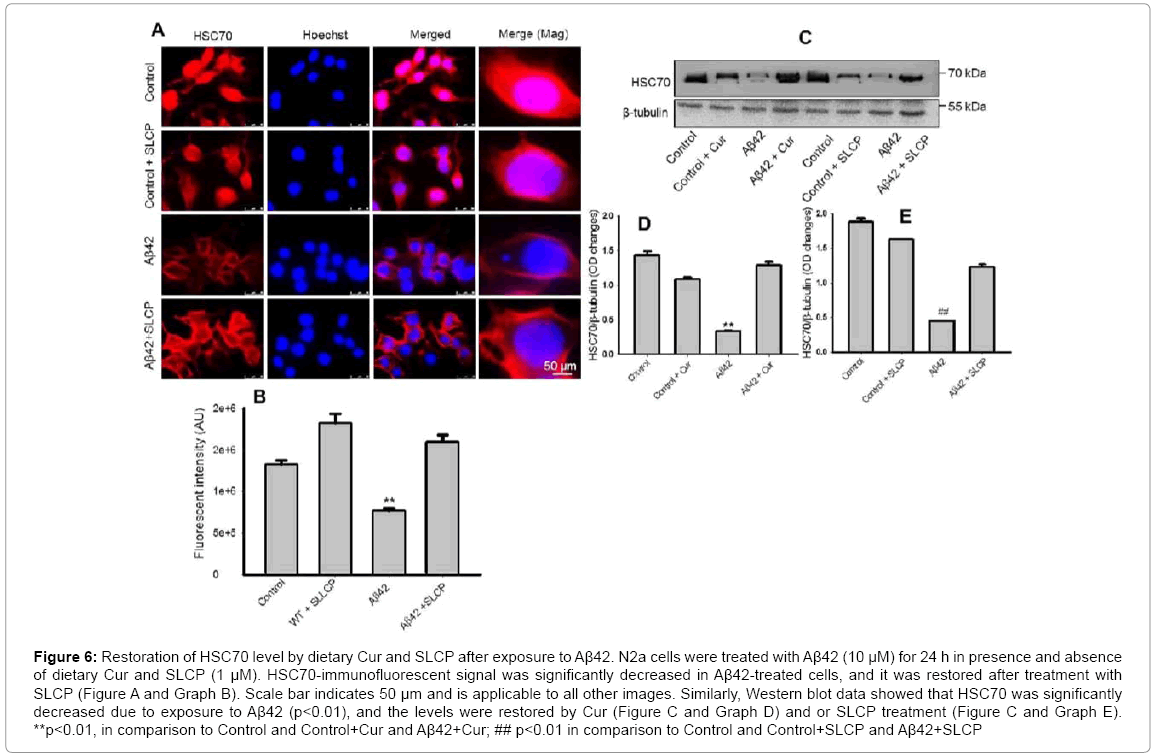
HSC70 is a highly conserved chaperone, which is involved in CMA for recognition of small, soluble misfolded protein substrates. The immunofluorescent signal of HSC70 was significantly less (p<0.01) in Aβ42-treated neurons and SLCP restored its level (Figure 6A and Graph B). We also found similar finding in our Western blot experiment, such that HSC70 was significantly downregulated (p<0.01) after exposure to Aβ42 and Cur (Figure 6C and Graph D) and or SLCP restored its level (Figure 6C and Graph E).
Figure 6: Restoration of HSC70 level by dietary Cur and SLCP after exposure to Aβ42. N2a cells were treated with Aβ42 (10 μM) for 24 h in presence and absence of dietary Cur and SLCP (1 μM). HSC70-immunofluorescent signal was significantly decreased in Aβ42-treated cells, and it was restored after treatment with SLCP (Figure A and Graph B). Scale bar indicates 50 μm and is applicable to all other images. Similarly, Western blot data showed that HSC70 was significantly decreased due to exposure to Aβ42 (p<0.01), and the levels were restored by Cur (Figure C and Graph D) and or SLCP treatment (Figure C and Graph E). **p<0.01, in comparison to Control and Control+Cur and Aβ42+Cur; ## p<0.01 in comparison to Control and Control+SLCP and Aβ42+SLCP
LAMP2A level was upregulated by Aβ42 but restored by Cur or SLCP treatment
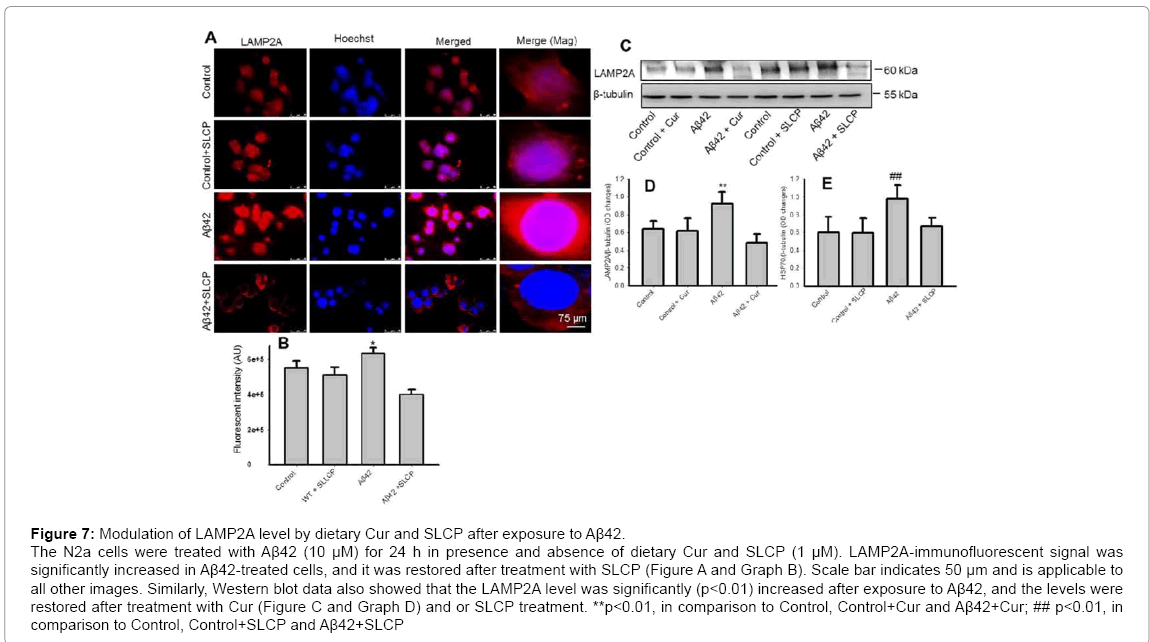
Lysosome associated membrane protein 2A (LAMP2A) is an important protein for internalization of small, soluble misfolded proteins into the lysosomal lumen, so is significantly involved in CMA. When we performed immunofluorescent study, we observed that the Aβ-treated cells increased its level and both Cur and SLCP decreased it (Figure 7A and Graph B). Similarly, Western blot data showed that LAMP2A level was significantly increased (p<0.01) after exposure to Aβ42, and it was decreased by Cur (Figure 7C and Graph D) and SLCP treatment (Figure 7C and Graph E).
Figure 7: Modulation of LAMP2A level by dietary Cur and SLCP after exposure to Aβ42. The N2a cells were treated with Aβ42 (10 μM) for 24 h in presence and absence of dietary Cur and SLCP (1 μM). LAMP2A-immunofluorescent signal was significantly increased in Aβ42-treated cells, and it was restored after treatment with SLCP (Figure A and Graph B). Scale bar indicates 50 μm and is applicable to all other images. Similarly, Western blot data also showed that the LAMP2A level was significantly (p<0.01) increased after exposure to Aβ42, and the levels were restored after treatment with Cur (Figure C and Graph D) and or SLCP treatment. **p<0.01, in comparison to Control, Control+Cur and Aβ42+Cur; ## p<0.01, in comparison to Control, Control+SLCP and Aβ42+SLCP
CHIP level was downregulated after exposure to Aβ42 and restored by cur and/or SLCP treatments
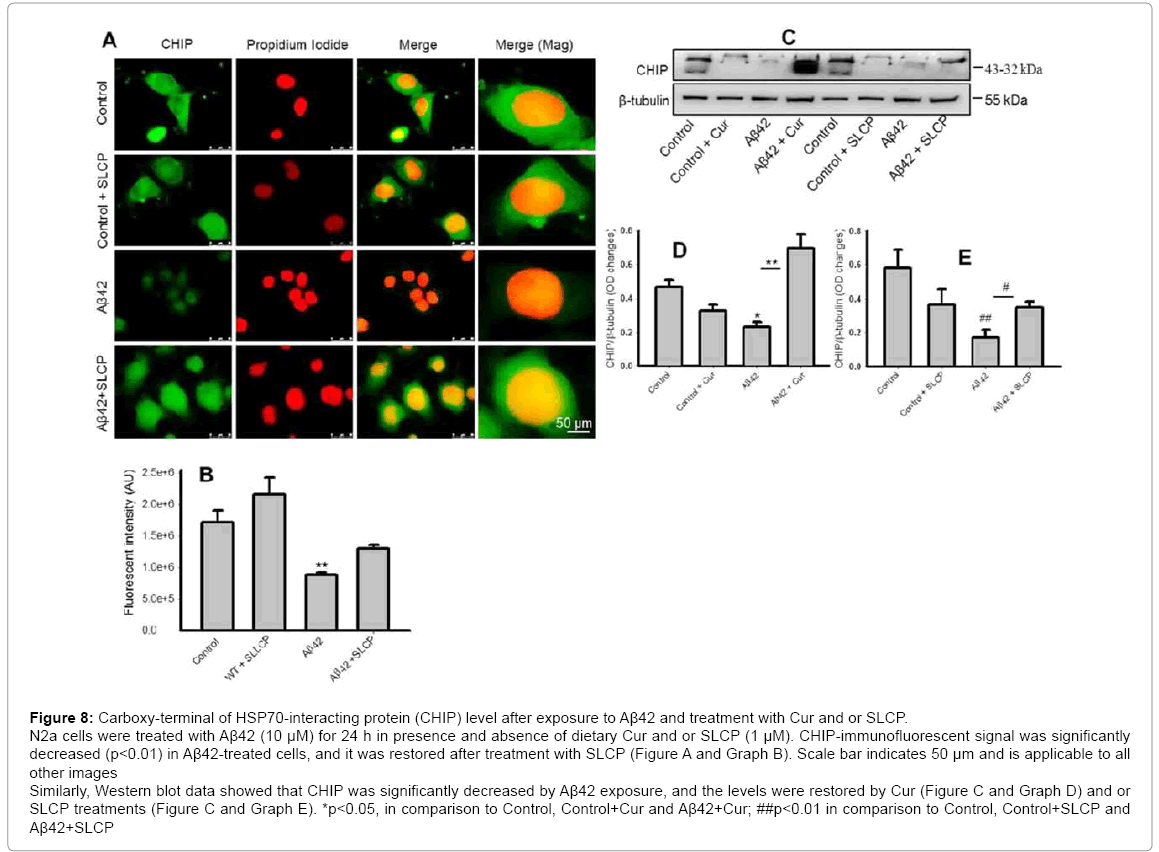
The carboxyl terminus of HSP70-interacting protein (CHIP) is one of the members of proteasome system. It acts as a link between the HSC70 and proteasome systems, thus is involved in maintaining the protein homeostasis in the cytosol. In the present study, we observed that immunofluorescence signal of CHIP was decreased after Aβ treatment, but restored by SLCP treatment (Figure 8A and Graph B). Similarly, our Western blot data revealed that CHIP level was significantly lowered in Aβ42-treated cells (p<0.05), but restored by treatment with Cur (Figure 8C and Graph D) or SLCP (Figure 8B and Graph D).
Figure 8: Carboxy-terminal of HSP70-interacting protein (CHIP) level after exposure to Aβ42 and treatment with Cur and or SLCP. N2a cells were treated with Aβ42 (10 μM) for 24 h in presence and absence of dietary Cur and or SLCP (1 μM). CHIP-immunofluorescent signal was significantly decreased (p<0.01) in Aβ42-treated cells, and it was restored after treatment with SLCP (Figure A and Graph B). Scale bar indicates 50 μm and is applicable to all other images Similarly, Western blot data showed that CHIP was significantly decreased by Aβ42 exposure, and the levels were restored by Cur (Figure C and Graph D) and or SLCP treatments (Figure C and Graph E). *p<0.05, in comparison to Control, Control+Cur and Aβ42+Cur; ##p<0.01 in comparison to Control, Control+SLCP and Aβ42+SLCP
Discussion
Proteolytic pathways play pivotal roles in disposing misfolded protein debris, such as soluble oligomers, the predominant toxic species of Aβ and p-tau found during neuronal death in AD [11,13,39,40]. Dysfunction of protein degradation pathways and failure of autophagic mechanism have strongly linked to neuronal death in AD [6,39,41]. The primary goal of this study was to determine whether Cur and or SLCP could restore dysfunction of molecular chaperones and autophagy lysosomal pathways after exposure to Aβ42, In Vitro. Therefore, we have investigated different HSPs using human neuroblastoma cell line (SH-SY5Y) and mouse neuroblastoma (N2a), along with different autophagy markers after exposure to Aβ42. We observed that the Aβ- induced down-regulation of HSPs was ameliorated by dietary Cur and or SLCP treatment. Similarly, ALP markers were also modulated by dietary Cur and or SLCP.
Although the mechanistic details of neuroprotection by Cur in AD are not yet clear, one possibility is that it is targeting some common endogenous protein clearance pathways, such as those using molecular chaperones (HSPs) and ALP and/or proteasome systems [11,13,26].
Indeed, induction of HSPs has emerged as a potential strategy for the treatment of neurodegenerative diseases [42,43]. Therefore, the modulatory roles of Cur on molecular chaperones and ALP markers were investigated in the present study with human neuroblastoma cells (SH-SY5Y), which were exposed to Aβ42. We observed significant downregulation of HSPs in these cells and also found that the Cur treatment prevented this.
We also treated the cells with THC, which is one of the major metabolites of Cur in our body. It lacks α, β-unsaturated carbonyl moiety and is more stable in our body fluids [31]. Interestingly, it also strongly binds with Aβ-plaques, similar to Cur. To determine whether THC is superior to Cur or has similar effects on restoration of HSPs, we treated the SH-SY5Y cells with THC along with Cur. Our findings suggested that THC is equally as effective as Cur in restoration of HSPs after Aβ42 treatment, In Vitro.
Although HSP90 is one of the main HSPs involved with protein misfolding, several other functionally related co-chaperones, such as CDC37 and P23 are involved in controlling the function of HSP90 [44]. In the present study, we investigated the levels of CDC37 (an inhibitor of HSP90) and P23 (a positive regulator of HSP90). Interestingly we found that CDC37 was significantly increased after Aβ42 treatment, and lower concentrations of Cur (1, 0.1 and 0.01 μM) and THC (1 μM) normalized CDC37 levels, which may explain why low levels of HSP90 are observed after Aβ42 exposure. In contrast, P23 was also upregulated by Aβ42, which may be due to decrease HSP90 level. The Cur treatment (1 μM) was able to induce P23 further, indicating that perhaps Cur can dissociate CDC37 from HSP90 protein complex, thus restoring the normal function of HSP90. These observations suggested that there was dysregulation of HSP90 co-chaperones, whereas Cur treatment significantly improved their levels by decreasing CDC37 and increasing P23 level (Figure 1) [45].
When we treated SH-SY5Y cells with different concentrations (in μM: 10-0.01) of Cur and THC with Aβ42, we observed that all these concentrations of Cur and THC induced HSP90 and HSP70. However, Cur at 10 μM concentration decreased the HSP90 level but the 1 μM concentration did not affect the HSP90 levels. The HSP90 level may have been reduced at 10 μM (Figure 2), because this concentration can induce neuronal death [46,47]. In our experiment, we observed that higher concentration (≤ 10 μM) induced neuronal apoptosis, whereas lower concentration especially nanomolar to 1 μM of Cur is neuroprotective. It has been reported that higher concentration of Cur can induce neuronal apoptosis and act as an anti-cancer agent [46-48]. Although the mechanistic details of how Cur restored HSPs after Aβ42 exposure, or how they induce HSPs in SH-SY5Y cells, is still unclear to us, one possible explanation may be that Cur treatments confer neuroprotection by secreting several growth factors, including BDNF, NGF and IGF [49- 51]. Although we did not investigate these neurotropic factors in the present study, we found Cur restores BDNF in other animal models of neurodegenerative diseases. These growth factors may help protect against Aβ-induced neuronal death by producing enough of HSPs to cope with adverse conditions or help proteasome systems to degrade the aggregates. However, we also found similar results when we treated mouse neuroblastoma cells (N2a) with Cur and SLCP after exposure to Aβ42 (Figure 3), suggesting that induction of heat shock response by Cur is not specific to human cortical neurons, but, rather, may also affect other neuronal cells.
Although HSPs can help proteasome systems to degrade misfolded protein aggregates, large Aβ species, including oligomers, protofibrils and fibrils, cannot be degraded by ubiquitin protease system (UPS), because such protein debris is too large to pass through the narrow proteasome barrel [52,53]. In this case, macroautophagy plays a greater role in degrading different species of Aβ and p-tau [54-56]. Abnormalities or failure of the autophagic-lysosomal system have been noted in neurons of the AD brain. Recently [6] suggested the dual roles of Hsp70 include functioning as a molecular chaperone for damaged proteins and as a guardian of lysosomal integrity. The impairments of ALP and stabilization may be driven by the calpain-mediated cleavage of carbonylated HSP70 and this causes lysosomal permeabilization and/or ruptures which leads to release of the cell degradation enzyme, cathepsins (calpain-cathepsin hypothesis). Therefore, the combined effects of Aβ-induced calpain activation and reactive oxygen species (ROS)-induced 4-hydroxy-2-nonenal (HNE) generation, can cause HSP70 carbonylation and cleavage, which ultimately lead to the lysosomal rupture, accounting for ALP failures in the AD brain [6]. Moreover, HSP70 dysfunction causes aggregation of phosphorylated tau and disturbances of the axonal transport [6], which lead to neurodegeneration by disturbing maturation of autophagosomes and destabilization of late endosomes/lysosomes [57].
In this context, we measured the amount of microtubule-associated protein 1A/1B-light chain 3 (LC3A/B), which is a marker for monitoring macroautophagy [58]. In fact, carboxyl terminal cleavage of LC3 can form LC3-I, which is then conjugated with phosphatidylethanolamine to form LC3-II. The LC3-II tightly binds to the autophagosomal membranes. Therefore, the amount of LC3-II clearly correlates with the number of autophagosomes, and increased expression of this protein reflects enhancement of autophagic activity [58,59]. In the present study, we observed that LC3A/B-II was significantly increased, due to Aβ42 exposure, and both Cur and SLCP further increased its expression, which indicates that Cur and or SLCP have roles in increasing autophagy to dispose Aβ from the cells (Figure 4) [60,61], some inhibitors (e.g. rapamycin) can be used to monitor the levels of LC3A/B-II after Aβ2 exposure and Cur and/or SLCP treatment. However, our main focus in the present study was to investigate whether Cur and/or SLCP have roles in modulating LC3A/B-II levels after Aβ42 exposure.
Similarly, beclin-1 levels also regulate the autophagy and membrane trafficking involved in several physiological and pathological processes, and under cellular stress conditions, it is increased [62]. It also interacts with vacuolar sorting protein 34 (VPS34), a class III phosphatidylinositol-3 kinase and regulates apoptosis and autophagy mechanisms [62]. In the present study, we observed that beclin-1 was increased by Aβ42 and that Cur treatment did not change this level, but SLCP significantly decreased it (Figure 5). Beclin-1 level is increased during stress and induces apoptosis, and, as a potent antioxidant, Cur and/or SLCP can reduce oxidative stress, thus may decreasing beclin-1 levels and reducing apoptotic death. However, unlike the effects of SLCP, Cur was unable to reduce beclin-1. Currently, we do not have a clear explanation to resolve this discrepancy. However, our findings suggest that SLCP may have greater potency, because of its greater cellular permeability, relative to dietary Cur, which contains a solid lipid coating. In addition, we did not explore whether VSP34 levels interacted with beclin-1 in regulating autophagy. Therefore, further research in this area may show such interactions for regulation of levels of beclin-1.
It has been shown that relatively small, soluble Aβ and p-tau can undergo degradation by CMA, without involving the HSPs and macroautophagic pathways [63]. In CMA, the HSC70 and LAMP2A proteins play a major role in recognizing these protein substrates [16,14,64]. In fact, CMA interacts with macroautophagy, via the upregulation of HSC70 and LAMP2A [65-68]. However, in the present study, we found that HSC70 level was significantly reduced after exposure to Aβ42 (Figure 7), which was also observed in 12 and 18 months old 3x Tg AD mice by others [64,67,69]. Because HSC70 is important for recognition of misfolded aggregates, reduction levels of HSC70 is directly associated with impairment of CMA [70,71]. Importantly, we found that Cur and/or SLCP restored the HSC70 levels, though the mechanistic details for this finding are still not clear.
In our study we also found that the LAMP2A was increased by Aβ42 and that Cur and or SLCP treatments maintained these levels in their normal ranges. LAMP2A expression may depend on HSC70 level, as decreases in HSC70 levels, due to Aβ42 exposure, may increase LAMP2A expression as a compensatory mechanism to increase binding with lower levels of HSC70-substrate complex. Increased LAMP2A levels after exposure to Aβ42, produces increased CMA. One reason for increased LAMP2A after Aβ exposure is because of downregulation of HSC70. When HSC70 levels decrease due to Aβ42 exposure, this may increase the expression of LAMP2A which then binds to the low levels of HSC70, along with substrate proteins. However, when HSC70 was restored by Cur and/or SLCP, LAMP2A levels returned to normal, suggesting that Cur and/or SLCP may be able to maintain stability of CMA system during Aβ42-induced stress. These phenomena indicate that HSC70 and LAMP2A are tightly regulated in the control cells and become dysregulated in disease conditions [66,70]. Although several other CMA markers are involved in autophagic regulation, we found that HSC70 and LAMP2A are sensitive markers to investigate the autophagy mechanism.
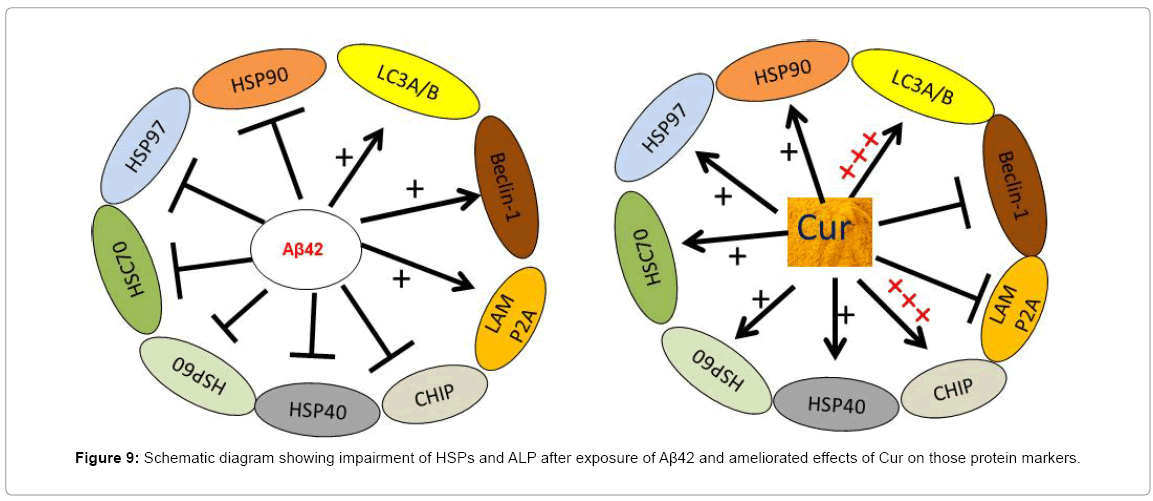
CHIP also has role in the turnover of Aβ and plays an important role in regulating misfolded protein load in cells, such as p-tau [72]. It is an E3 ubiquitin ligase protein, which interacts with HSC70-HSP70 and HSP90 and helps substrate ubiquitination and degradation of misfolded protein by the proteasome system [73]. CHIP serves as a link between the folding-refolding machinery in proteasome degradation pathways and has a direct role in a particular protein quality control pathway [74]. Over-expression of CHIP can decrease Aβ levels and may stabilize levels of amyloid precursor protein (APP) [75]. In our study, we observed that CHIP level was significantly downregulated by Aβ42 exposure (Figure 8). Due to Aβ exposure, HSP70 and HSC70 were downregulated, which may decrease CHIP levels. Furthermore, when levels of HSP70 and HSC70 were restored by Cur and or SLCP treatment, these treatments also maintained the CHIP levels, because, CHIP-dependent protein degradation is HSP70-dependent (Figure 9).
Conclusion
Taken together, Cur can modulate, as well as rectify, the dysregulation of molecular chaperones and autophagy lysosomal pathways as a means of protecting neurons from Aβ42-induced cell death. Therefore, curcumin might be a promising compound for AD therapy by providing neuroprotection, as well as maintenance of protein degradation pathways. Our findings may be helpful in understanding the neuroprotective mechanisms of autophagic pathways that are involved in the prevention of the type of neurodegeneration observed in AD.
Acknowledgement
This work was supported by the Field Neurosciencess Institute, St. Mary’s of Michigan and the Neuroscience Program at Central Michigan University. We thank to Verdure Science (Noblesville, IN) for donating the solid-lipid curcumin particles for this study.
References
- Harrison RS, Sharpe PC, Singh Y, Fairlie DP (2007) Amyloid peptides and proteins in review. Rev Physiol Biochem Pharmacol 159: 1-77.
- Merlini G, Westermark P (2004) The systemic amyloidoses: Clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med 255: 159-178.
- Selkoe DJ (2004) Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6: 1054-1061.
- Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med 2: a006338.
- Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, et al. (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112: 24-49.
- Yamashima T (2013) Reconsider Alzheimer's disease by the 'calpain-cathepsin hypothesis'--a perspective review. Prog Neurobiol 105: 1-23.
- Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp Mol Med 47: e147.
- Eskelinen EL, Saftig P (2009) Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793: 664-673.
- Takalo M, Salminen A, Soininen H, Hiltunen M, Haapasalo A (2013) Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am J Neurodegener Dis 2: 1-14.
- Bala K, Tripathy BC, Sharma D (2006) Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology 7: 81-89.
- Maiti P, Manna J, Veleri S, Frautschy S (2014) Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. Biomed Res Int 2014: 495091.
- Wilhelmus MM, de Waal RM, Verbeek MM (2007) Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer’s disease. Mol Neurobiol 35: 203-216.
- Maiti P, Manna J (2016b) Dysregulation of autophagy lysosomal pathway in Alzheimer’s disease: Role of curcumin. JSM Alzheimer ’s Disease and Related Dementia 3: 1026.
- Wang G, Mao Z (2014) Chaperone-mediated autophagy: Roles in neurodegeneration. Transl Neurodegener 3: 20.
- Wu H, Chen S, Ammar AB, Xu J, Wu Q, et al. (2015) Crosstalk between macroautophagy and chaperone-mediated autophagy: Implications for the treatment of neurological diseases. Molecular Neurobiology 52: 1284-1296.
- Cuervo AM, Wong E (2014) Chaperone-mediated autophagy: Roles in disease and aging. Cell Res 24: 92-104.
- Prasad S, Aggarwal BB (2011) Turmeric, the golden spice: From traditional medicine to modern medicine. In: Herbal Medicine: Biomolecular and Clinical Aspects.
- Ono K, Hasegawa K, Naiki H, Yamada M (2004) Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res 75: 742-750.
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, et al. (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques and reduces amyloid in vivo. J Biol Chem 280: 5892-5901.
- Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 595: 105-125.
- Ray B, Lahiri DK (2009) Neuroinflammation in Alzheimer’s disease: Different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol 9: 434-444.
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, et al. (2008) Curcumin structure-function, bioavailability and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 326: 196-208.
- Cole GM, Teter B, Frautschy SA (2007) Neuroprotective effects of curcumin. Adv Exp Med Biol 595: 197-212.
- Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R (2008) Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein - a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem 104: 1553-1564.
- Hoppe JB, Frozza RL, Pires EN, Meneghetti AB, Salbego C (2013) The curry spice curcumin attenuates beta-amyloid-induced toxicity through beta-catenin and PI3K signalling in rat organotypic hippocampal slice culture. Neurol Res 35: 857-866.
- Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, et al. (2013) Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic and behavioral deficits in aged human tau transgenic mice. J Biol Chem 288: 4056-4065.
- Maiti P, Manna J (2015) Dietary curcumin: A potent natural polyphenol for neurodegenerative diseases therapy. MOJ Anat Physiol 1: 00026.
- Maiti P, Dunbar GL (2016c) Rationale for curcumin therapy in Alzheimer’s disease. ARC Journal of Neuroscience 1: 10-16.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: Problems and promises. Mol Pharm 4: 807-818.
- Kumar A, Ahuja A, Ali J, Baboota S (2010) Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst 27: 279-312.
- Aggarwal BB, Deb L, Prasad S (2014) Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 20: 185-205.
- Frautschy SA, Cole GM (2010) Why pleiotropic interventions are needed for Alzheimer's disease. Mol Neurobiol 41: 392-409.
- Hu S, Maiti P, Ma Q, Zuo X, Jones MR, et al. (2015) Clinical development of curcumin in neurodegenerative disease. Expert Rev Neurother 15: 629-637.
- Maiti P, Hall TC, Paladugu L, Kolli N, Learman C, et al. (2016a) A comparative study of dietary curcumin, nanocurcumin and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5x-familial Alzheimer's disease mice. Histochem Cell Biol 146: 609-625.
- Cox KH, Pipingas A, Scholey AB (2015) Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J Psychopharmacol 29: 642-651.
- DiSilvestro RA, Joseph E, Zhao S, Bomser J (2012) Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J 11: 79.
- Nahar PP, Slitt AL, Seeram NP (2015) Anti-inflammatory effects of novel standardized solid lipid curcumin formulations. J Med Food 18: 786-792.
- Maiti P, Lomakin A, Benedek GB, Bitan G (2010) Despite its role in assembly, methionine 35 is not necessary for amyloid beta-protein toxicity. J Neurochem 113: 1252-1262.
- Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, et al. (2015) Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease. Curr Alzheimer Res 12: 32-46.
- Cecarini V, Bonfili L, Cuccioloni M, Mozzicafreddo M, Angeletti M, et al. (2016) The fine-tuning of proteolytic pathways in Alzheimer's disease. Cell Mol Life Sci 73: 3433-3451.
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, et al. (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11: 457-470.
- Hay DG, Sathasivam K, Tobaben S, Stahl B, Marber M, et al. (2004) Progressive decrease in chaperone protein levels in a mouse model of Huntington's disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet 13: 1389-1405.
- Bagatell R, Whitesell L (2004) Altered Hsp90 function in cancer: A unique therapeutic opportunity. Mol Cancer Ther 3: 1021-1030.
- Smith JR, Workman P (2009) Targeting CDC37: An alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle 8: 362-372.
- Salminen A, Ojala J, Kaarniranta K, Hiltunen M, Soininen H (2011) Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer's disease. Prog Neurobiol 93: 99-110.
- Dhandapani KM, Mahesh VB, Brann DW (2007) Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem 102: 522-538.
- Freudlsperger C, Greten J, Schumacher U (2008) Curcumin induces apoptosis in human neuroblastoma cells via inhibition of NF kappaB. Anticancer Res 28: 209-214.
- Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of curcumin, a component of golden spice and its miraculous biological activities. Clin Exp Pharmacol Physiol 39: 283-299.
- Franco-Robles E, Campos-Cervantes A, Murillo-Ortiz BO, Segovia J, López-Briones S, et al. (2014) Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl Physiol Nutr Metab 39: 211-218.
- Liu D, Wang Z, Gao Z, Xie K, Zhang Q, et. al., (2014) Effects of curcumin on learning and memory deficits, BDNF and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res 271: 116-121.
- Qin XY, Cheng Y, Cui J, Zhang Y, Yu LC (2009) Potential protection of curcumin against amyloid beta-induced toxicity on cultured rat prefrontal cortical neurons. Neurosci Lett 463: 158-161.
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305: 1292-1295.
- Levine B, Klionsky DJ (2004) Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463-477.
- Funderburk SF, Marcellino BK, Yue Z (2010) Cell "self-eating" (autophagy) mechanism in Alzheimer's disease. Mt Sinai J Med 77: 59-68.
- Nilsson P, Saido TC (2014) Dual roles for autophagy: degradation and secretion of Alzheimer’s disease a beta peptide. Bioessays 36: 570-578.
- Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19: 983-997.
- Patterson KR, Ward SM, Combs B, Voss K, Kanaan NM, et al. (2011) Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of pre-existing tau aggregates on fast axonal transport. Biochemistry 50: 10300-10310.
- Tanida I, Ueno T, Kominami E (2008) LC3 and autophagy. Methods Mol Biol 445: 77-88.
- Hansen TE, Johansen T (2011) Following autophagy step by step. BMC Biol 9: 39.
- Sahebkar A (2016) Autophagic activation: A key piece of the puzzle for the curcumin-associated cognitive enhancement? J Psychopharmacol 30: 93-94.
- Klionsky DJ, Cuervo AM, Seglen PO (2007) Methods for monitoring autophagy from yeast to human. Autophagy 3: 181-206.
- Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18: 571-580.
- Wang Y, Mandelkow E (2012) Degradation of tau protein by autophagy and proteasomal pathways. Biochem Soc Trans 40: 644-652.
- Cuervo AM, Dice JF (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1: 570-583.
- González-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, et al. (2005) The apoptosis/autophagy paradox: Autophagic vacuolization before apoptotic death. J Cell Sci 118: 3091-3102.
- Kaushik S, Massey AC, Cuervo AM (2006) Lysosome membrane lipid microdomains: Novel regulators of chaperone-mediated autophagy. EMBO J 25: 3921-3933.
- Kaushik S, Massey AC, Mizushima N, Cuervo AM, et al. (2008) Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell 19: 2179-2192.
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM, et. al. (2006) Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci U S A 103: 5805-5810.
- Cuervo AM, Dice JF (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273: 501-503.
- Kaushik S, Cuervo AM (2012) Chaperone-mediated autophagy: A unique way to enter the lysosome world. Trends Cell Biol 22: 407-417.
- Kaushik S, Cuervo AM (2012) Chaperones in autophagy. Pharmacol Res 66: 484-493.
- Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, et al. (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13: 703-714.
- Atkin G, Paulson H (2014) Ubiquitin pathways in neurodegenerative disease. Front Mol Neurosci 7: 63.
- McDonough H, Patterson C (2003) CHIP: A link between the chaperone and proteasome systems. Cell Stress Chaperones 8: 303-308.
- Kumar P, Ambasta RK, Veereshwarayya V, Rosen KM, Kosik KS, et al. (2007) CHIP and HSPs interact with beta-APP in a proteasome-dependent manner and influence abeta metabolism. Hum Mol Genet 16: 848-864.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4684
- [From(publication date):
February-2017 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 3715
- PDF downloads : 969