Cultivation of Methylocystis hirsuta in Bubble Columns Packed with Pall Rings Utilizing Methane as Carbon Source
Received: 28-Aug-2018 / Accepted Date: 17-Sep-2018 / Published Date: 20-Sep-2018 DOI: 10.4172/2155-952X.1000285
Keywords: Greenhouse gas; Methanotrophs; Single cell protein; Methylocystis hirsuta; Pall rings; Bubble column.
Introduction
Worldwide population growth, along with its consequent increasing demand for food, is responsible for the current global protein scarcity, making necessary the search for new protein sources. Since the 1950s, the prospective uses of non-conventional resources, such as Single-Cell Protein (SCP), have been studied. SCP comprises either dry microbial cells or total protein extracted from a mixed or pure culture that can serve as feeding supplement for animals or even humans [1,2].
Compared to more traditional sources, the use of SCP presents some advantages: higher rates of protein synthesis in prokaryotes, high content of protein, lipids, carbohydrates, nucleic acids, vitamins, minerals and some essential amino acids, mainly lysine and methionine, and independence on seasonal factors [2,3]. A proper choice of microorganism for SCP must consider the product destination, as well as the unique properties of each microbial species [4].
Since microorganisms can use different types of substrates, a wide range of materials, residual or not, can serve as feedstock for SCP processes. Oil & Gas industry, for example, can provide natural gas (typically 94 mole % of CH4) for SCP production [5-7]. In this case the reactor design should allow an efficient mass transfer of CH4 to the liquid phase where microorganisms are suspended. This would require knowledge about microbial metabolism and composition, cell growth kinetics, culture media, aeration rate, temperature and pressure [8].
Although not many microbial species are capable of metabolizing CH4, there is a group of methanotrophic bacteria that exhibit this ability. These bacteria are able to assimilate methane through the action of the enzyme methane monooxygenase, which converts methane to methanol, followed by the action of methanol dehydrogenase, transforming methanol to formaldehyde. The generated formaldehyde can follow two distinct metabolic pathways to be converted in biomass. In metanotrophic bacteria Type I it follows the ribulose monophosphate (RMP) pathway and in Type II it follows the serine pathway. As examples of Type I families we have Methylomonas and Methylobacter, while as Type II it is possible to mention the families Methylosinus and Methylocystis [9-11]. Additionally, their cell composition is suitable for animal feeding after adequate treatments. These bacteria also have the potential to be applied in bioremediation, polyhydroxybutyrate (PHB) production and nitrogen fixation [11-13]. Some species of methanotrophs are currently used for animal feeding, fully approved by the European Union [14].
An important parameter widely used to evaluate system’s capacity to transfer gas to liquid phase, is the volumetric mass transfer coefficient, KLα, which can be calculated by different methods [15,16]. This coefficient was originally introduced for oxygen, whose concentration in liquid phase can be easily measured by dissolved oxygen electrodes. It can be estimated for other gases, such as methane, allowing an assessment of the system's capacity to transfer CH4 to the microorganism cells. KLα values are related to several factors, such as the feed rate of the gas mixture, the residence time of the gas and the presence of fillings in the bioreactor, what increases the tortuosity of the gases, through the liquid column as well as their residence time in the liquid phase. These parameters should be investigated to improve the efficiency of the mass transfer during the process, allowing a better use not only of oxygen but also of the substrate methane.
Considering all factors related to the cultivation of methanotrophs and the use of a new bioreactor system, some other parameters should be investigated in order to optimize cell growth and obtain higher yields in protein production. Culture media that use a single carbon source, such as methane, require supplementation with vitamins, cofactors and other nutrients, which are necessary to achieve higher yields and rates of growth. It is also important to investigate the use of substances that allow greater availability of gases to the liquid phase, such as tensoactives, establishing essential interaction between the hydrophobic and hydrophilic substances that are in the continuous and discontinuous phases.
These factors along with high mass transfer coefficients will make it possible to obtain higher cell density in smaller time intervals. The present work aims at estimating KLα values for oxygen and methane as well as evaluating fermentation conditions for the methanotrophic bacteria Methylocystis hirsuta, so as to maximize the cell concentration for a new bioreactor design.
Materials and Methods
KLα measurement by the gassing-out method
Assays were carried out using the gassing-out method (or degasification) which consists of bubbling N2 through the system so as to remove all dissolved oxygen (DO) from the liquid phase. After that, airflow is initiated, and values of DO were measured every 15 seconds. The KLα value for each system was calculated with the following classical equation based on the mass balance of oxygen:
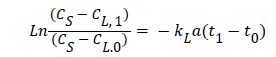
where CS is the saturation concentration of O2, CL,1 and t1 are, respectively, the values of DO and time at the end of exponential phase and CL,0 and t0 are, respectively, the values of DO and time at point 0.
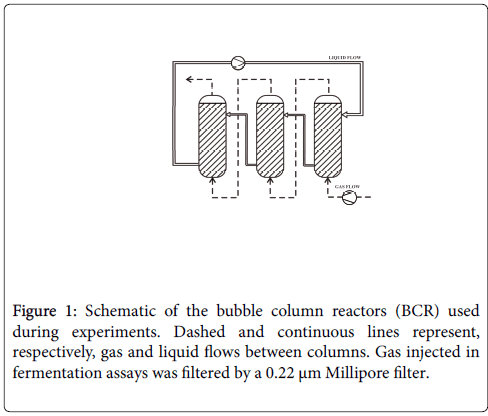
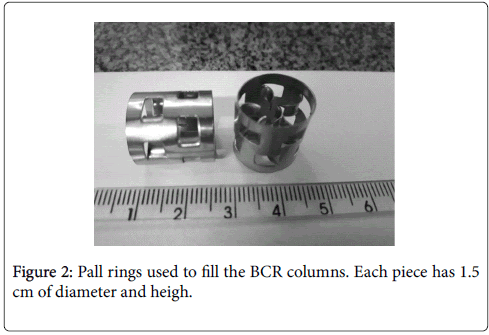
The reactor used to evaluate KLα was a system of three interconnected glass bubble columns packed with Pall rings of stainless steel with approximately 1.5 cm of diameter and height. The packing was aimed at promoting turbulence in the liquid phase, breaking up bubbles and improving the mass transfer from the gas to the liquid. Efficiency of the fillings in increasing the value of KLα of the system was observed when comparing the results with and without the use of Pall rings (Figures 1 and 2).
Considering that dissolved methane electrodes are not available commercially, values of KLα for methane were estimated on the basis of KLα for oxygen (KLα)o2), using the following correlation proposed by Yu et al. [15] for pneumatically agitated reactors:
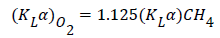
Fermentation assays
Culture medium used to evaluate Methylocystis hirsuta (ATCC BAA-1344) growth was a modified version of NMS 1306, recommended by the American Type Culture Collection (ATCC). Preliminary assays were carried out in shake flasks with controlled atmosphere to investigate fermentation conditions and possible influences in cell growth. The modified NMS 1306 composition is shown in Table 1.
| Solutions | Components | Mass |
|---|---|---|
| NMS (1000 mL) | MgSO4 . 7H2O | 1.0 g |
| CaCl2 . 6H2O | 0.20 g | |
| KNO3 | 1.0 g | |
| KH2PO4 | 0.272 g | |
| Na2HPO4 . 12H2O | 0.717 g | |
| CuSO4 | 1.4 mg | |
| Traces element solution | 0.5 mL | |
| Traces element solution (1000 mL) |
EDTA | 500.0 mg |
| FeSO4 . 7H2O | 200.0 mg | |
| ZnSO4 . 7H2O | 10.0 mg | |
| MnCl2 . 4H2O | 3.0 mg | |
| H3BO3 | 30.0 mg | |
| CoCl2 . 6H2O | 20.0 mg | |
| CaCl2 . 2H2O | 1.0 mg | |
| NiCl2 . 6H2O | 2.0 mg | |
| Na2MoO4 . 2H2O | 3.0 mg |
Table 1: Modified NMS1306 composition.
Full scale assays were carried out in the previously described BCR system, consisting of three 1 L columns in series (total volume of 3 L) and using the modified NMS 1306. Assays with and without filling were done to evaluate the interference in cell growth. All subsequent experiments were done with the BCR system filled with Pall rings.
Previous experiments in shaken flasks indicated that yeast extract and casaminoacids are effective sources of metabolic supplements to M. hirsuta. This was fully confirmed in a second fermentation in the BCR system, using the modified NMS1306 supplemented with yeast extract and casaminoacids (0.2% (w/v) each).
Following the assays described above, soybean oil was used in order to speed up the solubilization of methane in water.
Soybean oil acts as a tensioactive agent, increasing both the transfer rate of methane to the liquid as well as the availability of substrate for the microorganisms. Assay conditions were exactly the same as previous fermentations in BCR, except for the additional use of 1% (v/v) of soybean oil. In both cases, medium flow rates were such as to maintain the total volume of liquid, as well as the concentrations of supplements, at suitable levels for the microorganism growth.
The BCR system allowed for temperature monitoring only (around 30°C). The air-methane mixture flow rate was 4 L/min and its composition was maintained in 30% methane and 70% air. This proportion was optimized in a separate experiment, not reported here.
Results
KLa measurement by the gassing-out method
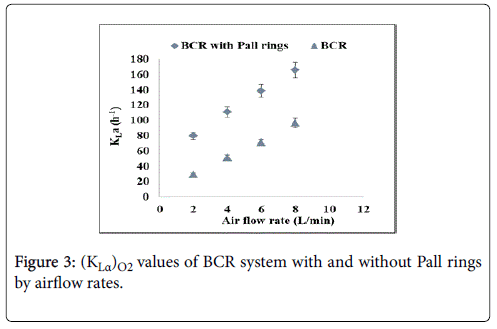
As expected, for both configurations the highest values were those for higher airflow rate, possibly related to the higher levels of turbulence/mixture in the columns. Calculated values of ((KLα)O2) were 166.1, 138.7, 111.2 and 79.9 h-1 for the BCR filled with Pall rings and 96.9, 71.1, 51.9 and 29.6 for the BCR without fillings with 8, 6, 4 and 2 L/min of airflow rate, respectively. Results of ((KLα)O2) assays are shown in Figure 3.
Fermentation assays
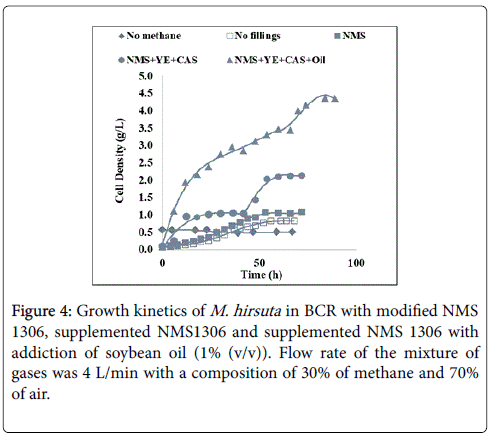
Final cell concentration obtained with pure mineral media was only 1.1 g/L with fillings and 0.8 without the Pall rings. With supplements it increased by almost two fold (2.1 g/L) and with oil addition cell concentration achieved the highest value (4.5 g/L), a gain, respectively, of 91% and 300% as compared to the pure mineral medium with fillings. The “No methane” curve shows growth kinetics of the microorganism without methane supply, which confirms the use of methane as exclusive carbon source even in the presence of supplements. Figure 4 presents the growth kinetics of M. hirsuta using the BCR system.
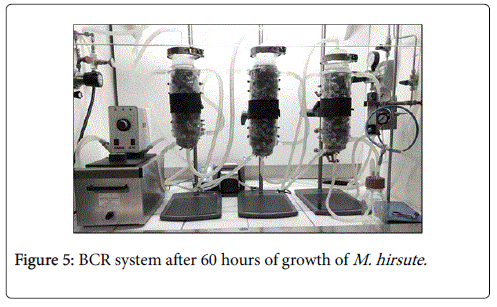
During supplemented NMS1306 assay, cell growth ceased possibly due to the depletion of supplements present in culture medium and cell concentration reached 1.0 g/L in 12 hours. After 30 hours without any further cell growth, the system was fed with supplemented medium to restore the initial levels of yeast extract and casaminoacids, and cell concentration rose to 2.1 g/L. On completion of fermentation, a sample of the culture medium was examined in a microscope (LEICA DSC295). Only small gram negative cells were present, confirming that the assay wasn’t contaminated. Figure 5 shows the BCR system after 60 hours of fermentation.
Discussion
The gain in KLa values using Pall rings reached 71, 95, 114 and 170% for 8, 6, 4 and 2 L/min which show the influence of the fillings in aeration efficiency, especially in situations of low turbulence and reduced air supply to the liquid phase. The presence of Pall rings is associated with tortuosity of bed passages and an increased gas hold up, with the gas evenly distributed across the bed section. Bubble coalescence, known to be detrimental for the process, was minimized while bubble breakage was promoted. Overall, the mass transfer from the bubble phase to the liquid improved as shown by the high values of. was estimated for the BCR filled with Pall rings using Yu et al. [17] correlation, and the following values were obtained: 147.6, 123.3, 98.9 and 71.1 for 8, 6, 4 and 2 L/min. These indicate an improved capacity for the developed bioreactor to efficiently supply oxygen and methane required for cell metabolism, actually making the new system a promising one for the cultivation of microorganisms in general.
In fermentation assays, observe the gains of adding supplements and oil to the NMS 1306 medium, either in relation to processing time or final concentrations of cells. Fermentation time to reach 1 g/L was reduced from 60 to about 20 and 12 hours, respectively, for the supplemented medium (with yeast extract and casaminoacids) and for supplemented medium plus soybean oil. Methane, a hydrophobic gas with very low water solubility, had its transference to the liquid facilitated by the presence of a tension active, increasing the contact area between gas (bubbles) and liquid, that is, improving its solubilization rate. The low cost of soybean oil in Brazil and its use in the food industry makes it an excellent choice of surfactant for SCP cultivation.
Traditional bubble column type reactors are known to be in disadvantage when compared to mechanically stirred and airlift reactors, which allow a more efficient circulation and homogenization of the culture medium. However, in this work, the use of packed bubble columns in series has increased the efficiency of methane transfer rate to the liquid, as seen in Figure 3, roughly in proportion to the increase in the gas flow rate. The increased methane residence time associated to the packing, provided a fermentation system of easy design, low operational cost compared to stirred reactors, and high efficiency in the mass transfer to the liquid phase.
Final cell concentrations achieved in BCR were much higher than those obtained in shake flasks (previously investigated, data not shown). These results reinforce the utmost importance of enhanced gas mass transfer promoted by this system, resulting in an increased availability of methane (substrate) and oxygen in the liquid phase. Mass transfer of methane to the liquid phase stands as a critical aspect of this process, leaving open the development of techniques for even more efficient contact between gas and liquid. Cell concentrations obtained, in addition to high KLα values, makes the innovative BCR a promising new configuration of bioreactor for cultivation of methanotrophic bacteria, with the significant competitive advantage of lowering the operational costs since mechanical agitation is not required.
References
- Kuhad RC, Singh A, Tripathi KK, Saxena Rk, and Eriksson L (1997) Microorganisms as an alternative source of protein. Nutr Rev 55: 65–75.
- Nasseri AT, Rasoul-Amini S, Morowvat MH, Ghasemi Y (2011) Single Cell Protein: Production and Process. Am J Food Technol 6: 103-116.Â
- Adedayo MR, Ajiboye EA, Akintunde JK, Odaibo A (2011) Single Cell Proteins: As Nutritional Enhancer. Adv Appl Sci Res 2:396-409.
- Miller M, Litsky W (1976) Single Cell Protein in Industrial Microbiology. McGraw Hill Inc. Indiana, Mishawaka.
- Bowman JP (2006) The Families Methylococcaceae and Methylocystaceae. The Prokaryotes 266-289
- Durre P and Eikmanns BJ (2015) C1-carbon sources for chemical and fuel production by microbial gas fermentation. Curr Opin Biotechnol 35: 63-72.
- Vary PS and Johnson MJ (1967) Cell yields of bacteria grown on methane. Appl Microbiol 15: 1473–1478.
- Garcia-Ochoa F, Gomes E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol Adv 27: 153–176.
- Anthony, C (2011) How half a century of research was required to understand bacterial growth on C1 and C2 compounds; the story of the serine cycle and the ethyl malonyl-CoA pathway. Science progress 94: 109-137.
- Hanson RS and Hanson TE (1996) Methanotrophic bacteria. Microbiol Reviews 60: 439 - 471.
- Khmelenina VN, Rozova ON, But SY, Mustakhimov II, Reshetnikov AS, et al. (2015) Biosynthesis of Secondary Metabolites in Methanotrophs: Biochemical and Genetic Aspects (Review). Appl Biochem Microbiol 51: 150-158.
- Wilson JT and Wilson BH (1985) Biotransformation of trichloroethylene in soil. Appl Environ Microbiol 49: 242 - 243.
- Wendlandt KD, Jechorek M, Helm J (2001) Producing Poly-3-Hydroxybutyrate with a High Molecular Mass from Methane. J Biotechnol 86: 127-133.
- Auman AJ, Speake CC, Lidstrom ME (2011) nifH Sequences and Nitrogen Fixation in Type I and Type II Methanotrophs. Appl Environ Microbiol 67: 4009-4016.
- Strong PJ, Kalyuzhnaya M, Silverman J, Clarke WP (2016) A methanotroph-based biorefinery: Potential scenarios for generating multiple products from a single fermentation. Bioresour Technol 215: 314-323.
- Cerri MO, Esperança MN, Badino AC, Ribeiro MPA (2016) A new approach for KLa determination by gassing-out method in pneumatic bioreactors. J Chem Technol Biotechnol 91: 3061-3069.
- Yu Y, Ramsay JA, Ramsay BA (2006) On-line estimation of dissolved methane concentration during methanotrophic fermentation. Biotechnol Bioengineering 95: 788-793.
Citation: Fernandes RP, Simoes ACP, Heigl C, Modesto LFA, Pereira Jr N, et al. (2018) Cultivation of Methylocystis hirsuta in Bubble Columns Packed with Pall Rings Utilizing Methane as Carbon Source. J Biotechnol Biomater 8: 285. DOI: 10.4172/2155-952X.1000285
Copyright: © 2018 Pereira Jr N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3386
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 2577
- PDF downloads: 809