Review Article Open Access
Crustacean Molting: Regulation and Effects of Environmental Toxicants
Neelima Hosamani*, Srinivasa Reddy B and Ramachandra Reddy PDepartment of Biochemistry, Yogi Vemana University, Kadapa-516 003, AP, India
- Corresponding Author:
- Neelima Hosamani
Department of Biochemistry
Yogi Vemana University, Kadapa-516 003, India
Tel: 08562-225426
E-mail: neelimalife@gmail.com
Received Date: August 18, 2017; Accepted Date: September 06, 2017; Published Date: September 11, 2017
Citation: Hosamani N, Reddy SB, Reddy RP (2017) Crustacean Molting: Regulation and Effects of Environmental Toxicants. J Marine Sci Res Dev 7:236. doi:10.4172/2155-9910.1000236
Copyright: © 2017 Hosamani N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
In crustaceans the growth of the animal occurs by shedding of old exoskeleton and formation of new exoskeleton. Immediately after ecdysis the newly synthesized cuticle up takes water to expand new exoskeleton thereby size. Molt cycle in crustaceans is under the control of several regulatory hormones, internal and external factors. The predominant hormones molt inhibiting hormone (MIH) and ecdysteroids act in a controversy manner to one another in regulation of molt. It is also identified that the methyl farnesoate (MF) induces molting by inducing the synthesis and release of ecdysteroids from Y-organs. Besides several other hormones and internal molecules like opioids and neurotransmitters along with toxicants (xenobiotics, chemicals and metals) are also involved in the regulation of crustacean molting. Toxicity of aquatic pollutants leads to retardation of growth and delays molting, besides influence mortality and causes huge loss to crustacean farming. This review presents the advances in the field of crustacean molting and its regulation.
Keywords
Crustacean Hormones; Ecdysteroids; Methyl Farnesoate; Halloween Genes
Contents
• Introduction
• Endocrine regulation of Molting
• Inhibition of MIH on phantom (phm) gene expression in Y-organ
• Toxicity of chemicals that mediated endocrine disruption on growth
Introduction
Crustaceans especially crabs are rich in protein and also known as poor man's protein. Worldwide population was increasing year by year and feeding billion people through aquaculture. So crustacean rearing has become significant and developed culture methods. On the other hand crustacean industry has its own complications like availability of quality yield etc., in addition to lack of ways to enhance the growth of animal. In crabs the vegetative growth (somatic growth instead of reproductive) of the animal is regulated by special process called molting that is shedding of old exoskeleton and synthesizing of new exoskeleton, which is required for ever growing body size. In natural molt cycle animal is allowed to undergo molt and varies depends on species, season and is mediated by Ecdysteroids secreted from Y-organ (YO) [1]. Molting cycle takes nearly 120 days. In order to reduce the molt duration nowadays induced molting is practiced. One of the classical methods employed for induced molting is by eyestalk ablation-unilateral bilateral extirpation of eyestalk. This eyestalk ablation shut downs all the inhibitory hormones of molting and allows animal to molt.
An inhibitory hormone that regulates molt belongs to CHH family neuropeptides are synthesized from neurosecretory cells, located in medulla terminalis X-organ (XO) Sinus gland of eyestalk. Among the inhibitory hormones, Molt inhibiting hormones (MIH), a type II peptide secreted from XO sinus gland suppresses ecdysteroid synthesis by YO[2].
In addition to above limitations of molting, one of the most prevailing problem nowadays is contamination of harmful chemicals lead (Pb) copper (Cu), Zinc (Zn) etc. due to urbanization increased the use of these metals in various ways. Several publication evidences clearly state the chemicals released into water bodies. As it sediments in fatty tissues because of high lipophilicity of organ chlorine. Some of compound as polychlorinated biphenyls, DDT, HCB are observed to get incorporated in fatty tissues as observed in shore crab C. maenas. Heptachlor epoxide, dieldrin, endosulfan, chlorane, DDT and metabolites HCHs were found to accumulate in C. granulate [3].
Endocrine Regulation of Molting
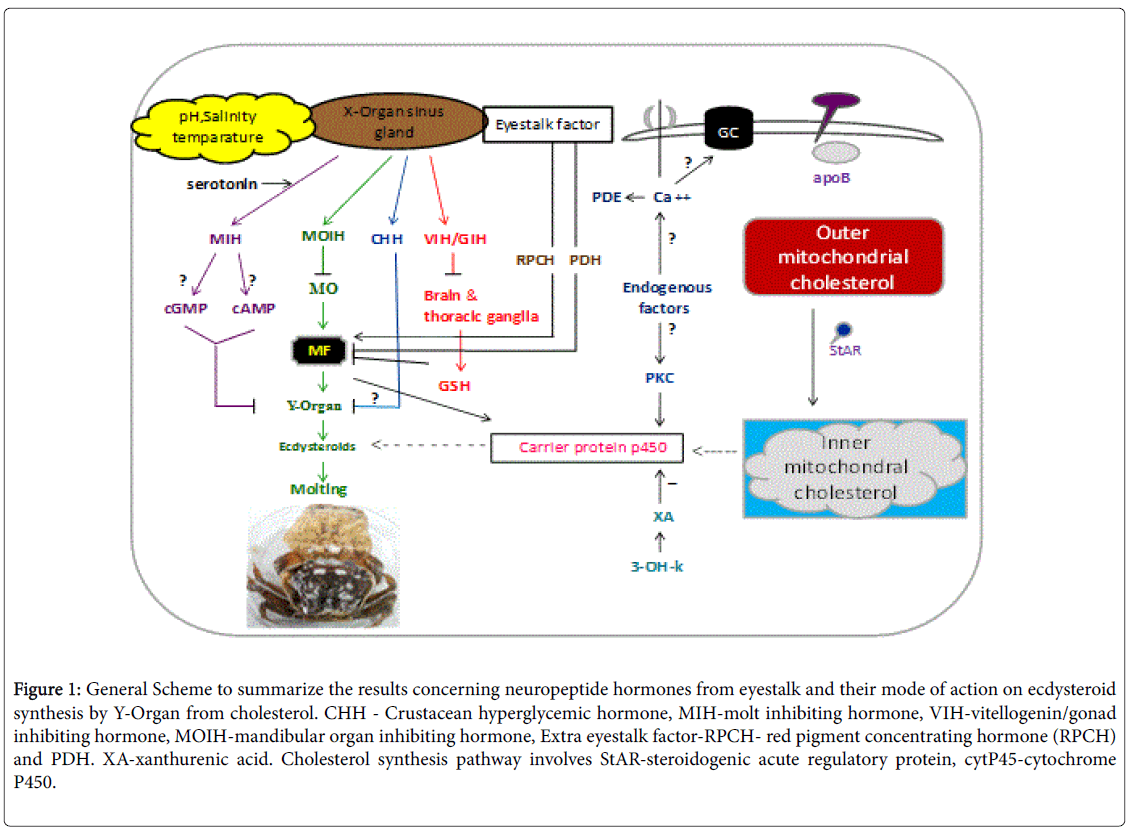
The neurosecretory system of the ES consists of a group of peptidergic neurons clustered in the medulla terminalis X-Organ (MTXO) and their bulbous axonic terminals that constitute the SG, which is a neurohemal organ that releases a number of peptide hormones into the hemolymph [4]. Molting in decapod crustaceans is controlled by the eyestalk X-organ/sinus gland complex, which secretes molt-inhibiting hormone (MIH), a neuropeptide that inhibits ecdysteroid production by a pair of Y-organs (YOs) located in the cephalothorax [5] and serves as the linkage between neurological signaling and steroidal control of processes such as molting and embryo development [6], CHH (crustacean hyperglycemic hormone) were identified from American lobster (H. americanus ) that contributed to regulating carbohydrate metabolism [7]. However, one hormone also contributed to the regulation of molting [7]; while, the other stimulated oocyte maturation [8]. MOIH (mandibular organ inhibiting hormone) negatively regulates the secretion of methyl farnesoate from the mandibular organ and its associated regulatory activities and GIH (Gonadal inhibiting hormone) also called vitellogenin-inhibiting hormone (VIH) negatively regulates aspects of gonadal maturation [9]. In addition to the above neuropeptides the XO sinus gland and the neurotransmitters like Xanthurenic acid, 3- hydroxy L-kynurenin and serotonin. Thus the process of molting is under the control of eyestalk peptide hormones secreted by XO sinus gland [1]. The involvement of eyestalk peptides and other molecules secreted by eyestalk on regulation of molting is described below as shown in Figure 1.
Figure 1: General Scheme to summarize the results concerning neuropeptide hormones from eyestalk and their mode of action on ecdysteroid synthesis by Y-Organ from cholesterol. CHH - Crustacean hyperglycemic hormone, MIH-molt inhibiting hormone, VIH-vitellogenin/gonad inhibiting hormone, MOIH-mandibular organ inhibiting hormone, Extra eyestalk factor-RPCH- red pigment concentrating hormone (RPCH) and PDH. XA-xanthurenic acid. Cholesterol synthesis pathway involves StAR-steroidogenic acute regulatory protein, cytP45-cytochrome P450.
Role of molt inhibiting hormone (MIH) on molting
The surgical extirpation of the eyestalk ablation results in a shortened molt cycle interval, while the implantation of the eyestalk ablation contents restores this interval. A factor has been implied that normally inhibits the molting process and it has been named the MIH. It belongs to class II peptide and separated into two subgroups A and B. Subgroup A acts physiologically as MIH, B regulates gonadal maturation in addition to molting in Callinectes sapidus [10]. Main function of MIH is to regulate ecdysteroid pathway by inhibiting conversion of ketodiol and 25 deoxyecdysone by binding to receptors on epidermis of YO [5]. MIH induces an increase in cAMP and cGMP, with subsequent activation of protein kinases in YOs in vitro; a small transient increase in cAMP may precede a larger sustained increase in cGMP [11]. It mediates inhibition of ecdysteroids from YO by binding to receptor guanylyl cyclase cGMP [12,13]. The effect of the aptly named MIH on YOs has been investigated in many decapod species, including the European shore (green) crab (Carcinus maenas), the blackback land crab (Gecarcinus lateralis), and the South African spiny lobster [14-16]. MIH levels alter on molt, significantly low on premolt and rises during post and intermolt stages.
Responsiveness of YO to MIH differs through molt stage. Whereas, Ca++ increase during premolt and enhance the activation of two intra cellular enzymes, protein kinase C and phosphodiesterase (PDE). Both PKC and PDE stimulate the increase of ecdysteroid levels on premolt stage. But this is reversed on incubation of YO with inhibitor of PKC and PDE IBMX which authentically supress ecdysteroid secretion and drops their levels at postmolt stage and intermolt stage [17]. But this is reversed on addition of Calcium ionosphere (A23187).
Role of Ecdysteroids on molting
Crustaceans appear to have the same enzymes for ecdysteroid biosynthesis as insects. An inhibitor of steroid 5-reductase, L-645390, blocks the conversion of cholesterol to 7DC in the YO of M. mercenaria [18]. The 5-reductase that converts 4-diketol to 5-diketol is a cytosolic enzyme in YO cells that requires NADPH for activity [19]. Orthologs of nvd, nmg/sro, spo, phm, dib, sad, and shd have been identified in the Daphnia pulex genome [20-22], and a cDNA encoding Phm has been cloned from Kuruma prawn, Marsupenaeus japonicus [23]. Nvd and spo are located adjacent to each other in the D. pulex genome [20]. The M. japonicus Phm and D. pulex Phm have five conserved motifs present in insect Phm (WxxxR, GxE/DTT/S, ExxR, PxxFxPE/DRF, and PFxxGxRxCxG/A heme-binding motif), five of six substrate recognition sites (SRS 1, 2/3, 4, and 5), and N-terminal ER-targeting and Pro/Gly-rich sequences [23]. Mj-Phm is a target of eyestalk neuropeptides, as its expression in the YO is increased as much as 7-fold during premolt and is decreased about 2.5-fold by sinus gland extract and recombinant MIH [23]. None of the crustacean Halloween enzymes have been characterized biochemically.
A member of the clade 4 of cytochrome P450 enzymes has been cloned from O. limosus (CYP4C15) and C. maenas (CYP4C39) [24,25]. Both genes encode proteins with hydrophobic N-terminal ERtargeting and Pro/Gly-rich sequences characteristic of microsomal cytochrome P450 enzymes [24,25]. CYP4C15 is expressed in the YO and the protein is associated with the ER [24,26]. Moreover, CYP4C15 expression in the YO is increased during premolt and is decreased by MIH [24,26]. These data suggest that they are involved in ecdysteroid biosynthesis.
Major ecdysteroids identified in crustaceans are of ecdysone (E), 20-OH-E (20-hydroxyecdysone), PoA (PonasteroneA), 25dE (25- deoxyecdysone), 3dE (3-deoxyecdysone). 25dE is the precursor of PoA. Ecdysteroids varies through molt stages. Experimental evidence shows that in eyestalk ablated animals levels of 20-OH-E is the major ecdysteroid present. At early premolt stage the ratio of ecdysone levels are high compare to 20-OH-E, than to late premolt stage. At late premolt in the hemolymph the titers of PoA decline more rapidly than those of ecdysone and 20-OH-E. During postmolt and intermolt stages 20-OH-E and PoA level increases. Between premolt and intermolt YO also secrete ecdysone and 25dE a precursor to PoA. PoA is an active molting hormone can be seen throughout all the three stages on additional to 20-OH-E.
Role of methyl farnesoate (MF) on molting
Methyl farnesoate (MF) is a sesquiterpenoid compound found in decapod crustaceans, and is structurally similar to the juvenile hormone (JH) of insects. However, MF differs from juvenile hormone (JH III) in containing an epoxide moiety at the terminal end. Crustaceans appear to lack epoxidase and S-adenosyl-methioninedependent methyltransferase, which convert farnesoic acid (FA) to JH III [27]. Therefore, crustaceans lack JH III, and MF is the end product of sesquiterpenoid biosynthesis. Farnesoic acid rather than MF is the secretory product from mandibular organ (MO) immediately converted in to MF by the action of enzyme farnesoic acid O-methyl transferase (FAOMeT) and is under negative control of MOIH derive from XO sinus gland complex at the terminal end of the eyestalk [28]. The MOIH peptide hormone suppresses the production of MF. In insects, JH III is the major hormone related to metamorphosis, gonad maturation, and molting [29,30].
In M. rosenbergii the levels of MF rise during premolt stage and decline during postmolt stage [31] and also accelerates molt in P. clarkii [31] and crab O. senex senex [32]. Olmstead and Leblanc [33] found antagonist of JH as methoprene decreases molt frequency in D. magna. This clearly indicates that MF modulates ecdysteroids.
Role of crustacean hyperglycemic hormone (CHH) on molting
Another eyestalk neuropeptide with MIH activity is the crustacean hyperglycemic hormone (CHH), which is so named for its role in elevating glucose levels in the hemolymph [34]. CHH may inhibit molting in response to certain environmental stresses reviewed [35]. The CHHs are the most abundant neuropeptides in the SG. Their central role on the regulation of carbohydrate metabolism has been reviewed [36]. CHH family peptides are the pleotropic hormones with multifunctional and involves in biological activities like blood glucose regulation, molting and inhibition of methyl farnesoate synthesis, lipid metabolism regulation, vitellogenin and ovarian maturation. CHH amino acid sequence was first determined in species like shore crab C. maenas. As it is separated into two subgroups A and B. Both the isoforms A and B have a hyperglycemic effect, in additional to that A subgroup possess molt inhibiting activity and B subgroup stimulate oocyte growth [37]. Both their values vary according to molt and reproductive cycle. In the species like C. maenas CHH shows sequence homology with MIH. This confirms that CHH has inhibitory effect on ecdysteroidogenesis even though 20 times slower than MIH. Binding of CHH to the specific receptor guanylyl cyclase II (GC II) on the membrane of YO elevated the levels of cGMP and inhibits regulation of ecdysteroidogenesis [38].
Role of Vitellogenesis/Gonad inhibiting hormone (VIH) on molting
In crustacean females, the late phase of gonadal maturation to form mature ova is called vitellogenesis. Usually the inhibitory role of GIH on vitellogenesis is observed in females, at the same time the occurrence of GIH in males is also recorded and this provides the additional role of GIH in males, assumed to be regulating molt [39]. Structural similarities among GIH and MIH in accordance to number of cysteine residues, their location provide the existence of homogenesity or similarity [40]. Thus similarity between GIH and MIH support the hypothesis phenomena of involvement of VIH in molting. GIH might have a molt-inhibiting function because female lobsters molt only after hatching of their larvae when the CHH and GIH hemolymph levels are low in H. americanus [41].
Molting and reproduction are complex hormone mediated process, and are also regulated by several other internal and external factors [42]. The similarity of VIH, GIH with that of MIH confers the involvement of induction of molting at previtellogenic stage in C. quadricarinatus [43].
Role of opioids on molting
Mancillas et al. [44] first declared the presence of opioid as small peptides in various species like spiny lobster Panulirus interruptus, red swamp, and in crayfish P. clarkia by using various techniques like immunohistochemistry, RIA, HPLC. Fingerman et al. [45] reported the presence of two types of peptides methionine-enkephalin and leucineenkephalin from neuroendocrine complex of eyestalk of fiddler crab U. pugilator. Later, based on experimental evidences proved the role of opioids like methionine-enkephalin as a neurotrasmitter that regulates the secretion of hyperglycemic hormone in fiddler crab U. pugilator , movement of red pigment molecules that regulates chromatophores and on ovarian maturation in U. pugilator . Recently confirmed that opioids, leucine-enkephalin have another crucial role on regulation of molting in the fresh water crab O. senex senex [46]. Complete descriptions of opioids were represented in review [47].
Influence of other eyestalk factors
Role of Xanthurenic acid, 3-OH-K on molting: Biogenic amines and peptide neuroregulators are known to modulate the release of some neuropeptide hormones from the SG [48]. These XA and 3-OH-K are also secreted by XO sinus gland and fully identified by MS and NMR and their structures. Among them the 3-OH-K is the circulating form and which converts into an active XA form by the action of an enzyme amino transferase [49]. Aminotransferases present in hemolymph, Eyestalk and YO. The inhibitory action of both XA and 3-OH-K is studied to mediate through binding their respective receptors present on the YO [49]. There by suppress the secretion of Ecdysteroids from YO. By interfering with the synthetic co enzymes like Cytochrome C (Cyt C) and Cytochrome P450 (CytP450) at the site of iron porphyrin [50]. But the supressive action of XA, 3-OH-K is studied to differ from species to species and also in stage specific [51].
Role of serotonin on molting: Serotonin (5-hydroxytryptamine or 5HT) is a neurotransmitter secreted by XO sinus gland of Eyestalk. The released serotonin is studied to stimulate the release of molt inhibiting hormone from eyestalk, thus have inhibitory role on molting. The inhibitory role of serotonin is by stimulating the release of variable hormones of molting such as PDH, CHH, GSH, MIH [52] and Neuro depressing hormone. On the other hand it is having suppressive role on MF secretion at the same time the progression of ovarian maturation by serotonin was observed in P. clarkia, white pacific shrimp L. vannamei [53] and tiger shrimp P. semisulcatus [54]. Further the suppressive role of serotonin on growth was evidenced by where the shrimp fed with Mannon oligosacharide 3 g kg-1 showed progressed molting compared with that of shrimp fed with serotonin. This supports the inhibitory role of serotonin on molting. But more recently Sainath and Reddy [55] stated that serotonin has no effect on molting in O. senex senex. Thus in depth and clarification studies are required in this direction.
MIH Inhibition of Phantom (Phm) Gene Expression on YO
Though it is well known that the process of molting is regulated by MIH, unfortunately the exact mechanism of action at gene expression level of MIH is unknown. Limited results persist regarding the involvement of Halloween genes and their regulation in molting [23]. YO ecdysteroid synthetic pathway suggest that the P450 monooxygenases, encoded by the Halloween genes Phantom (phm), Disembodied (dib), Shadow (sad), and Shade (shd) can bind multiple substrates. Phm apparently can hydroxylate 5β-diketol or 5β-ketodiol at C25; Dib apparently can hydroxylate 5β-diketol, 3D2, 22dE, 5β- ketodiol, or 5β-ketotriol at C22; Sad apparently can hydroxylate 3D2dE, 2,25dE, or 2dE at C2; and Shd apparently can hydroxylate 25dE, 3DE, or ecdysone at C20. However, the specificities of the Phm, Dib, Sad, and Shd enzymes are such that the C25→C22→C2→C20 order of hydroxylations is maintained [56]. These Halloween genes are studied to express in various parts of body like prothoracic gland, fat body, midgut etc. [57]. Unlike in crustaceans, in insects the prothoracic gland hormone known as prothorasicotropic hormone (PTTH) is studied for its positive regulation of molting [58]. Further the identification of Halloween gene orthologues in Daphnia ecdysteroidogenic pathway [21] represents the involvement of Halloween genes in molting of crustaceans. Based on the above studies the involvement of phm genes (Member of Halloween gene family) in molting and their expression levels at different molt stages were studied and were observed to regulate in stage specific manner [23]. The expression of phm was confined to YO at all stages of molting though minor expression was observed in ovaries at mature stages which was the basis for selecting the phm gene expression as the limitation in the above studies. The results clearly proposed that the levels of phm expression were high during pre-molt stages and expression was suppressed during the intermolt stages, which was due to the inhibitory action of MIH mediated by binding to the receptors on YO [23]. On the other hand the expression of phm in insects was observed in ovary also suggesting that on degradation of pro thoracic gland during metamorphosis the ovary adopts the function of ecdysteroidogenesis [59]. In addition to the difference in the expression levels of phm gene the levels of Cyp4c15 (type of cytochrome P450) were also regulated though the role of Cyp4c15 in molting was not known. This provides an idea that the MIH suppresses the molting by affecting the CYP gene expression in Y-organs. The role of Halloween genes in insect molting is mediated by initiating the transcription factor βFTZ-F1 observed in Drosophila [60] Similarly though the Orthologues of EcR, USP, FTZ-F1 were found in crustaceans but their possible contribution in molting is not clear and which needs further research in this direction [61].
Toxicity of Chemicals that Mediated Endocrine Disruption on Growth
Ecdysteroids regulate aspects of embryo development, growth (molting), and reproduction (perhaps vitellogenin synthesis). Accordingly, chemicals that interfere with ecdysteroid signaling have the potential to elicit profound adverse effects on crustacean populations. Chemicals with anti-ecdysteroidal activity in crustaceans have been identified that function as either ecdysteroid synthesis inhibitors or ecdysteroid receptor antagonists. Chemicals with antiecdysteroidal activity include many of the classic estrogen receptor agonists of vertebrate. However, studies with ecdysteroid-responsive insect cells have demonstrated that non-steroidal EcR agonists are rare [62]. The binding of an environmental chemical to the EcR will more likely result in inhibition of ecdysteroid signaling.
Testosterone exposure causes abnormal embryo development of daphnids similar to that observed with fenarimol [63]. Administration of exogenous 20-hydroxyecdysone protected embryos against this toxicity of testosterone indicating that testosterone interfered with normal ecdysteroid signaling. Additional studies indicated that testosterone elicited anti-ecdysteroidal activity by inhibiting the EcR [64]. The binding of an environmental chemical to the EcR will more likely result in inhibition of ecdysteroid signaling.
Among chemicals shown to elicit 20-hydroxyecdysone-like activity in crustaceans are ponasterone A and RH 5849. Ponasterone A is a steroid that was first isolated from plants that has high-ecdysteroid activity in insects [65]. Exposure of D. magna to ponasterone A stimulated premature ecdysis [66]. RH 5849 accelerated molting; and, in the barnacle B. amphitrite, RH 5849 enhanced attachment and metamorphosis of the larvae [67]. Recent development of a crustacean EcR reporter gene construct may stimulate screening efforts aimed at identifying chemicals that harbor this activity [68]. Several studies have reported effects of environmental chemicals that are consistent with interference with ecdysteroid signaling, though a precise mechanism of action was not established. The chemicals 4- nonylphenol [69], propiconazole [70], and bisphenol A [71], have elicited effects in crustaceans consistent with anti-ecdysteroidal activity. The fungicides propiconazole [72] and fenarimol [73] inhibit cytochromeP450 (CYP) enzymes that are critical to ecdysteroid synthesis. Both of these chemicals may inhibit ecdysteroidsynthesis through this enzyme inhibition. The 4-Nonylphenol is an antagonist of the insect ecdysteroidreceptor in vitro [62]. Bisphenol A was proposed to elicit anti-ecdysteroidal activity through a receptor cross-talk mechanism [71,74]. Thus, it is mechanistically plausible that all of these compounds elicit toxicity to crustaceans via perturbations in ecdysteroid signaling. Relatively few studies have been performed that evaluated perturbations in ecdysteroid signaling in crustaceans by xenobiotics.
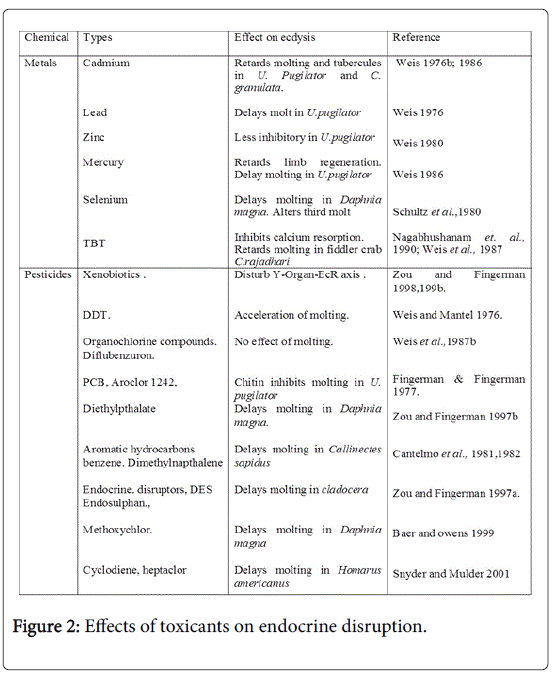
Axenobioticis a foreign chemical substance found within an organism that is not normally, naturally produced by or expected to be present within that organism and which affects the organism. In this regard the crustacean industry nowadays facing many problems due to increasing urbanization day by day i.e. contamination of water bodies by harmful pesticides, metals, plastic and sewage, from agriculture and industrial activity which are getting assimilated and affecting the normal physiological process of aquatic organism as shown in (Figure 2). Various estrogenic compounds such as Arochlor 1242, PcB29, DES, endosulphan, diethyl phthalate and are detected in the fatty tissues and are studied to affect molting [75]. It is found to effect or delay molting either by disrupting the synthetic pathway of chitin of exoskeleton by degrading the enzyme chitobiase (N-acetyl-β-glucosaminidase) or by disturbing the ecdysteroid receptor axis (EcR) which heterodimerize with crustacean retinoid X receptor (RXR) [76] an central event in molting.
Adverse effects of some of Pesticides such as Organochlorine compound DDT, PCB, HCHs on molting were studied and found to accumulate in tissues of burrowing crabs C. granulata [77]. On the other hand no effect on molting on exposure of carbamate, Malathion and parathion and progressive molting on exposure to DDT was studies in U. pugilator .
Diflubenzuron (Dimilin) a chitin inhibitor was found to increase mortality near ecdysis on exposure to higher concentrations [78]. Baer and owens [79] investigated that Aroclor 1242, 2,4,5-trichlorobiphenyl (PCB29), diethylpthalate and Methoxyclor decreases chitobibiase activity at epidermis and results in inhibition of molting in D. magna (Snyder and Mulder, 2001). Delay in molting by various pesticides Heptachlor in H. americanus [80] and dioxins, dibenzofurans, benzene and dimethylnapthalene in C. sapidus are also reported. Similarly Feeding with 2,3,7,8-TCDD dioxin found to retards regeneration and molting in C. sapidus. Some endocrine modulators, estrogenic agents like DES and endosulfan are observed to delay the molting in Cladoceran. Diet containing sodium pentachlorophenate, 2,4,5- trichlorophenol or 2,4,6-trichlorophenol was found to retards limb regeneration, in Palaemonetes pugio but does not alters molt cycle [81].
Though metals such as sodium, potassium, calcium and magnesium are required for normal physiological functions of organism some heavy metals like cadmiun, zinc, mercury, manganese, chromium, cobalt, nickel and selenium are very toxic to flora and fauna. Heavy metals are observed to interfere with the biochemical events involved in physiological process. These heavy metals interfere with hormones and manipulate their release and thus affect the physiological events like molting, limb regeneration, blood glucose levels and reproduction. In crayfish Astacus leptodactylus exposure to cadmium caused impairment of nuclear pycnosis, mitochondrial dis organization, abnormal development and collapse of Golgi vesicles and fragmentation of endoplasmic reticulum by accumulating in central nervous system, thus affecting the normal physiological metabolism [82]. On exposure of cadmium results delayed molt in eyestalk ablation C. granulata [83] and P. clarkii [84]. On feeding 10 ppm cadmium for 10 days caused damage to neurosecretory cells in brain eyestalk ganglia and also observed that males developed resistance when compared to females [85]. Cadmium when fed in combination with lead and mercury got accumulated in the brain and inhibited central nervous system, sensory ganglia, and sulphydryl group containing enzymes in crayfish P. clarki . Zinc is also observed to have profuse effect on limb generation when combined with methyl mercury than alone and in combination with cadmium.
In addition to the effects of cadmium and zinc other heavy metals like Selenium delays molting in Daphnia magna, arsenic (in the form of CCA: (chromated copper arsenate) retarded regeneration in U. pugilator in a dose dependant manner, chromium affects the neurosecretory cells in brain and thoracic ganglian of the shrimp, Penaeus monodon and Lead, retards limb regeneration and molting of U. pugilator are also reported.
Organometallic compound Tributyltin (TBT) extensively used in antifouling paints also retards molting and produces abnormalities in regenerates. It gets interfered in the calcium reabsorption, an essential event in the molting and inhibits the exoskeleton formation as observed in C. rajadhari [86]. Reddy et al. [87] found the impact of TBT at initial and final stages of limb regeneration in freshwater prawn C .rajadhari. Further Reddy et al. [88] proposed that low dosage of TBT will not show any effect on first two molts, it shows significant changes after third molt.
Acknowledgement
The authors combinely thank the University Grants Commission (UGC) for providing financial assistance in the form of project F.No 41-582/2012(SR) sanctioned to Dr. P. Ramachandra Reddy.
References
- Neelima H, Ramachandra Reddy P, Sreenivasula Reddy P (2016) Natural and Induced (Eyestalk Ablation) Molt Cycle in Freshwater Rice Field Crab Oziothelphusa senex senex. J. Aquac Res Development 7: 4.
- Sirinart Techo, J Sook chung (2015) Ecdysteroid Regulate the levels of Moult inhibiting hormone expression in the Blue crab, Callinectes sapidus. PLOS One 10: e0117278.
- Menone ML, Bortulos A, Botto F, Aizpun de Moreno JE, Moreno VJ, et al. (2000) Organochlorine contaminants in a coastal lagoon in Argentina: an Analysis of sediment, crabs, and cordgrass from two different habitats. Estuaries 23: 583-592.
- Skinner D (1985) Molting and regeneration. In: Bliss DE, Mantel LH. (Eds.), The Biology of the Crustacea, Academic Press, New York 9: 43-146.
- Gäde G, Marco HG (2006) Structure, function, and mode of action of select arthropod neuropeptides. Stud Nat Prod Chem. 33:69-140.
- Chang ES (1985) Hormonal control of molting in decapod crustacea. Am Zool 25:179-185.
- Chang ES, Prestwich GD, Bruce MJ (1990) Amino acid sequence of a peptide with both molt-inhibiting and hyperglycemic activities in the lobster, Homarus americanus. Biochem Biophys Res Commun 171: 818-826.
- Tensen CP, Janssen KPC, Van Herp R (1989) Isolation, characterization and physiological specificity of the crustacean hyperglycemic factors from the sinus gland of the lobster Homarus americanus (Milne-Edwards). Invert Reprod Dev 16: 155-164.
- Huberman A (2000) Shrimp endocrinology. A review. Aquaculture 2000: 191-208.
- Zmora N, Sagi A, Zohar Y, Chung JS (2009) Molt-inhibiting hormone stimulates vitellogenesis at advanced ovarian developmental stages in the female blue crab, Callinectes sapidus 2: novel specific binding sites in hepatopancreas and cAMP as a second messenger. Saline Systems 5: 6.
- Covi JA, Chang ES, Mykles DL (2009) Conserved role of cyclic nucleotides in the regulation of ecdysteroidogenesis by the crustacean molting gland. Comp Biochem Physiol. 152: 470-477.
- Sajal Shrivastava and Adline Princy S (2014) Crustacean hyperglycemic hormone family Neurohormones: A prevailing tool to decipher the physiology of crustaceans. Indian journal of Geo- Marine sciences. 43: 434-440.
- Covi J, Gomez A, Chang S, Lee K, Chang E, et al. (2008) Repression of Y-organ ecdysteroidogenesis by cyclic nucleotides and agonists of NO-sensitive guanylyl cyclase. In: Morris S, Vosloo A, editors. Fourth Meeting of Comparative Physiologists and Biochemists in Africa Mara 2008“Molecules to Migration: The Pressures of Life. Bologna: Monduzzi Editore International. 37-46.
- Lachaise A, Le Roux A, Hubert M, Lafont R (1993) The molting gland of crustaceans: localization, activity, and endocrine control (a review) J Crustac Biol 13:198-234.
- Marco HG, Stoeva S, Voelter W, Gäde G (2000) Characterization and sequence elucidation of a novel peptide with molt-inhibiting activity from the South African spiny lobster, Jasus lalandii. Peptides 21: 1313-1321.
- Lee KJ, Kim HW, Gomez AM, Chang ES, Covi JA, et al (2007a) Molt-inhibiting hormone from the tropical land crab, Gecarcinus lateralis: cloning, tissue expression, and expression of biologically active recombinant peptide in yeast. Gen Comp Endocrinol 150: 505-513.
- Spaziani E, Jegla TC, Wang WL, Booth JA, Connolly SM, et al. (2001) Further studies on signaling pathways for ecdysteroidogenesis in crustacean Y-organs. Am Zool 41: 418-429.
- Spaziani E, Wang WL (1993) Biosynthesis of ecdysteroid hormones by crustacean Y-organs: conversion of cholesterol to 7-dehydrocholesterol is suppressed by a steroid 5-reductase inhibitor Mol Cell Endocrinol 95: 111-114.
- Blais C, Dauphin-Villemant C, Kovganko N, Girault JP, Descoins C, et al. (1996) Evidence for the involvement of 3-oxo-4 intermediates in ecdysteroid biosynthesis. Biochem J 320: 413-419.
- Niwa R, Namiki T, Ito K, Shimada-Niwa Y, Kiuchi M, et al. (2010) Non-molting glossy/shroud encodes a short-chain dehydrogenase/ reductase that functions in the “Black Box” of the ecdysteroid biosynthesis pathway. Development 137: 1991-1999.
- Rewitz KF, Gilbert LI (2008) Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evol Biol 8: 60.
- Markov GV, Tavares R, Dauphin-Villemant C, Demeneix BA, Baker ME, et al. (2009) Independent elaboration of steroid hormone signaling pathways in metazoans. Proc Natl Acad Sci 106: 11913-11918.
- Asazuma H, Nagata S, Nagasawa H (2009) Inhibitory effect of molt-inhibiting hormone on Phantom expression in the Y-organ of the kuruma prawn, Marsupenaeus japonicus, Arch. Insect Biochem. Physiol 72: 220-233.
- Dauphin-Villemant C, Böcking D, Tom M, Maibeche M, Lafont R (1999) Cloning of a novel cytochrome P450 (CYP4C15) differentially expressed in the steroidogenic glands of an arthropod, Biochem. Biophys. Res. Comm. 264 : 413-418.
- Rewitz K, Styrishave B, Andersen O (2003) CYP330A1 and CYP4C39 enzymes in the shore crab Carcinus maenas: sequence and expression regulation by ecdysteroids and xenobiotics, Biochem. Biophys. Res. Comm. 310: 252-260.
- Aragon S, Claudinot S, Blais C, Maibeche M, Dauphin-Villemant C (2002) Molting cycle-dependent expression of CYP4C15, a cytochrome P450 enzyme putatively involved in ecdysteroidogenesis in the crayfish, Orconectes limosus. Insect Biochem. Mol. Biol. 32: 153-159.
- Hui JHL, Hayward A, Bendena WG, Takahashi T, Tobe SS (2010) Evolution and functional divergence of enzymes involved in sesquiterpenoid hormone biosynthesis in crustaceans and insects. Peptides 31: 451-455.
- Nagaraju GPC (2007) Is methyl farnesoate a crustacean hormone. Aquaculture 272: 39-54.
- Bellés X, Martin D , Piulachs MD (2005) The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol 50: 181-199.
- Tsubota T, Minakuchi C, Nakakura T, Shinoda T, Shiotsuki T (2010) Molecular characterization of a gene encoding juvenile hormone esterase in the red flour beetle, Tribolium castaneum. Insect Mol Biol 19: 527-535.
- Laufer H, Demir N, Pan X, Stuart JD, Ahl JSB (2005) Methyl farnesoate controls adult male morphogenesis in the crayfish (Procambarus clarkii). J Insect Physiol 51:379-384.
- Reddy PR, Nagaraju GPC, Reddy PS (2004) Involvement of methyl farnesoate in the regulation of molting and reproduction in the freshwater crab Oziotelphusa senex senex. J Crust Biol 24: 511-515.
- Ohmstead AW, Leblanc GA (2001) Low exposure concentration effects of methoprene on endocrine regulated process in the crustacean Daphnia magna. Toxical Sci 62 :268-273.
- Zarubin TP, Chang ES, Mykles DL (2009) Expression of recombinant eyestalk crustacean hyperglycemic hormone from the tropical land crab, Gecarcinus lateralis, that inhibits Y-organ ecdysteroidogenesis in vitro. Mol Biol Rep. 36: 1231-1237.
- Chang ES (2005) Stressed-out lobsters: crustacean hyperglycemic hormone and stress proteins. Integr Comp Biol 45:43-50.
- Keller R, Sedlmeier D (1998) A metabolic hormone in crustaceans: the hyperglycemic neuropeptide. In: Läufer, H., Downer, R.G.H. (Eds.), Endocrinology of selected invertebrate types vol. II A.R. Liss, New York 315-326.
- Chang ES (1991) Crustacean molting hormones, cellular effects, role in reproduction and regulation by molt inhibiting hormone. In: DeLoach PF, DoughertyWJ, DavidsonMA (editors).Frontiers of Shrimp Research Amsterdam: Elsevier Science 83-105.
- Ernest S, Chang, Donald L Mykles (2011) Regulation of crustacean molting: A review and our perspectives. General and Comparative Endocrinology 172: 323-330.
- De Kleijn DPV, Coenen T, Laverdure AM, Tensen CP, Van Herp F (1992) Localization of messenger RNAs encoding crustacean hyperglycemic hormone and gonad inhibiting hormone in the X-organ sinus gland complex of the lobster Homarus americanus. Neuroscience 51: 121-128.
- Swetha CH, Sainath SB, Reddy PR , Reddy PS (2011) Reproductive Endocrinology of Female Crustaceans: Perspective and Prospective. J. Marine Sci. Res. Development 11-13.
- De Kleijn DP, Janssen KP, Waddy SL, Hegeman R, Lie W (1998) Expression of the crustacean hyperglycemic hormones and the gonad inhibiting hormone during the reproductive cycle of the female American lobster Homarus americanus. Journal of Endocrinology 156: 291-298.
- Srinivasa Reddy, sridevi vaadala, Neelima Hosamani (2016) Regulation of vitellogenesis by selected endocrine modulators in crab Oziothelphusa senex senex. With special reference to Methyl farnesotae. Aquaculture Reports 3: 24-30.
- Joly Ghanawi, Patrick Saoud I (2014) Molting, reproductive biology, and hatchery management of redclaw crayfish Cherax quadricarinatus (von Martens 1868) Aquaculture volume 358-359: 183-195
- Mancillas JR, Ginty JF, Selverston AI, Karten H, Bloom FE (1981) Immunocytochemical localization of enkephalin and substance P in retina and eyestalk neurones of lobster. Nature 293: 576-578.
- Fingerman M, Hanumante M, Kulkarni G, Ikeda R, Vacca L (1985) Localization of substance P-like, leucine-enkephalin-like, methionine-enkephalin-like, and FMRF amide-like immunoreactivity in the eyestalk of the fiddler crab, Uca pugilator, Cell. Tissue Res 241: 473-477.
- Kishori B, Sreenivasula reddy P (2003) Influence of leucine enkephalin on Moulting and vitellogenesis in the freshwater crab Oziotelphusa senex senex. Brill 76: 1281-1290.
- Nagabhushanam R, Sarojini R, Reddy RS, Fingerman M (1995) Opioid peptides in invertebrates: Localization, distribution and possible functional roles. Curr Sci 69: 659-671.
- Lüschen WS, Willig A, Jaros PP (1993) The role of biogenic amines in the control of blood glucose level in the decapod crustacean, Carcinus maenas L. Comp Biochem Physiol C 105: 291-296
- Naya Y, Ohnishi M, Ikeda M, Nakanishi K, Miki W (1989) What is molt-inhibiting hormone? The role of an ecdysteroidogenesis inhibitor in the crustacean molting cycle. Proceedings of the National Academy of Sciences 86: 6826-6829.
- Ohnishi M, Nakanishi K, Naya Y (1991) Down-regulation of ecdysteroidogenesis: a physiological dynamics of prothoracic and Y-glands. Chimicaoggi 9: 53-56.
- Naya Y, Kishida MK, Sugiyma M, Murata M, Miki W, et al. (1988) Endogenous inhibitor of ecdysone synthesis in crabs. Experientia 44: 50-52.
- Mattson MP, Spaziani E (1985) Characterization of molt inhibiting hormone (MIH) action on crustacean Y-organ segments and dispersed cells in culture and a bioassay for MIH activity. Journal of Experimental Zoology 236: 93-101.
- Vaca AA, Alfaro J (2000) Ovarian maturation and spawning in the white shrimp, Penaeus vannamei, by serotonin Injection. Aquaculture 182: 373-385.
- Aktas M, Kumulu M (2005) Gonadal maturation and spawning in Penaeus semisulcatus Hann, 1844 by hormone injection. Turkish. Journal of Zoology. 29: 193-199.
- Sainath SB, Reddy PS (2011) Effects of selected biogenic amines on reproduction in the freshwater edible crab, Oziotelphusa senex senex. Aquaculture. 13: 144-148.
- Truman JW (2005) Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitam Horm 73: 1-30.
- Rewitz KFR, Rybczynski JT, Warren, Gilbert LI (2006) Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 36:188-199.
- Rybczynski (2005) Prothoracicotropic hormone. In: Gilbert L.I., Iatrou,K., Gill, S., editors. Comprehensive molecular insect science, Vol.3.Oxford: Elsevier. P. 61-123.
- Brown MRD, Sieglaff H, Rees HH (2008) Gonadal ecdysteroidogenesis in Arthropoda: occurrence and regulation. Annu. Rev Entomol 54: 105-125.
- Parvy JP, Blais C, Bernard F, Warren JT, Petryk A, et al. (2005) A role for bFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Dev Biol 282: 84-94.
- Mykles DL (2011) Ecdysteroid metabolism in crustaceans. J Steroid Biochem Mol Biol 127: 196-203.
- Dinan L, Bourne P, Whiting P, Dhadialla TS, Hutchinson TH (2001) Screening of environmental contaminants for ecdysteroid agonist and antagonist activity using the Drosophila melanogaster B11 cell in vitro assay. Environ Toxicol Chem 20: 2038-2046.
- LeBlanc GA, Mu X, Rider CV (2000) Embryotoxicity of the alkylphenol degradation product 4-nonylphenol to the crustacean Daphnia magna. Environ Health Perspect 108:1133-1138.
- Mu X, LeBlanc GA (2004b) Synergistic interaction of endocrine disrupting chemicals: model development using an ecdysone receptor antagonist and a hormone synthesis inhibitor. Environ Toxicol Chem 23:1085-1091.
- Nakanishi K, Koreeda M, Saski S, Chang ML, Hsu HY (1966) Insect hormones. The structure of ponasterone A, and insect-moulting hormone from the leaves of the Podocarpus nakaii hay. Chem Commun 24:915-917.
- Baldwin WS, Bailey R, Long KE, Klaine S (2001) Incomplete ecdysis is an indicator of ecdysteroid exposure in Daphnia magna. Environ Toxcol Chem 20: 1564-1569.
- Clare AS, Rittschof D, Costlow JD (1992) Effects of the nonsteroidal ecdysone mimic RH 5849 on larval crustaceans. J Exp Zool 262: 436-440
- Yokota H, Sudo Y, Yakabe Y (2005) Development of an in vitro binding assay with ecdysone receptor of mysid shrimp. In SETAC Annual Meeting Proceed.
- LeBlanc GA, Mu X, Rider CV (2000) Embryotoxicity of the alkylphenol degradation product 4-nonylphenol to the crustacean Daphnia magna. Environ Health Perspect 108:1133-1138.
- Kast-Hutcheson K, Rider CV, LeBlanc GA (2001) The fungicide propiconazole interferes with embryonic development of the crustacean Daphnia magna. Environ Toxicol Chem 20:502-509
- Mu X, Rider CV, Hwang GP, Hoy H, LeBlanc GA (2005) Covert signal disruption: anti-ecdysteroidal activity of bisphenol A involves cross-talk between signaling pathways. Environ Toxicol Chem 24:146-152
- Ronis MJJ, Celander M, Badger TM (1998) Cytochrome P450 enzymes in the kidney of the bobwhite quail (Colinus virginianus): induction and inhibition by ergosterol biosynthesis inhibiting fungicides. Comp Biochem Physiol 121: 221-229.
- Williams DR, Fisher MJ, Rees HH (2000) Characterization of ecysteroid 26-hydroxylase: an enzyme involved in molting hormone inactivation. Arch Biochem Biophys 376: 389-398.
- Mu X, LeBlanc GA (2004a) Cross communication between signaling pathways: juvenoid hormones modulate ecdysteroid activity in a crustacean. J Exp Zool 301A:793-801.
- Zou E, Fingerman M (1997a) Synthetic estrogenic agents donot interfere with sex determination but do inhibit molting of a cladoceran, Daphnia magna. Bull Environ Contam Toxicol 58: 596-602.
- Chung ACK, Durica DS, Clifton SW, Roe BA, Hopkins P (1998) Cloning of crustacean ecdysteroid receptor and retinoid-X receptor gene homologs and elevation of retinoid-X receptor mRNA by retinoic acid. Mol Cell Endocrinol 139: 209-227.
- Menone ML, Bortulos A, Botto F, Aizpun de Moreno JE, Moreno VJ, et al. (2000) Organochlorine contaminants in a coastal lagoon in Argentina: an Analysis of sediment, crabs, and cordgrass from two different habitats. Estuaries. 23: 583-592.
- Weis JS, Cohen R, Kwiatkows J (1987b) Effects of diflubenzuron on limb regeneration and molting in the fiddler crab, Uca pugilator. Aquat Toxicol 10: 279-290.
- Baer KN, Owens KD (1999) Evaluation of selected endocrine disrupting compounds on sex determination in Daphnia magna using reduced photoperiod and different feeding rates. Bull Environ Contam Toxicol 62: 214-221.
- Snyder MJ, Mulder EP (2001) Environmental endocrine disruption in decapod crustacean larvae: Hormone titers, cytochrome P450, and stress protein responses to heptachlor exposure. Aquat Toxicol 55: 177-190.
- Rao KR, Fox FR, Conklin PJ, Cantelmo AC (1981) Comparative toxicology and pharmacology of chlorophenols: Studies on the grass shrimp, Palaemonetes pugio. In: Vemberg FJ, Calabrese A, Thurberg FP and Vernberg WB (editors). Biological monitoring of marine pollutant,. Academic Press, New York. 37-72.
- Serfozo J (1993) Necrotic effects of the xenobiotics accumulation in the central nervous system of a crayfish Astacus leptodactylus Eschz. ActaBioI Szeged 39: 23-38.
- Rodriguez EM, Greco LSL, Medesani DA, Laufer H, Fingerman M (2002) Effect of methyl farnesoate, alone and in combination with other hormones, on ovarian growth of the red swamp crayfish, Procambarus clarkia, during vitellogenesis. Gen Comp Endocrinol 125: 34-40.
- Reddy PS, Devi M, Sarojini R, Nagabhushanam R Fingerman M (1994) Cadmium chloride induced hyperglycemia in the red swamp crayfish, Procambarus clarkii: Possible role of crustacean hyperglycemic hormone. Compo. Biochem Physiol 107: 57-61.
- Weis JS, Kim K (1988) Tributyltin is a teratogen in producing deformities in limbs of the fiddler crab, Uca pugilator. Arch Environ Contam Toxicol 17: 583-587.
- Nagabhushanam R, Reddy PS, Sarojini R (1990) Tributyltin oxide induced alterations in exuvial weight and calcium content of the prawn, Caridina rajadhari. Proc Anim. Sci 99: 397-400.
- Reddy PS, Sarojini R, Nagabhushanam R (1991) Impact of tributyltin oxide (TBTO) on limb regeneration of the prawn, Caridina rajadhari, after exposure to different time intervals of amputation. J Tiss Res 1: 35-39.
- Reddy PS, Nagabushanam R, Sarojini R (1992) Retardation of moulting in the prawn, Caridina rajadhari, exposed to tributyltin oxide (TBTO). Proc Nat Acad Sci India 62: 353-356.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 20456
- [From(publication date):
October-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 18835
- PDF downloads : 1621