Crosstalk between Cytokine RANKL and AhR Signalling in Osteoclasts Controls Bone Homeostasis
Received: 13-May-2017 / Accepted Date: 18-May-2017 / Published Date: 25-May-2017 DOI: 10.4172/2576-3881.1000114
6659Short Communication
The aryl hydrocarbon receptor (AhR), which acts as a transcription factor when bound by a ligand, regulates a variety of physiological processes, most notably cellular activities related to immune responsivity. Observations of disrupted bone tissue healing and slowed endochondral ossification in AhR −/− mice indicate strongly that AhR also modulates skeletal remodeling, though the signaling mechanism through which AhR affects osteoclastogenesis remains to be resolved. Recent studies have shown that receptor activator of nuclear facor kappa-B ligand (RANKL) causes rapid upregulation of AhR expression in bone marrow-derived osteoclasts. Ligand activated AhR by the smoke toxin BaP increased osteoclast differentiation in a receptor AhR-dependent manner. Mitochondrial biogenesis in osteoclasts is subject to regulation by AhR. AhR involvement in osteoclastogenesis makes AhR a potential therapeutic target for the treatment of inflammatory and metabolic bone diseases. The present review highlights the recently uncovered critical role that RANKL–AhR–c-Fos signaling plays in osteoclastogenesis.
A bone metabolic switch
The aryl hydrocarbon receptor (AhR) is a basic helix-loop-helix/ Pas-Arnt-Sim family transcription factor that becomes activated when bound by a ligand [1,2]. AhR ligands include various environmental toxins as well as endogenous molecules [1,3]. Inactive cytosolic AhR is found in a protein complex, and ligand-activated AhR translocates to the nucleus where it forms a dimer with its nuclear translocator protein Arnt [2,4]. The AhR-Arnt dimer then regulates target genes by binding xenobiotic response element sequences in their promoter regions; AhR target genes include drug-metabolizing cytochrome P450 (Cyp) enzymes and the steroid biosynthesis enzyme NAD(P)H:quinone oxidoreductase [2].
Carcinogens in smoke have been shown to degrade bone tissue via influences on osteoclastogenesis [5-8]. A key culprit, the toxin benzo[a]pyrene (BaP), stimulates osteoclastogenesis by binding AhR and thereby triggering activation of several Cyp1 isoforms [9-12]. However, the downstream signaling pathways mediating these effects, particularly in the context of bone repair, are unclear. Moreover, the levers controlling AhR expression in osteoclasts have not been demonstrated.
Skeletal homeostasis
Bone tissue is formed by osteoblasts, which are derived from mesenchymal stem cells, and bone resorption is conducted by osteoclasts, which are multinucleated giant cells formed by the fusion of macrophages [13]. The skeleton is a dynamic tissue whose constant remodeling depends upon the balance of osteoclast-mediated bone resorption and osteoblast-mediated bone formation. Various diseases have been related to an osteoblast-osteoclast imbalance, including osteoporosis, rheumatoid arthritis, gum disease, and metastatic bone cancer [14,15].
Osteoclasts become terminally differentiated and activated in response to binding of receptor activator of nuclear factor kappa B ligand (RANKL), a tumor necrosis factor (TNF) family cytokine, to its receptor RANK [16-18]. RANKL binding of RANK induces c-Fos expression, and c-Fos then enables the downstream induction of NFATc1 (nuclear factor of activated T-cells, cytoplasmic 1), which modulates the terminal differentiation of osteoclasts [15,19]. The importance of c-Fos in osteoclastogenesis is evidenced by the fact that mice deficient in c-Fos exhibit osteopetrosis [19,20].
RANKL and epigenetic signaling pathways involved in osteoclast differentiation
RANKL_binding of RANK in osteoclast precursor cells triggers the activation of several mitogen-activated protein kinase (MAPK) pathways, including signaling by TNF receptor-associated factor, family mediated c-jun N-terminal kinase (JNK), p38, and nuclear factor-kappa B (NF-κB) [21]. Osteoclast precursors express the macrophage colony-stimulating factor (M-CSF) receptor c-Fms, and stimulation by M-CSF activates MAPK pathways such as Akt and extracellular signal-regulated kinase (ERK) signaling [22]. In a recent study examining whether AhR activation may involve RANKL or MCSF in AhR−/− cells, we found that the absence of AhR disrupted activation of downstream cell signaling pathways in response to RANKL, but not M-CSF, indicating that AhR promotion of osteoclast differentiation may be mediated by way of sensitization of osteoclast precursors to RANKL [23].
Emerging evidence suggests that epigenetic machinery plays an essential role in the normal development and tissue homeostasis in mammals. For instance, recent observation revealed that inhibition of the catalytic activity of histone H3 lysine 9 (H3K9) methyltransferases G9a and its partner modifier GLP is able to suppresses the RANKLassociated osteoclast differentiation [24]. In addition, as aberrant osteoclast formation during critical illness was also linked to global histone hypo-methylation [25], these findings suggested that establishment and maintenance of heterochromatin status at particular loci in the osteoclasts is critical for their development. Strikingly, as G9a/GLP complex maintains DNA methylation [26] and their associated H3K9me2 is also involved in the protection of DNA methylation during DNA replication [27], proper patterns of DNA methylation, another heterochromatin marker, might also be required for the normal development of osteoclasts. Therefore, investigation the status of H3K9me as well as DNA methylation in the mature osteoclasts and their precursors might shed light on the indispensable epigenetic mechanisms that involved in the regulation of their development.
AhR knockout and overexpression mouse phenotypes
Systemic or osteoclast-targeted AhR gene knockout results in increased bone mass together with reduced bone resorption [28-30], whereas osteoblast-targeted AhR knockout does not alter bone phenotype [29]. Moreover, AhR-overexpressing mice exhibit excessive bone resorption [31]. Treatment of wild-type (WT) mice, but not osteoclast-selective AhR −/− mice, with the AhR ligand 3- methlcholanthrene (3MC) increases bone resorption, thereby reducing bone mass [29,30]. Such findings suggest that pathological bone resorption may involve AhR and further implicate AhR as a potential therapeutic target in the treatment of bone-degrading diseases such as osteoporosis and rheumatoid arthritis.
Involvement of osteoclast RANKL and AhR in immune regulation
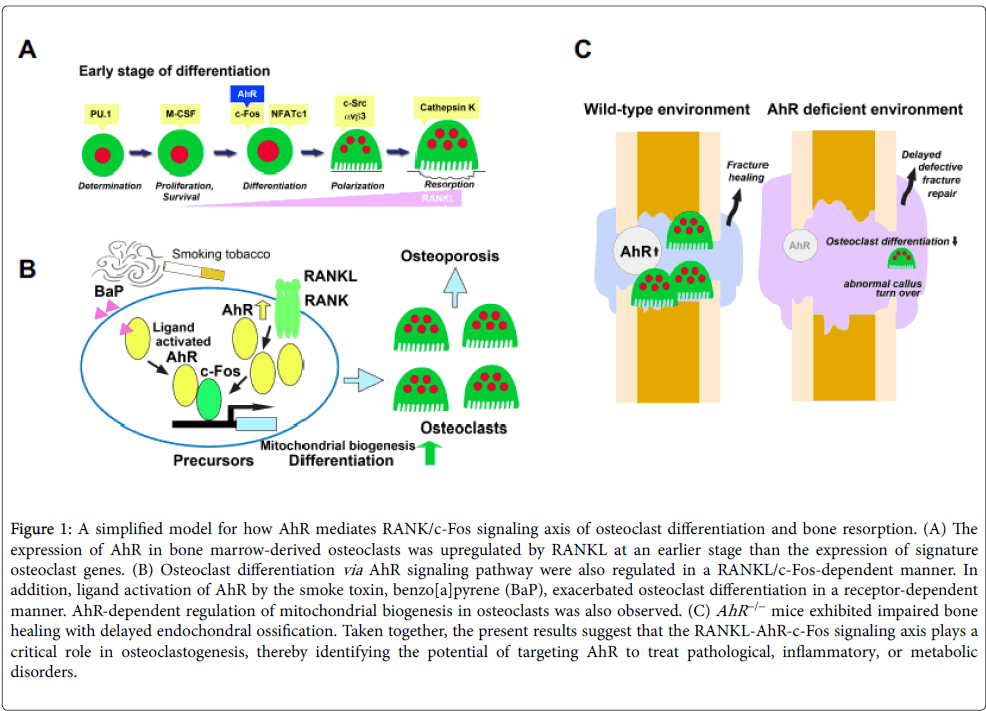
AhR signaling has been implicated strongly in immune modulation. Notably, AhR has been shown to affect dendritic cell function and the balance between regulatory T cells and Th17 cells [3,32,33]. AhR has been shown to control mast cell differentiation and homeostasis [34] and to be a positive regulator of osteoclastogenesis in vitro [28,29]. Additionally, AhR expression has been shown to be upregulated in dendritic cells and macrophages following treatment with immune response trigger molecules, such as lipopolysaccharide and CpG DNA [33,35]. Recently, we showed that AhR expression was upregulated in bone marrow macrophages (BMMs), similar to c-Fos, 24 h after RANKL treatment. Together with the aforementioned finding that AhR −/− osteoclastic cells have disrupted RANKL-stimulated osteoclastogenic signaling, but normal responsivity to M-CSF [23], these findings suggest that impaired osteoclastogenesis of AhR −/− BMMs may be consequent to RANKL signaling pathway disruption (Figure 1A).
Figure 1: A simplified model for how AhR mediates RANK/c-Fos signaling axis of osteoclast differentiation and bone resorption. (A) The expression of AhR in bone marrow-derived osteoclasts was upregulated by RANKL at an earlier stage than the expression of signature osteoclast genes. (B) Osteoclast differentiation via AhR signaling pathway were also regulated in a RANKL/c-Fos-dependent manner. In addition, ligand activation of AhR by the smoke toxin, benzo[a]pyrene (BaP), exacerbated osteoclast differentiation in a receptor-dependent manner. AhR-dependent regulation of mitochondrial biogenesis in osteoclasts was also observed. (C) AhR −/− mice exhibited impaired bone healing with delayed endochondral ossification. Taken together, the present results suggest that the RANKL-AhR-c-Fos signaling axis plays a critical role in osteoclastogenesis, thereby identifying the potential of targeting AhR to treat pathological, inflammatory, or metabolic disorders.
AhR mediates c-Fos activation in osteoclastogenesis and BaP-induced bone loss
There have been conflicting several reports on the induction of c- Fos by AhR ligands in mammals. One is that AhR ligands repress the E2-induced expression of c-Fos [36], the other is that AhR ligands themselves induce the expression of c-Fos [37]. It has also has been reported that a time-course chromatin immunoprecipitation assay indicated that ER-a, AhR and p300 were recruited to c-Fos promoter, presumably upon the binding of 3MC to AhR in MCF-7 cells [38].
In osteoclast differentiation, RANKL activates c-Fos [39], which triggers the expression of NFATc1 [40]. Examining whether AhR plays a role in RANKL/c-Fos/NFATc1 osteoclastogenesis, we found that, compared to control BMMs, BMMs induced to express c-Fos by retroviral gene transfer exhibited a dramatic increase in the number of multinucleated osteoclasts [23]. In the same study, we also showed that RANKL stimulation of BMMs failed to induce osteoclast differentiation in the absence of the AhR–c-Fos complex. These findings provide strong evidence in support of the hypothesis that AhR plays an important role in c-Fos mediated regulation of osteoclast differentiation.
AhR ligands, including the smoke toxin BaP, stimulate osteoclastogenesis through activation of Cyp1 enzymes [9-12,28]. BaP exposure increases c-Fos levels in RANKL-stimulated WT BMMs, but fails to induce c-Fos expression in AhR −/− BMMs [23]. These findings provide hints regarding the relationship between smoking and bone metabolism, provide insight into c-Fos–AhR-axis-mediated osteoclastogenesis, and support the pursuit of AhR-targeted therapies for osteoporosis (Figure 1B).
Control of mitochondrial biogenesis via AhR signaling
Reactive oxygen species released from mitochondria stimulate osteoclast differentiation by inducing Ca2+ level oscillations and NFATc1 activation [41]. Mitochondrial biogenesis during osteoclast development requires peroxisome proliferator-activated receptorgamma coactivator 1β (PGC-1β), a key mitochondrial oxidative energy metabolism factor [42-47]. Recent experimental results have shown that RANKL induction of PGC-1β mRNA and protein expression is suppressed in AhR −/− cells [23]. Additionally, the AhR −/− mouse phenotype includes defective calcium signaling and mitochondrial function leading to mast cell deficiency as well as enhanced intracellular reactive oxygen species and apoptosis [34]. Lack of AhR also diminishes basal mitochondrial biogenesis in osteoclasts [23]. Together, these findings indicate that AhR may play an important role in mitochondrial biogenesis (Figure 1B).
AhR in bone healing
Fracture healing is a complex process that requires the coordinated interplay of many cell types, growth factors, extracellular matrix components, and mechanobiological input. In non-stabilized fractures, healing occurs via generation of a periosteal callus that bridges the fracture site. AhR involvement in bone repair has been examined in a semi-stabilized fracture-healing model, wherein an early inflammatory phase is followed sequentially by a soft callus reparative phase, a hard callus phase, and a bone-remodeling phase that can last several weeks [48]. In a study employing this semi-stabilized, fracture-healing model, AhR −/− mice were found to have impaired endochondral bone healing relative to WT mice [23], suggesting that AhR may play an important role in bone fracture healing. In particular, the callus tissue in AhR −/− mice contained a greater amount of cartilage for a longer period time, and the mice suffered from defective long-term bone remodeling. Compared with WT calluses, reduced bone remodeling was accompanied by a reduction in the number of tartrate-resistant acid phosphatase-positive cells in AhR −/− calluses [23]. Notably, the inflammatory response to fracture is delayed in mice lacking cyclooxygenase-2 or TNF-α receptors [49,50]. These findings are consistent with the notion that AhR may be involved in various inflammation-driven disorders, ranging from septic shock to psoriasis and rheumatoid arthritis [51-53].
In addition to their well-known resorption activity in bone remodeling, osteoclasts may also regulate the formation of new bone [54,55]. Despite their abnormal bone repair following injury and increased local metabolic demands, AhR −/− mice do show normal bone formation under basal conditions (Figure 1C). A convergence of recent findings indicates that early osteoclast differentiation involves cooperative actions of AhR and c-Fos, and that AhR may control bone remodeling through the regulation of osteoclast differentiation. Future studies should explore the therapeutic potential of AhR-targeted therapy for support of fracture repair.
Acknowledgment
This work was supported by the grant provided by JSPS KAKENHI (Grant Numbers. 25713063, 15K15757 to T.I., 16H05511 to R.A., 15K15676, 16H02690 to N.I.), The Ichiro Kanehara Foundation, Suzuken Memorial Foundation, The Nakatomi Foundation, and Smoking Research Foundation.
References
- Abel J, Haarmann-Stemmann T (2010) An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem 391: 1235-1248.
- Fujii-Kuriyama Y, Mimura J (2005) Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun 338: 311-317.
- Veldhoen M, Duarte JH (2010) The aryl hydrocarbon receptor: fine-tuning the immune-response. Curr Opin Immunol 22: 747-752.
- Petrulis JR, Perdew GH (2002) The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact 141: 25-40.
- Szulc P, Garnero P, Claustrat B, Marchand F, Duboeuf F, et al. (2002) Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab 87: 666-674.
- Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL (2000) Smoking and bone metabolism in elderly women. Bone 27: 429-436.
- Porter SE, Hanley EN Jr (2001) The musculoskeletal effects of smoking. J Am Acad Orthop Surg 9: 9-17.
- McKee MD, DiPasquale DJ, Wild LM, Stephen DJ, Kreder HJ, et al. (2003) The effect of smoking on clinical outcome and complication rates following Ilizarov reconstruction. J Orthop Trauma 17: 663-667.
- Pitts JN, Jr Van Cauwenberghe KA, Grosjean D, Schmid JP, Fitz DR, et al. (1978) Atmospheric reactions of polycyclic aromatic hydrocarbons: facile formation of mutagenic nitro derivatives. Science 202: 515-519.
- Hankinson O (2005) Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys 433: 379-386.
- Martin LA, Byrd SK, Milofsky RE (2003) Rapid communication: effects of tobacco processing on the quantity of benzo[a]pyrene in mainstream smoke. J Toxicol Environ Health A 66: 1283-1286.
- Besaratinia A, Kleinjans JC, Van Schooten FJ (2002) Biomonitoring of tobacco smoke carcinogenicity by dosimetry of DNA adducts and genotyping and phenotyping of biotransformational enzymes: a review on polycyclic aromatic hydrocarbons. Biomarkers 7: 209-229.
- Novack DV, Teitelbaum SL (2008) The osteoclast: friend or foe? Annu Rev Pathol 3: 457-484.
- Drake MT (2013) Osteoporosis and cancer. Curr Osteoporos Rep 11: 163-170.
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337-342.
- Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289: 1504-1508.
- Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12: 17-25.
- Takayanagi H(2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7: 292-304.
- Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, et al. (2000) Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 24: 184-187.
- Wagner EF, Matsuo K (2003) Signalling in osteoclasts and the role of Fos/AP1 proteins. Ann Rheum Dis 62 Suppl 2: ii83-85.
- Takayanagi H, Kim S, Taniguchi T (2002) Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis research 4 (Suppl 3): S227-232.
- Ross FP, Teitelbaum SL (2005) alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev 208: 88-105.
- Izawa T, Arakaki R, Mori H, Tsunematsu T, Kudo Y, et al. (2016) The Nuclear Receptor AhR Controls Bone Homeostasis by Regulating Osteoclast Differentiation via the RANK/c-Fos Signaling Axis. J Immunol 197: 4639-4650.
- Tsuda H, Zhao N, Imai K, Ochiai K, Yang P, et al. (2013) BIX01294 suppresses osteoclast differentiation on mouse macrophage-like Raw 264.7 cells. Bosn J Basic Med Sci 13: 271-275.
- Owen HC, Vanhees I, Gunst J, Van Cromphaut S, Van den Berghe G (2015) Critical illness-induced bone loss is related to deficient autophagy and histone hypomethylation. Intensive Care Med Exp 3: 19.
- Zhang T, Termanis A, Özkan B, Bao XX, Culley J, et al. (2016) G9a/GLP Complex Maintains Imprinted DNA Methylation in Embryonic Stem Cells. Cell Rep 15: 77-85.
- Rose NR, Klose RJ (2014) Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta 1839: 1362-1372.
- Iqbal J, Sun L, Cao J, Yuen T, Lu P, et al. (2013) Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci USA 110: 11115-11120.
- Yu TY, Kondo T, Matsumoto T, Fujii-Kuriyama Y, Imai Y (2014) Aryl hydrocarbon receptor catabolic activity in bone metabolism is osteoclast dependent in vivo. Biochem Biophys Res Commun 450: 416-422.
- Yu TY, Pang WJ, Yang GS (2015) Aryl hydrocarbon receptors in osteoclast lineage cells are a negative regulator of bone mass. PLoS One 10: e0117112.
- Wejheden C, Brunnberg S, Larsson S, Lind PM, Andersson G, et al. (2010) Transgenic mice with a constitutively active aryl hydrocarbon receptor display a gender-specific bone phenotype. Toxicol Sci 114: 48-58.
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, et al. (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453: 65-71.
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, et al. (2010) Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA 107: 19961-19966.
- Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, et al. (2013) Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood 121: 3195-3204.
- Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, et al. (2009) Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med 206: 2027-2035.
- Astroff B, Eldridge B, Safe S (1991) Inhibition of the 17 beta-estradiol-induced and constitutive expression of the cellular protooncogene c-fos by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the female rat uterus. Toxicol Lett 56: 305-315.
- Puga A, Nebert DW, Carrier F (1992) Dioxin induces expression of c-fos and c-jun proto-oncogenes and a large increase in transcription factor AP-1. DNA Cell Biol 11: 269-281.
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, et al. (2003) Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423: 545-550.
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, et al. (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266: 443-448.
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, et al. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889-901.
- Kim MS, Yang YM, Son A, Tian YS, Lee SI, et al. (2010) RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem 285: 6913-6921.
- Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann H, Bjursell M, et al. (2006) Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4: e369.
- Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J et al. (2006) Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4: 453-464.
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM (2007) PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 104: 5223-5228.
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, et al. (2007) The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35-46.
- Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, et al. (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15: 259-266.
- Wei W, Wang X, Yang M, Smith LC, Dechow PC, et al. (2010) PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11: 503-516.
- Le AX, Miclau T, Hu D, Helms JA (2001) Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res 19: 78-84.
- Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, et al. (2003) Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 18: 1584-1592.
- Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, et al. (2002) Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest 109: 1405-1415.
- Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, et al. (2009) Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol 29: 6391-6400.
- Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, et al. (2011) Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci USA 108: 14222-14227.
- Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, et al. (2014) Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 40: 989-1001.
- Teti A (2013) Mechanisms of osteoclast-dependent bone formation. Bonekey Rep 2: 449.
- Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, et al. (2013) Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 123: 3914-3924.
Citation: Izawa T, Arakaki R, Ishimaru N (2017) Crosstalk between Cytokine RANKL and AhR Signalling in Osteoclasts Controls Bone Homeostasis. J Cytokine Biol 2: 114. DOI: 10.4172/2576-3881.1000114
Copyright: © 2017 Izawa T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4126
- [From(publication date): 0-2017 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3246
- PDF downloads: 880