Review Article Open Access
Cross-Roads in Research on Neurodegenerative Diseases
Frank O Bastian*
Professor of Animal Science, Louisiana State University Agricultural Center, Baton Rouge, LA, USA
- Corresponding Author:
- Frank O. Bastian
Professor of Animal Science
Louisiana State University
Agricultural Center, Baton Rouge
LA 70803, LA 70803, USA
Tel: 504-610-9177
E-mail: fbastian@agcenter.lsu.edu
Received date: January 20, 2014; Accepted date: February 27, 2014; Published date: March 15, 2014
Citation: Bastian FO (2014) Cross-Roads in Research on Neurodegenerative Diseases. J Alzheimers Dis Parkinsonism 4:141. doi: 10.4172/2161-0460.1000141
Copyright: © 2014 Bastian FO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) is the most prevalent of the neurodegenerative amyloid diseases and is characterized by accumulation of amyloid-β and tau. Recent studies have indicated that AD may be a brain infection. Although AD has not been shown to be transmissible, the burning issue is whether the potential infectious causes are the misfolded amyloid proteins themselves, or an unidentified microorganism. The idea of a replicating protein (prion) evolved from research on the transmissible spongiform encephalopathies (TSE) represented by Creutzfeldt-Jakob disease in humans. Prions are now suspect in all of the amyloid related neurodegenerative diseases. However, the popularized prion theory is controversial since there is convincing evidence that spiroplasma, a wall-less prokaryote, is involved in the pathogenesis of TSE, and may represent the trigger mechanism. Interest in bacterial involvement in AD has surfaced from discovery that most bacteria produce biofilm and that components of the biofilm experimentally induce misfolded amyloid proteins. The recent discovery of H. pylori in AD has brought this controversy to a head. In this review we will discuss involvement of bacteria as candidate causal agent/s for the neurodegenerative diseases, and relate the evidence to involvement of spiroplasma in the pathogenesis of the TSEs as a model for these neurodegenerative diseases.
Keywords
Spiroplasma; Alzheimer’s disease; Parkinson’s disease; Prion; Alpha-synuclein; Creutzfeldt-Jakob disease; Biofilm
Introduction
The neurodegenerative diseases include Alzheimer’s (AD), Parkinson’s (PD), frontotemporal dementia (FTD), posttraumatic stress disorders (PTSD), including dementia pugilistica, and chronic traumatic encephalopathy (CTE) seen in athletes, and soldiers after episodes of traumatic brain injury. All of these diseases present with dementia and show accumulation within the brain of misfolded diseasespecific amyloid proteins. The deposition of misfolded amyloid proteins is also a manifestation of Creutzfeldt-Jakob disease, a transmissible spongiform encephalopathy (TSE) in humans with a significant animal disease reservoir including scrapie in sheep, chronic wasting disease (CWD) in deer, and bovine spongiform encephalopathy (BSE) or ‘mad cow disease’ in cattle. All transmissible spongiform encephalopathies show accumulation of ‘prion’ amyloid deposits considered to be novel self-propagating proteins [1]. The pathogenic mechanism in TSEs involves accumulation of misfolded or unfolded proteins that trigger the ‘unfolded protein response’ (UPR), which is a protective cellular mechanism whereby protein translation is shut down and chaperone proteins are recruited [2]. The unfolded protein response and autophagy are important cellular homeostatic mechanisms that affect cell survival or lead to cell death in the TSEs [2,3]. The toxicity of the prion amyloid proteins builds up with continued stimulation of the UPR and leads to neuro-degeneration and synapse loss [3].
Presumably the same phenomenon is operative in AD and PD, although the neuronal damage is not as severe as seen in TSEs. This comparison brings up the idea that all neurodegenerative diseases are potentially infectious, with the focus on the amyloid deposits themselves by extension of the prion replicating protein theory [4]. However, the UPR response and disruption of autophagy are known to occur after the buildup of misfolded amyloid proteins associated with intracellular viruses and bacteria [3]. This phenomenon is operative in plant virus infection [5]. Furthermore, there are problems with the concept of replicating prion amyloid in TSEs, since there is convincing evidence of consistent involvement of spiroplasma, a wallless bacterium, in TSE-affected brains and eyes. Thus Spiroplasma spp. are candidate causal agents in CJD and the animal TSEs [1,6,7]. Recognition of conventional bacterial or viral involvement in the pathogenesis of the neurodegenerative diseases could lead to new diagnostic and therapeutic approaches. This treatise encourages a more balanced research and funding approach to investigating the exciting possibility of the infectious nature of AD.
The Linking of AD to Prion Diseases
By definition, ‘prions are infectious proteins that can transmit biologic information by propagating protein misfolding and aggregation’ [8]. The mechanism proposed is the seeding of PrP(c) (a normal host protein) by the abnormally folded prion amyloid, but there is no definitive proof that this actually happens [9]. The introduction of small amounts of misfolded insoluble hyperphosphorylated Tau protein leads to conversion of soluble Tau into insoluble neurofibrillary tangles (NFTs), which are a component of AD pathology [10]. This seeding process along with discovery of trans-synaptic transmission of Tau [11] are consistent with the pathogenic mechanisms proposed in prion diseases, therein leading to the all encompassing ‘concept of disease-causing amyloidogenic proteins’ [10,12,13]. While there is no convincing evidence of transmissibility in AD and PD, the idea is emerging that prions including all the amyloid proteins associated with neurodegenerative diseases, namely Tau, amyloid-β and alphasynuclein, are infectious. Although there is binding between beta amyloid oligomers and PrP(c), there are no functional consequences [14]. Injection of AD-affected brain tissue into animals leads to amyloid-β deposits that progressively increase over time [15]. The increase may simply be due to amyloid self-assembly following the seeding by the foreign protein [16]. There is continuous production of prion amyloid in experimental prion infection in vitro, even after the infectious particles are eliminated [17]. The interpretation is that an environmental infectious agent initiates the process, and may no longer be present in late stages of the disease. Consideration must be given to the much dissimilarity between AD and the TSEs, exemplified by recognition that the precursor and propagated proteins differ in TSE and AD, as well as clinical manifestations and pathological findings [18]. Populations receiving growth hormone have been susceptible to TSE, but not to AD or PD [19] suggesting that there are major differences in the pathogenesis of each disease. Furthermore, there are basic problems with the prion concept in TSEs, which raises the issue regarding the significance of association of enteric bacteria with AD.
Problems with the Prion Concept
Since prions are becoming a major focus of research efforts in trying to determine underlying pathogenic factors in AD and other neurodegenerative diseases, the basic concept of replication of proteins as cause of disease has to be critically evaluated. Prions accumulate in at least 90% of CJD cases (DeArmond, personal communication) and appear to be important in the pathogenesis of CJD and the other TSEs, possibly by toxicity of the prion amyloid on neurons. Others have hypothesized that the prion-like amyloid protein provides the template for the infectious agent/s [3]. On the other hand the prion concept in TSEs remains controversial after 30 years. The mechanism whereby a prion amyloid protein is able to misfold a host protein is not established [9] and the theory does not satisfactorily explain the multiple disparate strains involved [20,21]. Manuelidis has shown in several publications that prion accumulation is not directly related to TSE infectivity [17]. Similarly the protein misfolding cyclic amplification (PMCA) technique [22] that has become a standard in detecting small quantities of prion in suspect CJD cases has raised issues whether the prion is a separate marker from infectivity. Repeated sonication of test brain samples in the presence of excess host PrP(c) leads to accumulation of prion amyloid [23].
However, there is no corresponding increase in infectivity suggesting another factor is involved [24]. False positives in controls of the PMCA test are often ignored as evidence of spontaneous generation [25]. Nevertheless, the prion concept has become the sole explanation of causality in TSEs and alternate theories have been discounted. We propose that the prion may not be the surrogate marker for infectivity in brains affected with TSE. Instead our laboratory has presented data showing that spiroplasma, a wall-less bacterium, is involved in the pathogenesis of TSE.
Spiroplasmosis and the TSEs
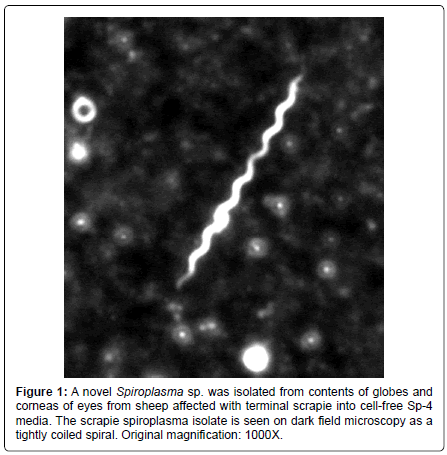
The spiroplasmosis concept arose from ultrastructural findings of spiroplasma inclusions in a CJD brain biopsy [26], which has been confirmed by several laboratories in autopsy tissues [27,28]. Succeeding experiments have shown a clear association of spiroplasma with the TSEs. Spiroplasma ribosomal rDNA is found in brains from all forms of TSE [29]. Spiroplasma are isolated in cell-free culture from TSE affected brains [30] and eyes of sheep with terminal scrapie (Figure 1) [31]. Spiroplasma was found initially in mice fortuitously inoculated with rabbit tick isolates for purposes of screening for arboviruses [32]. Initially thought to be a slow virus infection, suckling mouse cataract agent (SMCA) strain of S. mirum inoculated intracranially (IC) into mice induced a persistent brain infection with development of neurological deterioration [33]. Experimental spiroplasma inoculation IC into rats was done to examine the neuropathological lesions and the bacterium was found to induce spongiform encephalopathy [34]. Spiroplasma was isolated from the rat tissues [35]. Spiroplasma show morphological variability and spiral forms, which are rare in the tissues [34]. The ultrastructural pathology is remarkably similar to lesions associated with CJD [34]. In a recent study, the spiroplasma laboratory strains isolated from rabbit ticks and passaged for many years in the laboratory induce clinical neurological disease ½ in deer at 3 months after IC inoculation [30]. The experimental spiroplasma infection in the deer is manifested by neurological deterioration with brain stem signs and wasting, which closely resembles naturally occurring chronic wasting disease (CWD) in deer and elk [5].
Sheep inoculated with the SMCA strain of S. mirum showed no clinical neurological deterioration at 16 months, but were probably significantly visually impaired since marked retinopathy was noted at autopsy [31]. Particularly exciting was the finding of bulbar lesions identical to natural scrapie or CWD in sheep and goats inoculated IC with spiroplasma isolates from brains affected with scrapie or CWD [30]. Subsequent experiments have shown that there is no immune cross-reactivity between the rabbit tick isolate strains and the spiroplasma isolates from TSE suggesting presence of a novel strain of spiroplasma in TSE-affected tissues [31]. Still it has been difficult to grasp that tiny bacteria possess the unusual biologic properties of the transmissible agent of TSE.
Biofilm, Spiroplasma and Prion
The breakthrough research is the discovery that spiroplasma induce biofilm formation [36]. Spiroplasma embedded in a polysaccharide matrix are resistant to 50% glutarraldehyde simulating an important biologic property of the TSE agent. This explains our unpublished data wherein spiroplasma shows significant resistance to radiation, and heat treatment near boiling temperatures, unlike other more conventional bacteria. Biofilm formation by spirolasma on stainless steel and nickel may be the mechanism responsible for iatrogenic transmission of CJD [36]. Spiroplasma biofilm forms readily on mica suggesting the tendency of the organisms to bind to clay particles. The long conjugate inter-spiroplasma connections observed in spiroplasma biofilm would provide a reliable means for communication in micro-colonies established in soil [37]. The location of scrapie or CWD infectivity in soil rich in clay [38] and the tendency of ruminants to ingest large amount of soil would explain the lateral transmission spread of CWD [39]. Bacteria in biofilm express different pathogenicity related proteins compared to planktonic organisms [40]. Furthermore, there is presence of curli fibers (functional amyloid) in spiroplasma biofilms [36] that bacteria use to attach and enter host cells [41]. Curli fibers also combine with host proteins and cause misfolding or amyloid formation [42,43] and induce amyloidosis when inoculated into susceptible mice [44]. This misfolded amyloid then increases by self-assembly [16]. In the case of neurodegenerative disease, amyloids may be the toxic element but the bacterium may be the trigger. Prion amyloid has not been documented as yet in experimental infection with laboratory strains of spiroplasma. However it is noteworthy that in vitro SMCA infection of bovine corneal endothelia cells or mouse neuroblastoma cell produces alpha-synuclein (Feng, personal communication). Alpha-synuclein formation by a bacterium interacting with host proteins is significant evidence that a conventional pathogen can trigger onset of neurodegenerative diseases. My inclination is that the novel spiroplasma isolates from scrapie or CWD are more likely to produce prion, since the bulbar lesions in experimental spiroplasmosis are more in line with natural disease than lesions associated with the laboratory strains [30]. The data supporting involvement of a Spiroplasma sp. in the causality of TSEs provides new possibilities for developing protocols for diagnosis and treatment of the TSEs.
The Genetic Connection
There is a genetic connection in CJD and a long llisting of susceptibility genes to CJD exists [45]. Gerstmann-Sträussler-Scheinker syndrome (GSS) and fatal familial insomnia (FFI) are genetic forms of TSEs attributed to mutations of the prion protein gene on chromosome 20 [46]. However, protease-resistant prion deposits are absent in some brains with characteristic GSS neuropathology [46]. GSS and FFI are not purely genetic since both heredity forms of CJD have been transmitted to animal models [46,47]. There is a rationale for spiroplasma being involved in heredity CJD cases. An important related observation is localization of infectivity to the ovary in scrapie in sheep [48] is important since spiroplasma show transovarian passage in Drosophilia [49]. Spiroplasma induces chromosome abnormalities in tissue culture infection (unpublished data) suggesting possible transformation of host genetic material. Spiroplasma are gene manipulators [50] and may be involved through the action of bacterial DNA binding proteins, or transformation or transfection wherein the bacterium inserts exogenous genetic material into animal cells [51]. The answer can only come from experimentation.
New Horizons in TSE Dx & Rx
Involvement of a bacterium in the pathogensis of CJD or other neurodegenerative diseases broadens the variables that can be addressed. It has been problematic trying to develop a diagnostic test for TSEs solely based upon the prion.
The quaking test developed at Rocky mountain laboratories [52] has proved unreliable according to the prion center (Gambetti, personal communication). The protein misfolding cyclic amplification test is difficult to control and has proved unreliable with controls being positive [25]. Evaluation of other brain proteins using the 14-3-3 test has proved unreliable with no better than 50% accuracy [53]. On the other hand, a serological test is possible for detection of a bacterial infection, especially since we have evidence that polyclonal scrapie specific antibodies react with spiroplasma proteins derived from cellfree cultures [54]. We have shown consistent presence of spiroplasma ribosomal DNA by PCR in TSE-affected brain tissues [29], and there are numerous spiroplasma proteins that are specific for this species [5]. Vaccine production has been considered problematic for TSEs since it may be impossible to generate antibodies against prion amyloid proteins without developing autoimmune response [55]. Antibodies against an extraneous spiroplasma infecting organism are more likely to succeed in development of a workable vaccine. Experimental spiroplasma infection localizes to the eye with development of severe degenerative retinopathy remarkably similar to retinopathy that occurs in terminal scrapie in sheep [31,56]. It is noteworthy that degenerative retinopathy has been described in AD [57] suggesting that a thorough ophthalmic examination is essential for evaluation of patients presenting with dementia. It is important that eyes from dementia patients be examined at autopsy to confirm the usefulness of ophthalmic examinations. Furthermore, the localization of spiroplasma to the eye in experimental spiroplasmosis in ruminants [31] suggests the eyes from these patients may be important for research studies. The evidence supporting spread of CWD by contamination of soil [58] makes control of CWD infection especially difficult in wild animal populations, even if we now know a Spiroplasma sp. is likely involved. There is need for a vaccine to protect susceptible cattle populations that mingle with deer in the fields. A diagnostic test based on spiroplasma could finally resolve the epidemiology of the transmissible agent/s
Conclusion
This review presents a major cross-road for research on AD and PD, and care must be taken to avoid the pitfalls of past research efforts on CJD and the animal TSEs. Failure to consider alternate theories in investigations of the TSEs has hindered progress in the TSE field. Our pioneer work shows a role for spiroplasma in the pathogenesis of the TSEs. We propose that spiroplasma acts as a trigger in TSE, while the prion amyloid is the byproduct of the infection. The prion amyloid may be the toxic element that causes the neurodegeneration in TSEs, but is unlikely to be the cause. This controversy is important for the future research direction in other potentially infectious neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Reports of involvement of conventional viruses or bacteria in neurodegenerative diseases [58,59] must be investigated since such associations may be useful for developing future diagnostic tests and treatment regimens. It is remarkable that curli fibers in bacterial biofilms are capable of misfolding host proteins [42,43], and the accumulation of alphasynuclein in tissue cultures infected with spiroplasma is a prime example. These experimental data provide further avenues of investigations in the neurodegenerative diseases. Of practical importance in the spiroplasmosis model is the localization of the infection to the eyes with development of retinal degeneration [31].
It is noteworthy that similar eye involvement is seen in naturally occurring TSEs and in neurodegenerative diseases [57] such as AD and PD suggesting that a thorough eye examination be done on any patient presenting with dementia.
References
- Bastian FO (1991) Creutzfeldt-Jakob Disease and Other Transmissible Spongiform Encephalopathies. Mosby–Year Book, New York, NY.
- Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, et al. (2012) Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature 485: 507-511.
- Lee DY, Lee J, Sugden B (2009) The unfolded protein response and autophagy: herpesviruses rule! J Virol 83: 1168-1172.
- Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216: 136-144.
- Ye C, Verchot J (2011) Role of unfolded protein response in plant virus infection. Plant Signal Behav 6: 1212-1215.
- Bastian FO (2005) Spiroplasma as a candidate agent for the transmissible spongiform encephalopathies. J Neuropathol Exp Neurol 64: 833-838.
- Bastian FO (2014) The case for involvement of spiroplasma in the pathogenesis of transmissible spongiform encephalopathies. J Neuropathol Exp Neurol 73: 104-114.
- Soto C, Estrada L, Castilla J (2006) Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci 31: 150-155.
- Eisenberg D, Jucker M (2012) The amyloid state of proteins in human diseases. Cell 148: 1188-1203.
- Guo JL, Lee VM (2011) Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286: 15317-15331.
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, et al. (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7: e31302.
- Braak H, Del Tredici K (2011) Alzheimer's pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol 121: 589-595.
- Westaway D, Jhamandas JH (2012) The P's and Q's of cellular PrP-Aβ interactions. Prion 6: 359-363.
- Forloni G, Sclip A, Borsello T, Balducci C (2013) The neurodegeneration in Alzheimer disease and the prion protein. Prion 7: 60-65.
- Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C (2012) De novo induction of amyloid-β deposition in vivo. Mol Psychiatry 17: 1347-1353.
- Takahashi Y, Mihara H (2004) Construction of a chemically and conformationally self-replicating system of amyloid-like fibrils. Bioorg Med Chem 12: 693-699.
- Miyazawa K, Kipkorir T, Tittman S, Manuelidis L (2012) Continuous production of prions after infectious particles are eliminated: implications for Alzheimer's disease. PLoS One 7: e35471.
- Jeffrey M (2013) Review: membrane-associated misfolded protein propagation in natural transmissible spongiform encephalopathies (TSEs), synthetic prion diseases and Alzheimer's disease. Neuropathol Appl Neurobiol 39: 196-216.
- Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, et al. (2013) Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70: 462-468.
- Bruce ME (1993) Scrapie strain variation and mutation. Br Med Bull 49: 822-838.
- Morales R, Abid K, Soto C (2007) The prion strain phenomenon: molecular basis and unprecedented features. Biochim Biophys Acta 1772: 681-691.
- Saá P, Castilla J, Soto C (2006) Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J Biol Chem 281: 35245-35252.
- Fujihara A, Atarashi R, Fuse T, Ubagai K, Nakagaki T, et al. (2009) Hyperefficient PrP Sc amplification of mouse-adapted BSE and scrapie strain by protein misfolding cyclic amplification technique. FEBS J 276: 2841-2848.
- Klingeborn M, Race B, Meade-White KD, Chesebro B (2011) Lower specific infectivity of protease-resistant prion protein generated in cell-free reactions. Proc Natl Acad Sci U S A 108: E1244-1253.
- Cosseddu GM, Nonno R, Vaccari G, Bucalossi C, Fernandez-Borges N, et al. (2011) Ultra-efficient PrP(Sc) amplification highlights potentialities and pitfalls of PMCA technology. PLoS Pathog 7: e1002370.
- Bastian FO (1979) Spiroplasma-like inclusions in Creutzfeldt-Jakob disease. Arch Pathol Lab Med 103: 665-669.
- Reyes JM, Hoenig EM (1981) Intracellular spiral inclusions in cerebral cell processes in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol 40: 1-8.
- Bastian FO, Hart MN, Cancilla PA (1981) Additional evidence of spiroplasma in Creutzfeldt-Jakob disease. Lancet 1: 660.
- Bastian FO, Dash S, Garry RF (2004) Linking chronic wasting disease to scrapie by comparison of Spiroplasma mirum ribosomal DNA sequences. Exp Mol Pathol 77: 49-56.
- Bastian FO, Sanders DE, Forbes WA, Hagius SD, Walker JV, et al. (2007) Spiroplasma spp. from transmissible spongiform encephalopathy brains or ticks induce spongiform encephalopathy in ruminants. J Med Microbiol 56: 1235-1242.
- Bastian FO, Boudreaux CM, Hagius SD, Bulgin MS, Sorensen-Melson SJ, et al. (2011) Spiroplasma found in the eyes of scrapie affected sheep. Vet Ophthalmol 14: 10-17.
- CLARK HF (1964) SUCKLING MOUSE CATARACT AGENT. J Infect Dis 114: 476-487.
- Clark HF (1974) The suckling mouse cataract agent (SMCA). A slow mycoplasma-like agent? Prog Med Virol 18: 307-322.
- Bastian FO, Purnell DM, Tully JG (1984) Neuropathology of spiroplasma infection in the rat brain. Am J Pathol 114: 496-514.
- Tully JG, Bastian FO, Rose DL (1984) Localization and persistence of spiroplasmas in an experimental brain infection in suckling rats. Ann Microbiol (Paris) 135A: 111-117.
- Bastian FO, Elzer PH, Wu X (2012) Spiroplasma spp. biofilm formation is instrumental for their role in the pathogenesis of plant, insect and animal diseases. Exp Mol Pathol 93: 116-128.
- Fries GF, Marrow GS, Snow PA (1982) Soil ingestion by dairy cattle. J Dairy Sci 65: 611-618.
- David Walter W, Walsh DP, Farnsworth ML, Winkelman DL, Miller MW (2011) Soil clay content underlies prion infection odds. Nat Commun 2: 200.
- Miller MW, Williams ES (2003) Prion disease: horizontal prion transmission in mule deer. Nature 425: 35-36.
- McAuliffe L, Ayling RD, Ellis RJ, Nicholas RA (2008) Biofilm-grown Mycoplasma mycoides subsp. mycoides SC exhibit both phenotypic and genotypic variation compared with planktonic cells. Vet Microbiol 129: 315-324.
- Gophna U, Barlev M, Seijffers R, Oelschlager TA, Hacker J, et al. (2001) Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect Immun 69: 2659-2665.
- Barnhart MM, Chapman MR (2006) Curli biogenesis and function. Annu Rev Microbiol 60: 131-147.
- Wang X, Chapman MR (2008) Curli provide the template for understanding controlled amyloid propagation. Prion 2: 57-60.
- Lundmark K, Westermark GT, Olsén A, Westermark P (2005) Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc Natl Acad Sci U S A 102: 6098-6102.
- Mead S (2006) Prion disease genetics. Eur J Hum Genet 14: 273-281.
- Collins S, McLean CA, Masters CL (2001) Gerstmann-Sträussler-Scheinker syndrome, fatal familial insomnia, and kuru: a review of these less common human transmissible spongiform encephalopathies. J Clin Neurosci 8: 387-397.
- Tateishi J, Kitamoto T (1995) Inherited prion diseases and transmission to rodents. Brain Pathol 5: 53-59.
- Hourrigan JL (1990) Experimentally induced bovine spongiform encephalopathy in cattle in Mission, Tex, and the control of scrapie. J Am Vet Med Assoc 196: 1678-1679.
- Anbutsu H, Fukatsu T (2006) Tissue-specific infection dynamics of male-killing and nonmale-killing spiroplasmas in Drosophila melanogaster. FEMS Microbiol Ecol 57: 40-46.
- Razin S, Yogev D, Naot Y (1998) Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 62: 1094-1156.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al. (2002) Molecular Biology of the Cell, 4th edition. Garland Science, New York, NY.
- Orrù CD, Wilham JM, Vascellari S, Hughson AG, Caughey B (2012) New generation QuIC assays for prion seeding activity. Prion 6: 147-152.
- Geschwind MD, Martindale J, Miller D, DeArmond SJ, Uyehara-Lock J, et al. (2003) Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol 60: 813-816.
- Bastian FO, Jennings RA, Gardner WA (1987) Antiserum to scrapie-associated fibril protein cross-reacts with Spiroplasma mirum fibril proteins. J Clin Microbiol 25: 2430-2431.
- Shoenfeld Y, Aron-Maor A (2000) Vaccination and autoimmunity-'vaccinosis': a dangerous liaison? J Autoimmun 14: 1-10.
- Barnett KC, Palmer AC (1971) Retinopathy in sheep affected with natural scrapie. Res Vet Sci 12: 383-385.
- Ikram MK, Cheung CY, Wong TY, Chen CP (2012) Retinal pathology as biomarker for cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry 83: 917-922.
- Honjo K, van Reekum R, Verhoeff NP (2009) Alzheimer's disease and infection: do infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement 5: 348-360.
- Kountouras J, Tsolaki M, Gavalas E, Boziki M, Zavos C, et al. (2006) Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 66: 938-940.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14493
- [From(publication date):
April-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9881
- PDF downloads : 4612