Critical Appraisal of Randomised Controlled Trials
Received: 21-Dec-2015 / Accepted Date: 28-Dec-2015 / Published Date: 04-Jan-2016 DOI: 10.4172/2471-9919.1000e114
41585Introduction
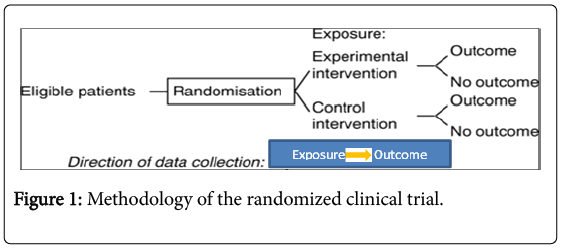
A randomized clinical trial is a type of study designed to evaluate therapeutic interventions and is often used to test the efficacy of a treatment approach in a population of patients or to gather information about potential adverse events of a particular procedure. Furthermore, patients are randomly allocated to receive one of various clinical interventions (Figure 1).
In order to practice with the most up-to-date knowledge of evidence-based medicine, we should take into account as well as implement the results of clinical research well designed and adequately conducted in specific settings and individuals, which represent part of the decision making process. Table 1 shows the checklists needed to make a critical analysis of randomised controlled trials [1-12].
| Appraisal questions | |||
|---|---|---|---|
| Did the trial address a clearly focused issue? (The population studied • The intervention given • The comparator given • The outcomes considered) | |||
| The study addresses an appropriate and clearly focused question. The assignment of subjects to treatment groups is randomized. An adequate concealment method is used. | |||
| The treatment and control groups are similar at the start of the trial. The only difference between groups is the treatment under investigation. Was allocation adequately concealed by a rigorous method (e.g. random numbers)? | |||
| Were all subjects who entered the trial accounted for at its conclusion? Were they analysed in the groups to which they were randomised, i.e. intention-to-treat analysis? | |||
| Were appropriate measures of baseline characteristics taken in all groups before the intervention, and were study groups shown to be comparable in all characteristics likely to influence outcome? Was there a baseline measure of performance and patient outcomes, and were study groups comparable in these at baseline? | |||
| Was the assignment of patients to treatments randomised? How was this carried out, some methods may produce broken allocation concealment • Was the allocation concealed from researchers? | |||
| Were patients, health workers and study personnel blinded? (Health workers could be; clinicians, researchers, etc. • Study personnel – especially outcome assessors) | |||
| Were the groups similar at the start of the trial? (Other factors that might affect the outcome such as age, sex, social class, these may be called baseline characteristics or clinical profile) | |||
| The only difference between groups is the treatment under investigation. What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed? | |||
| Aside from the allocated treatment, were groups treated equally? All the subjects are analysed in the groups to which they were randomly allocated (often referred to as intention to treat analysis). | |||
| Were all patients who entered the trial accounted for and were they analysed in the groups to which they were randomised? Where the study is carried out at more than one site, results are comparable for all sites. | |||
| Were all of the patients who entered the trial properly accounted for at its conclusion? (Was the trial stopped early? • Were patients analysed in the groups to which they were randomised?) | |||
| How large was the treatment effect? (What outcomes were measured? • Is the primary outcome clearly specified? • What results were found for each outcome? • Is there evidence of selective reporting of outcomes?) | |||
| How precise was the estimate of the treatment effect? (• What are the confidence limits? • Were they statistically significant?) | |||
| Is my patient so different to those in the study that the results cannot apply? | |||
| Is the treatment feasible in my setting? How well was the study done to minimise bias? | |||
| Taking into account clinical considerations, your evaluations of the methodology used, and the statistical power of the study, are you certain that the overall effect is due to the study intervention? | |||
| Will the potential benefits of treatment outweigh the potential harms of treatment for my patient? | |||
| What were the results? | |||
| Outcome event | |||
| Yes | NO | Total | |
| Experimental group | A | B | A+B |
| Control group | C | D | C+D |
| Experimental event rate=Risk of outcome event in experimental group=EER=a/(a+b) Control event rate=Risk of outcome event in control group=CER=c/(c+d) Relative risk reduction (RRR) = (CER-EER)/CER or 1-RR Absolute risk reduction (ARR) =CER-EER Number needed to treat (NNT) =1/ARR=1/(CER-EER) |
|||
| How large was the treatment effect? How the results were expressed (RRR, NNT, etc.). | |||
| Are the results of this study directly applicable to the patient group targeted by this guideline or clinical statement? | |||
| How great would the benefit of therapy be for my particular patient? | |||
| Can the results be applied to your organization? (Do you have any reason to believe that your population of interest is different to that in the trial • If so, in what way?) | |||
| Were all clinically important outcomes considered? (Is there any additional information you would like to have observed? • Was the need for this trial clearly described?) | |||
| Was the primary outcome measure valid (i.e., do two independent raters agree that this was a sensible and reasonable measure of performance or outcome)? Was the primary outcome measure reliable (i.e., do two independent raters agree on the nature and extent of change)? | |||
| Is it unlikely that the control unit of allocation (professional, practice, institution, community) received the intervention through contamination? Were outcomes measured by ‘blinded’ observers or were they objectively verified (e.g. quantitative measures recorded prospectively and independently)? | |||
| Was there complete follow-up of patient groups (ideally>95%)? Was follow-up continued for long enough for the primary outcome measure to show an impact and for sustainability to be demonstrated? | |||
| Are the benefits worth the harms and costs? (Even if this is not addressed by the trial, what do you think?) | |||
| Should policy or practice change as a result of the evidence contained within this trial? | |||
| Conflicts of interest are declared. | |||
High quality (++): Majority of criteria met. Little or no risk of bias.
Acceptable (+): Most criteria met. Some flaws in the study with an associated risk of bias.
Low quality (-): Either most criteria not met, or significant flaws relating to key aspects of study design.
Reject (0): Poor quality study with significant flaws. Wrong study type. Not relevant to guideline.
Table 1: Critical appraisal of randomised controlled trials.
Use of this checklist can improve the evaluation of randomised controlled trials.
References
- Guyatt G, Meade MO, Cook DJ, Rennie D (2014) Users' Guides to the Medical Literature: A Manual for Evidence-based Clinical Practice, Third edition. New York.
- Sackett DL, Richardson WS, Rosemberg WS, Rosenberg W, Haynes BR (2010) Evidence-Based Medicine: how to practice and teach EBM. Churchill Livingstone.
- Critical Appraisal Skills Programme (CASP), Public Health Resource Unit, Institute of Health Science, Oxford.
- Godin k, Dhillon M, Bhandari M (2011) The three-minute appraisal of a randomized trial. Indian J Orthop 45: 194–196.
- Abdelnoor M, Sandven I, Limalanathan S, Eritsland J (2014) Postconditioning in ST-elevation myocardial infarction: a systematic review,criticalappraisal, and meta-analysis ofrandomizedclinicaltrials. Vasc Health Risk Manag 10: 477–491.
- Hoffmann T, Bennett S, Del Mar C (2013). Introduction to evidence-based practice. Evidence Based Practice across the Health Professions. Sydney.
- Higgins JPT, Altman DG, Sterne JAC (2011) Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration.
- Fethney F (2010) Statistical and clinical significance, and how to use confidence intervals to help interpret both. Australian Critical Care 23: 93-97.
- Young JM, Solomon MJ (2009) How to critically appraise an article. Nature Clinical Practice Gastroenterology andHepatology 6: 2.
- Guyatt GH, Sackett DL, Cook DJ (1994) User’s Guide to the Medical Literature; II. How to use an article about therapy or prevention – B. What were the results and will they help me in caring for my patients? JAMA 271: 59-63.
Citation: Roever L, Oliveira BFG (2016) Critical Appraisal of Randomised Controlled Trials. Evidence Based Medicine and Practice 1: e114. DOI: 10.4172/2471-9919.1000e114
Copyright: © 2016 Roever L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15152
- [From(publication date): 4-2016 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 13810
- PDF downloads: 1342