Case Report Open Access
Creutzfeldt-Jakob Disease and Hashimotos Thyroiditis: A Case Report Illustrating Prion-Induced Encephalitis
| Luis Dabul1, Gerardo F Ferrer2, Susanna Oh3, Juan D Oms2, Marcos A Sanchez-Gonzalez1* and Rhaisa Dumenigo2 | |
| 1Division of Clinical and Translational Research, Larkin Community Hospital, South Miami, FL, USA | |
| 2Department of Psychiatry, Larkin Community Hospital, South Miami, FL, USA | |
| 3Department of Neurology, Larkin Community Hospital, South Miami, FL, USA | |
| *Corresponding Author : | Dr. Marcos A Sanchez-Gonzalez Division of Clinical and Translational Research Larkin Community Hospital 7000 SW 62nd Ave South Miami, Florida 33143, USA Tel: 3052847608 E-mail: masanchez@larkinhospital.com |
| Received: February 04, 2016 Accepted: February 11, 2016 Published: February 15, 2016 | |
| Citation: Dabul L, Ferrer GF, Oh S, Oms JD, Sanchez-Gonzalez MA, et al. (2016) Creutzfeldt-Jakob Disease and Hashimotos Thyroiditis: A Case Report Illustrating Prion-Induced Encephalitis. J Neuroinfect Dis 7:204. doi:10.4172/2314-7326.1000204 | |
| Copyright: © 2016 Dabul L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Sporadic creutzfelt-Jakob disease (sCJD) is a rare neurodegenerative disorder and the most common type of the prion diseases that affects humans. Its clinical manifestations are progressive dementia, myoclonus, visual or cerebellar disturbances, pyramidal dysfunction, and akinetic mutism. Laboratory findings include high levels of the 14-3-3 protein in cerebrospinal fluid and Electroencephalogram with periodic spikes at ≈ 1 Hz. We present a 51- year-old Hispanic woman with Hashimoto’s thyroiditis and a 1-year history of depression who developed rapidly progressive dementia. She required extensive diagnostic examinations and eventually was diagnosed with sCJD based on the World Health Organization diagnostic criteria. Real-time quaking-induced conversion (RT-QuIC) was positive, and thyroid peroxidase antibodies (TPO-AB) (2391 IU/ml) and 14-3-3 protein (3.8 ng/ml) were elevated. Her key findings included rapidly progressive dementia, ataxia, akinetic mutism, myoclonus, rigidity, visual disturbance, EEG (Electroencephalogram) with periodic sharp wave complexes at 1 Hz, and normal head CT scans. She progressively worsened and died 3 months after the onset. In sum, patients with CJD and Hashimoto’s thyroiditis should be (i) evaluated for autoimmune encephalitis, (ii) screened for a rise in TPO antibodies, and (iii) steroids administration should be considered as adjuvant therapy. This case illustrates a prion disease mediated immune system modulation which we believe is a potential underlying mechanism by which the 14-3-3 protein induces a steroid non-responsive autoimmune encephalitis.
| Keywords |
| Creutzfelt-jakob disease; Prion; Autoimmune encephalitis; Hashimoto’s thyroiditis; Prion-induced autoimmune disease |
| Abbreviations |
| sCJD: Sporadic Creutzfelt-Jakob Disease; CSF: Cerebrospinal Fluid; EEG: Electroencephalogram; WHO: World Health Organization; TPO-AB: Thyroid Peroxidase Antibodies; ANA: Antinuclear Antibodies; CT: Computerized Tomography; PCR: Polymerase Chain Reaction; DNA: Deoxyribonucleic Acid; RNA: Ribonucleic Acid; MRI: Magnetic Resonance Imaging; PO: Per Oral; B/L: Bilateral; TSH: Thyroid Stimulating Hormone; RPR: Rapid Plasma Reagin; HIV: Human Immunodeficiency Virus; HSV-1: Herpes Simplex Virus type 1; HSV-2: Herpes Simplex Virus Type 2; CMV: Cytomegalo Virus; HTLVI: Human T-cell Lymphotropic Viruses Type I; HTLV-II: Human T-cell Lymphotropic Viruses Type II; AFB: Acid Fast Bacilli; VDRL: Venereal Disease Research Laboratory; HE: Hashimoto's Encephalopathy; Ig: Immunoglobulin; CSR: Class Switch Recombination |
| Introduction |
| Creutzfeldt-Jakob disease (CJD), which is a rare neurodegenerative that affects humans, is part of the family of prion diseases known as transmissible spongiform encephalopathies [1]. There are four subtypes of CJDs: sporadic, familial, iatrogenic, and variant, with the majority of all disease cases reported as sporadic form (85%) [2]. CJD occurs at a rate of 1-1.5 people per million worldwide annually [3]. Interestingly, the risk of CJD increases with age, such that the annual rate of the disease increases to 3.4 cases per million in persons over 50 years of age, with death occurring at a median of 4-6 months after the onset of illness [4]. Although the exact nature of this infectious disease is poorly understood, we know that a non-nucleic acid, but rather a protein based particle is considered the causal entity. |
| The normal cellular human prion protein (PrPC) comprises 253 amino acids mainly structured as alpha helixes. In normal physiological conditions, PrPC is highly hydrophilic, sensitive to digestion, and noninfectious. Conversely, infective Prion protein (PrPSc) has the same primary amino acid sequence as its isoform PrPC with a different three- dimensional conformation. This conformational change makes PrPSc pathogenic, composed mainly of beta sheets making it hydrophobic and ultimately resistant to protease digestion [1]. Notably, PrPSc is neurotropic and its initial progression through the body before its final aggregation in the nervous system is unclear [5]. With several epidemiologic and ubiquitous features of prion diseases making them rare albeit devastating infectious neurological diseases, a prion is a host protein with an altered conformation. It evades antigen recognition and thus antibody production; it also lacks DNA or RNA making it undetectable by PCR [5]. Currently, there are still several challenges in diagnosing prion diseases partly because of their clinical resemblance to some neurological and immunological medical entities. |
| Accordingly, this case report illustrates a patient with an established diagnosis of probable sporadic CJD, which was based on the world health organization (WHO) clinical diagnostic criteria, with clinical and laboratory parameters that are typically found in autoimmune encephalitis. After a detailed examination of the present case, we concluded that it is plausible to propose a prion-induced autoimmune encephalopathy as an alternate mechanism of disease progression. The case describes the patient’s history of illness from the initial presentation to our hospital until death after 3 months. |
| Case Presentation |
| A 51-year-old Hispanic (Cuban) woman presented to the emergency room of our hospital complaining of unsteady gait, dizziness, and unexplained weight loss for the past two months. She felt dizzy when walking and could not keep her balance in addition to complaining of difficulty urinating, but denied dysuria and polyuria. During this initial presentation, she also experienced delusions of being poisoned such that wanted her blood to be cleaned. Her records revealed that within the same month of her presentation to our hospital, she had visited four other hospitals reporting similar symptoms. An MRI and CT scans of the brain, with and without contrast, had been done both of which were reported with normal findings. |
| After the patient was evaluated for ataxia, she was discharged home. Ten days later she was brought to the ER by her mother because of extreme anxiety and agitation. At the time, she also complained of difficulty focusing with her right eye. She was heavily sedated and ultimately was admitted to the hospital. On the second day of her admission, she became mute while her condition started to deteriorate developing progressive dementia, altered mental status, dysphagia to solid foods and medications, and responsive only to painful stimuli. |
| Her past medical history was significant for obsessive compulsive disorder, generalized anxiety disorder, depressive disorder, and Hashimoto’s thyroiditis, but had no known allergies. Her home medications were Alprazolam tabs 2 mg PO and Seroquel 100 mg PO both given at bedtime. Surgical history revealed a left breast lump biopsy and tonsillectomy. Family history revealed a paternal cousin with multiple sclerosis and a paternal grandmother with Alzheimer’s disease. Social history showed that the patient smoked 1 PPD for 33 years, and used recreational drugs such as cocaine and marijuana. In addition, the patient was a victim of rape at the age of 19 years old. She worked as a “call girl” and part of her job included traveling to different countries including Europe. |
| Her physical exam in the ER during her first admission was within normal limits. However, during her second admission (10 days later) the patient was alert, awake, and oriented to self only (AAO 1) becoming mute unable to complete the Mini-Mental State Examination. She developed a progressive descending decorticate posture with bilateral (B/L) flexion of the hands and later B/L flexion of the feet. She had a posture of flexion of her trunk at the waist. Her deep tendon reflexes were 3/4, motor strength was 5/5, and the sensation was decreased unilateral in the right upper and lower extremities. She had myoclonic jerks B/L in the upper and lower extremities with negative Babinski reflexes. The finger nose cerebellar test was abnormal. Eye examination showed B/L mydriasis with pupil diameter of 6 mm, and B/L involuntary horizontal eye movements of supra nuclear origin. |
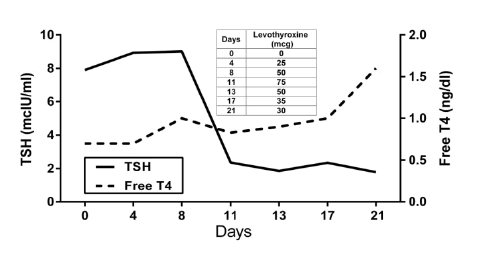
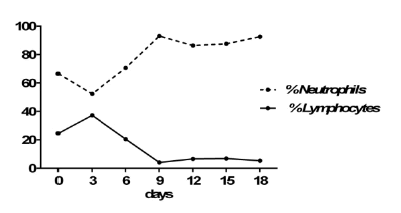
| Lab blood work was significant for elevated WBCs of 11.2 103/mcL without left shift (% Neutrophils 66.5; Table 1) and elevated lipase of 175.2 U/L (Table 2). Initial thyroid function evaluation showed an elevated TSH of 7.9 mcIU/ml and normal free T4 of 0.7 ng/dl (Figure 1). Thyroid peroxidase antibodies (TPO-AB) were elevated at 2391 IU/ml, and antithyroglobulin antibodies were normal at <20 U/ml. Antinuclear antibodies (ANA) were positive, CRP and rheumatoid factor were normal. Folic acid and Vitamin B12 levels were normal at 21.8 ng/ml and 514 pg/ml, respectively. Cultures from blood, CSF, and urine were all negative. Rapid plasma reagin (RPR) and human immunodeficiency virus (HIV) tests were negative. Herpes Simplex Virus type 1 (HSV-1) IgG antibodies were normal at 0.20 index value (IV) and Herpes Simplex Virus type 2 (HSV-2) IgG antibodies were elevated at 11.40 IV. CMV IgM antibodies were negative, but CMV IgG antibodies were positive at >10 u/ml. Arterial Blood Gasses showed a pH of 7.44, PaCO2 of 41.1 mmHg, and HCO3 -1 of 27.9 mEq/L. Urine toxicology was negative for barbiturates, cocaine, methadone, opiates, and phencyclidine. Urinalysis (UA) showed a large amount of blood and moderate bacteria. |
| Cerebrospinal fluid (CSF) studies showed an increased glucose of 72 mg/dl and normal protein of 26 mg/dl. Cytology specimen was acellular, negative for atypical and malignant-appearing cells. Positive real time quaking induced conversion (RT-QuIC), and elevated 14-3-3 protein (3.8 ng/ml), tau protein (4167 pg/ml), total lymphocyte count (4617 cells/ul), and absolute CD4 cell count (2632 cells/ul) were present. Arbo virus IgM and IgG antibodies were negative. HSV-1 and HSV-2 DNA PCR were negative. Human T-cell lymphotropic viruses type I (HTLV-I) and type II (HTLV-II) qualitative analysis was nonreactive. Antibodies to Borrelia burgdorferi were not detected. Cryptococcal antigen, Indian ink stain, acid-fast bacilli (AFB) smear and culture, and venereal disease research laboratory (VDRL) tests were negative. Electroencephalogram (EEG) showed periodic sharp wave complexes at 1Hz. CT scans done on two occasions were reported with normal findings (Figure 2). |
| Part of her medical treatment in the hospital included Decadron (Dexamethasone) at an initial dosage of 6 mg IV every 8 hours. She remained on steroid treatment for a total of 14 days, with a downward titration to a final dose of 2 mg every 12 hours. In addition, she was treated with Synthroid at an initial dose of 25 mcg, up to a maximum dose of 75 mcg, and titrated down to 30 mcg. The patient’s clinical condition did not show any improvement in this medical treatment. |
| The patient was diagnosed with CJD and the family was explained about the course and poor prognosis of the disease. The patient entered into hospice care, kept on morphine at 5 ml per hour for end of life comfort. She expired three months after the onset of her symptoms of dizziness and ataxia while becoming unresponsive and having an absence of respiratory movements. |
| Discussion |
| The WHO defines sporadic CJD into three categories: (i) possible, (ii) probable, and (iii) confirmed [6]. A diagnosis of probable CJD includes progressive dementia (<2 years duration) and myoclonus, visual or cerebellar disturbance, akinetic mutism, typical EEG (generalized periodic sharp wave complexes at ≈ 1 Hz), and or a positive 14-3-3 protein assay in CSF [6]. Patient presented in this case met criteria for a diagnosis of probable sporadic CJD because of her symptoms of rapidly progressive dementia, visual and cerebellar disturbance, and akinetic mutism. Furthermore, her EEG and CSF analysis were consistent with this diagnosis as CSF samples showed a high level of the 14-3-3 protein. In diagnosing CJD, the 14-3-3 protein in CSF has been reported as having a high sensitivity (86-90%) and intermediate specificity (40-68%) whereas the EEG a sensitivity of 44% and specificity of 92% [7]. In addition, prior studies have demonstrated that 14-3-3 protein has a sensitivity of 92% and a specificity of 80% in diagnosing sporadic CJD [8]. Consistent with a positive test in the present patient, CSF RT-QulC analysis has a sensitivity of 91% andv specificity of 98% in diagnosing sCJD [9], and CT scansare usually normal in patients with prion diseases, which also allow for excluding other neurological diseases [5]. |
| There are several reasons why this case illustrates a diagnostic challenge. First, CJD has an unusual presentation and is a disease that has a very low incidence. For example, Kamogawaet, et al. described a CJD case of a 74-year-old man with clumsiness and levitation of his right upper limb [10]. Second, in the present case the patient had preexisting psychiatry conditions (depression and anxiety disorders), making catatonic depression and anxiety disorders part of the initial differential diagnoses. Furthermore, psychiatric symptoms (i.e. agitation, hallucinations, anxiety, and depression) have been reported to be present in as high as 90% of patients with sporadic CJD [11]. Milanliouglu et al. described a case of patient with sporadic CJD who presented initially with symptoms of catatonic depression [12]. Finally, patient presented with depressive symptoms that were tractable to psychosocial stressors (unemployment, etc.) and physiological causes (Hashimoto’s thyroiditis). Because of this, it was unclear to us if CJD caused an exacerbation, or was indeed the primary cause of the patient’s psychiatric disorders. |
| The most common misdiagnoses of CJD have been neurodegenerative, autoimmune, paraneoplastic, infectious, and toxic or metabolic disorders [13]. The patient presented in this case had Hashimoto’s thyroiditis, which introduced an autoimmune disease as another diagnosis that could explain her symptoms. This presented another challenge in diagnosing the patient because of the possibility of Hashimoto’s encephalopathy (HE). HE has been reported as mimicking CJD [14] and as having an initial presentation of acute psychosis [15]. Furthermore, CJD can mimic autoimmune encephalitis, and antibodies can be found in the patient’s CSF [16]. It has been reported that Hashimoto’s thyroiditis is associated with encephalopathy, and that thyroid antibody titers do not correlate with the severity of symptoms [17]. HE is treatable with steroids with a high remission of up to 80% [17]. In addition, when anti-thyroid antibodies are present in patients with CJD, a trial of steroids is recommended [18]. Lastly, it is of extreme clinical significance to diagnose and treated appropriately patients with HE because of the good prognosis of the disease when compared to CJD. |
| The treatment of choice addressed both an autoimmune thyroiditis and encephalitis. She was treated with steroids (Dexamethasone) for a total of 14 days, showing a significant decrease in absolute lymphocyte count, which serves as evidence that there was a depression in the immune system response (Figure 3), albeit the clinical picture did not improve. As mentioned above, steroids are recommended in patients that have a diagnosis of CJD and have anti-thyroid antibodies. Furthermore, patients with HE treated with steroids have shown improvement within 1 week [19]. Lastly, her thyroid function was monitored and showed normalization of lab values after appropriate treatment. Unfortunately, she did not show any clinical symptomatic improvement with either treatment, and we attributed this to the presence of the prion disease. |
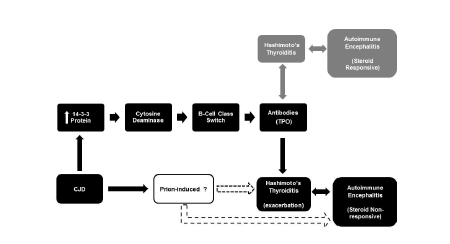
| The CSF analysis showed high levels of the 14-3-3 protein. The 14-3-3 protein plays a crucial role in immunoglobulin (Ig) class switch DNA recombination (CSR) [20]. It substitutes the constant region of the Ig heavy chain, giving rise to the different type of antibodies (IgG, IgE, IgA) without changing the structure of the antigen binding site [21]. Thus, we propose a potential underlying interplay between the 14-3-3 protein and dysregulation of patient’s immune system inducing steroid non-responsive autoimmune encephalitis (Figure 4). Further immuno-biological research studies are needed to investigate a molecular basis for the interaction between the 14-3-3 protein and the production of TPO antibodies. |
| We propose that in this patient the 14-3-3 protein and immune system interaction let to the production of high levels of TPO antibodies because of an underlying autoimmune disease manifested as Hashimoto’s thyroiditis. High levels of TPO antibodies are found in the blood and CSF of patients with HE [22,23]. In addition, there is a previously well study association between the autoimmune diseases Hashimoto’s thyroiditis and encephalitis [22]. Hence, the presence of a neurodegenerative disease of autoimmune origin, pathologically mediated through TPO antibodies, and induced by the prion disease should not be disregarded in this case. |
| The present study is limited by a lack of biopsy due to the patient’s and family wishes to not have the procedure done. Thus, we illustrated the case with a diagnosis of probable sCJD based on clinical, laboratory, and radiographic findings. |
| Conclusion |
| This case provides a description of a patient diagnosed with sporadic CJD from the onset of symptoms to death after 3 months. Patient presented with clinical symptoms and laboratory findings that are found in CJD and HE. In addition, diagnosing CJD can be very challenging because the disease can also manifest as psychiatric symptoms (depression, agitation, hallucinations) and neurologic symptoms (ataxia, akinetic mutism, and myoclonus). Interestingly, we hypothesize that there is an interaction between up-regulation of the immune system caused by the prion disease. This case can serve as a through studied model in the troublesome diagnosis of CJD in patients with Hashimoto’s thyroiditis, and provides enough evidence to warrant further investigation of the modulation of the immune system by prion diseases. |
References
- Ryou C (2007) Prions and prion diseases: fundamentals and mechanistic details. Journal of microbiology and biotechnology 17: 1059-1570.
- Prusiner SB (2001) Shattuck lecture--neurodegenerative diseases and prions. The New England journal of medicine344: 1516-1526.
- Lee J, Hyeon JW, Kim SY, Hwang KJ, Ju YR, et al. (2015)Review: Laboratory diagnosis and surveillance of Creutzfeldt-Jakob disease. Journal of medical virology 87(1): 175-186.
- Belay ED,Holman RC, Schonberger LB (2005) Creutzfeldt-Jakob Disease Surveillance and Diagnosis 41: 834-436.
- Erdtmann R, Sivitz L (2003)Advancing Prion Science: Guidance for the National Prion Research Program: Interim Report. Washington, USA.
- http://www.who.int/csr/resources/publications/surveillance/whocdscsrisr992.pdf
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, et al. (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132: 2659-2668.
- Muayqil T, Gronseth G, Camicioli R (2012) Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 79: 1499-1506.
- McGuire LI, Peden AH, Orru CD, Wilham JM, Appleford NE, et al. (2012) Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 72: 278-285.
- Kamogawa K, Ninomiya S, Okuda S, Matsumoto Y, Tomita H, et al. (2014) [A case of Creutzfeldt-Jakob disease presenting with arm levitation as an initial symptom]. Rinsho shinkeigaku 54: 803-808.
- Krasnianski A, Bohling GT, Harden M, Zerr I (2015) Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. The Journal of clinical psychiatry.
- Milanlioglu A, Ozdemir PG, Cilingir V, Ozdemir O (2015) Catatonic depression as the presenting manifestation of creutzfeldt-Jakob disease. J Neurosci Rural Pract 6: 122.
- Paterson RW, Torres-Chae CC, Kuo AL, Ando T, Nguyen EA, et al. (2012) Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol69: 1578-1582.
- Muramatsu T, Hamano T, Shirafuji N, Matsunaga A, Ikawa M, et al. (2013) Hashimoto's encephalopathy presenting periodic synchronous discharge, as a differential diagnosis for Creutzfeldt-Jakob disease. Rinsho shinkeigaku = Clinical neurology 53: 716-720.
- Guirgis H, Amar C (2014) A Case of Hashimoto's Encephalopathy Presenting With Acute Psychosis. The Journal of neuropsychiatry and clinical neurosciences. 26: E1-2.
- Zuhorn F, Hubenthal A, Rogalewski A, Onugoren MD, Glatzel M, et al. Creutzfeldt-Jakob disease mimicking autoimmune encephalitis with CASPR2 antibodies. BMC neurology 14: 227.
- Kothbauer-Margreiter I, Sturzenegger M, Komor J, Baumgartner R, Hess CW (1996) Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment. Journal of neurology 243: 585-593.
- Kondziella D, Hansen K, Gonzalez T, Gideon P, Christiansen I, Sellebjerg F (2012) [Anti-thyroid antibodies in two patients with subacute dementia, ataxia, and myoclonus]. Ugeskrift for laeger 174: 577-579.
- Anand KS, Garg J, Verma R, Chakraborty A (2014) Hashimoto's Encephalitis: Unusual Cause of Reversible Dementia. J Family Med Prim Care 3: 284-286.
- Lam T, Thomas LM, White CA, Li G, Pone EJ, et al. (2013) Scaffold functions of 14-3-3 adaptors in B cell immunoglobulin class switch DNA recombination. PloS one 8: e80414.
- Xu Z, Fulop Z, Zhong Y, Zan H, Casali P, et al. (2005) DNA lesions and repair in immunoglobulin class switch recombination and somatic hypermutation. Ann N Y Acad Sci 1050: 146-162.
- Hilberath JM, Schmidt H, Wolf GK (2014) Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): case report of reversible coma and status epilepticus in an adolescent patient and review of the literature. European journal of pediatrics 173: 1263-1273.
- Yoneda M (2013) [Hashimoto's encephalopathy and autoantibodies]. Brain and nerve = Shinkei kenkyu no shinpo 65: 365-376.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11805
- [From(publication date):
March-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10845
- PDF downloads : 960
