Cranial Nerve Examination for Neurogenic Dysphagia Patients
Received: 09-Aug-2017 / Accepted Date: 28-Aug-2017 / Published Date: 05-Sep-2017 DOI: 10.4172/2161-119X.1000319
Abstract
This cross-sectional study aimed to validate a cranial nerve assessment for swallowing against fiber optic-endoscopic examination of swallowing (FEES) in a group of neurological patients. A specific cranial nerve evaluation was designed, comprising semi-qualitative evaluations of the main five cranial nerves involved in swallowing. Eighty-five participants were consecutively recruited with the following inclusion criteria were: a) neurological diagnosis; b) ability to respond to simple verbal command; c) absence of citrus allergy and age<18 years. All participants first underwent cranial nerve examination (the “I&I” test), followed by a FEES conducted by a blinded otolaryngologist who completed the penetration aspiration scale (PAS) and the functional oral intake scale (FOIS). The results showed that I&I test had a high sensitivity (0.89) and specificity (0.93) for identify dysphagia in persons with neurologic disorders, with an area under ROC curve of 0.97. Significant differences were found from the comparison between I&I test scores and the three severity levels of PAS. In conclusion, the I&I test is a useful scale to detect the major deficits affecting the cranial nerves in patients with swallowing disorders. In addition, it allows defining the pathophysiological dysphagia symptoms, which are fundamental for planning the customized rehabilitation interventions.
Keywords: Cranial nerve; Patients; Otolaryngologist; Dysphagia
256207Introduction
Oropharyngeal dysphagia is commonly associated with neurologic disease, as well as other acute and chronic conditions. Incidence is up to 70% following stroke [1], up to 97% in Amyotrophic Lateral Sclerosis [2] and ranges from 55-82% in Parkinson’s disease depending on the method of assessment [3,4]. It is widely accepted that the presence of dysphagia is associated with pulmonary complications, increased hospital length of stay, dehydration, malnutrition and ultimately mortality [5]. Patients who aspirate food and liquids into the airways are at increased risk of developing pneumonia [6].
The usual process for diagnosing dysphagia includes the following steps: 1) a screening phase; 2) clinical assessment and 3) instrumental evaluation [7]. A screening swallowing assessment is a pass/fail procedure to identify individuals who require a quick and comprehensive assessment of swallowing function or a referral for other professional and/or medical services [8]. Generally, it is designed to identify patients at high risk of dysphagia at the early stage of symptoms, whereas a clinical assessment is a behavioural evaluation of tone and motility of oro-faccial structures, of swallowing mechanism and function using different consistencies of food and liquid [7,9,10]. Video fluoroscopic swallowing study (VFS) and video-endoscopic endoscopic evaluation of swallowing (VEES) are considered the gold standards for detecting dysphagia and aspiration as they detect the abnormal anatomy or physiology causing the problem [11]. In the last decades, most studies have focused on the validity of screening with the dysphagia test and/or questionnaire in order to rapidly detect symptoms of swallowing disorders at the first stage. On other hand, few studies have analysed the feasibility and efficacy of the clinical swallowing assessment scale. These assessments are fundamental for providing detailed information on the integrity of cranial nerves and oral-pharyngeal structures in order to guide neurological diagnosis and/or predict the swallowing complicacies [12]. In the literature, there are two main swallowing assessment protocols: the Mann Assessment of Swallowing Ability (MASA) [13] and the dysphagia outcome and severity scale [14]. Although these scales are important for understanding the clinical pathophysiology of swallowing they have not been validated in Italian and do not mentioned the status of cranial nerves involved in swallowing. It is well recognised that evaluation of the five cranial nerves is essential in understanding deficits in swallowing function and in predicting negative outcomes, mostly in patients with neurological disorders [15,16]. To our knowledge there is no published study concerning a comprehensive cranial nerve examination associated with swallowing deficit severities. The aim of this study is to validate a cranial nerve assessment for swallowing against fiber optic endoscopic examination of swallowing (FEES) in a group of neurological patients. This assessment comprises the semi-quantitative evaluations of the five cranial nerves in charge of swallowing. The second aim was to verify which cranial nerves were more comprised in dysphagic patients.
Materials and Methods
This cross sectional study was conducted from August 2015 to November 2016. The study was carried out in accordance with the amended Declaration of Helsinki and received the Approval of the Ethical Committee for clinical experimentation of the province of Venice, Italy. Informed consent was taken from all participants directly or from legal decision makers or proxies.
Participants
Participants were consecutively recruited at the Neurorehabilitation Department of Fondazione Ospedale San Camillo I.R.C.C.S. (Venice- Italy). The inclusion criteria were: a) confirmed neurological diagnosis including stroke, Parkinson’s disease (PD), head and neck cancer, amyotrophic lateral sclerosis (ALS), or traumatic brain injury (TBI); b) ability to respond to simple verbal command; c) absence of citrus allergy and; d) age<18 years.
All participants first underwent the cranial nerve examination that was video-recorded in order to allow inter-rater reliability. Secondly, the instrumental evaluation of swallowing (FEES) was conducted. The otolaryngologist in charge of the examination was blind to the cranial nerve outcomes.
Cranial nerve examination test (I&I)
The I&I was developed after a detailed review of cranial nerve examinations reported in literature, comprising of five nerves that are well recognised as being principally involved in the swallowing process, including the assessment of the trigeminal nerve (V° CN), facial nerve (VII° NC), glosso-pharyngeal nerve (IX° NC), vagus nerve (X° NC), hypoglossum nerve (XII° NC) and ansa cervicalis assessment (XII°-CIII° NC) [9,12,16,17]. In particular, V° CN carries tactile sensations from the face and the oral cavity. Its motor efferent component innervates the muscles of mastication (masseters, temporalis, medial and lateral pterygoid muscles) and mylohyoid muscles and anterior bellies of the digastric muscles, which allows anterior elevation of the hyoid laryngeal complex during the swallowing reflex. Thus, it plays a crucial role not only during the mastication oral phase but mainly in the oropharyngeal phase for the following reasons. Firstly, the sensory input triggers the swallowing reflex. Secondly, the anterior-superior motion during the elevation of the hyolaryngeal complex contributes to upper esophageal sphincter opening and thirdly, this movement narrows the laryngeal lumen in order to protect airways. In order to valuate it, we selected the following items which include motor and sensory assessments: Jaw opening against resistant, Jaw lateralization, Tactile sensitivity of the face and Tactile sensitivity of 2/3 anterior portion of the tongue.
The VII° NC innerves the facial muscles including the orbicularis oris e buccinators muscles; the posterior belly of digastric and the stylohyoid muscles and the sub-mandible and sublingual salivary glands. In addition, the sensory components of the VII° NC convey the taste sensations from the anterior two-thirds of the tongue and oral cavity. This nerve prevents food/liquid spillage from the mouth and contributes to the production of saliva during bolus preparation into the oral cavity. In addition, the contraction of poster digastric belly and stylohoid muscle allow the approximation of base of tongue and pharyngeal wall during oral preparation. In the I&I, we evaluated the integrity of this nerve through the following tasks: Eye Closure, Wrinkle eyebrows, Smile and Kiss.
The IX° NC supplies general sensation and taste from the posterior one-third of the tongue, soft palate and pharynx and visceral sensation from the carotid body and sinus. It has a small branchial motor component to the stylopharyngeus muscle, which elevates the pharynx during swallowing and a parasympathetic motor component of the parotid gland for salivary secretion. The pharyngeal plexus is formed by branches from the external laryngeal and pharyngeal nerves (CN X), as well as branches from CN IX in order to innervate all the muscles of the pharynx, including the superior, middle and inferior pharyngeal constrictor muscles, palatopharyngeus and salpingopharyngeus. The contraction of the pharyngeal wall propels the bolus, generating the pharyngeal wave. Although it is difficult to test this nerve, we selected the following items: Elevation of soft palate, Tactile sensitivity of 1/3 posterior portion of the tongue, Gag reflex.
The X°NC is the largest visceral sensory nerve of the cranial nerves. In swallowing it is involved in carrying the visceral sensation from the lower pharynx, larynx, trachea (including cough receptors) and esophagus. The motor efferent innervates the cricopharyngeal muscles, the palatoglossus and the intrinsic muscles of the larynx. It is responsible for laryngeal adduction and for triggering coughs, which prevent food/liquid inhalations in to the airways and upper esophageal sphincter opening in order for the bolus to flow in the esophagus. In the I&I test the vagus nerve was tested by evaluation of Voluntary Cough [18], Reflex Cough [19], Vocal Quality and Wet voice [20].
The hypoglossal nerve supplies all the intrinsic tongue muscles in all but one of the extrinsic muscles of the tongue, the exception being the palatoglossus muscle, which is supplied by CN X. The tongue muscles are fundamental for the preparatory phase of swallowing. The intrinsic ones act by changing the shape of the tongue. The extrinsic muscles act to pull the tongue forward and protrude it (genioglossus), elevate and retract the tongue (styloglossus), depress the tongue (hyoglossus), as well as move it from side to side. The tongue movements was assessed by these tasks: Lingual Protrusion, Lingual Elevation, Lingual Lateralization, Sliding tongue, Click of tongue [9,10].
The ansa cervicalis is a loop of nerves formed by nerve roots C1-C3. It branches out four muscular branches: the superior belly of the omohyoid muscle, inferior belly of omohyoid muscle and the sternohyoid. It stabilize the neck during the swallowing act, in order to evaluate it we examined Head control, Up-down, Rotation right-left and the Inclination right-left.
Examination of all these nerves was included in the I&I. Each nerve was assessed through a set of specific items, which are summarized in Table 1. In addition, we defined specific scoring criteria to limit discrepancies between administrators.
| Variable | Category | Absolute frequency | Relative frequency |
|---|---|---|---|
| Gender | M | 48 | 0.565 |
| F | 37 | 0.435 | |
| Diagnosis | Stroke | 34 | 0.4 |
| Head and Neck Cancer | 10 | 0.12 | |
| Parkinson’s disease (PD) | 14 | 0.16 | |
| Amyotrophic Lateral Sclerosis (ALS) | 17 | 0.2 | |
| Traumatic Brain Injury | 10 | 0.12 | |
| Tracheostomy | Yes | 33 | 0.39 |
| No | 52 | 0.61 | |
| PAS | No penetration/aspiration (PAS=1) | 19 | 0.22 |
| Penetration Events (PAS=2-5) | 42 | 0.5 | |
| Aspiration Events(PAS=6-8) | 24 | 0.28 | |
| FOIS | Total Oral Intake with no restrictions (FOIS=7) | 23 | 0.27 |
| Total oral intake with restrictions (FOIS=4-6) | 37 | 0.43 | |
| Tube dependent (FOIS=1-3) | 25 | 0.29 |
Table 1: Descriptive information and frequency of the sample group, showing specific information regarding the diagnosis, the presence of tracheostomy and the severity of dysphagia assessed by Penetration Aspiration Scale (PAS) and Functional Oral Intake Scale (FOIS).
Fiberoptic endoscopic examination of swallowing (FEES)
The FEES was completed by a senior otolaryngologist using a flexible laryngoscope with xenon light transnasally without local anaesthesia [18], which visualized images directly of the base of the tongue, pharynx and larynx. The examination was completed for all patients including different swallowing bolus trials [21]: bolus semisolid apple puree (3 cc) with methylene blue and bolus fluid milk (3 ml). For all patients we scored the presence of residues using the laryngeal penetration-aspiration scale (PAS) [22] for each consistency. The PAS ranges from 1 (material does not enter airway) to 8 (material enters the airway, passes below the vocal folds). For the purpose of this study, we selected the worse scores obtained during the examination to use as the reference standard for the statistical analysis.
Inter-rater reliability
We evaluated the inter-rater reliability using two speech-language pathologists (AC;IK). AC had three years of clinical experience and IK had 29 years of experience in dysphagia management. The I&I were taken from 35 consecutive patients using video-recorded assessments. The examiners underwent one-day training before the study started. Inter-rater reliability was evaluated using intra-class correlation coefficient (ICC).
Data analysis
Demographic data were summarized using standard descriptive statistics. Dysphagia was categorized into three levels of severity depending on the PAS (PAS 1=no penetration/aspiration; PAS 2-5=level of penetration; PAS 5-8=level of aspiration) and FOIS (levels 1-3: tube dependent; levels 4-6: total oral intake with restriction and modification; 7=total oral intake with no restrictions). Non-parametric ANOVA (Kruskal-Wallis test) was used to determine if there were statistically significant differences between the I&I test and i) PAS scores and ii) FOIS scores. We used Receiver operating characteristic (ROC) curves to determine the performance of the models in predicting dysphagia. The minimum distance criterion (Youden index) was used as a criterion for selecting the cut-off point. Sensitivity and specificity were calculated to verify the validity of the I&I test and the Youden index was used. We also completed subgroup analysis of persons with a tracheostomy. Inter-ratater reliability and inter-rater reliability were calculated using Intra-class Correlation Coefficient (ICC). The analyses were completed using the software R and the significance threshold was set at p-value=0.05.
Results
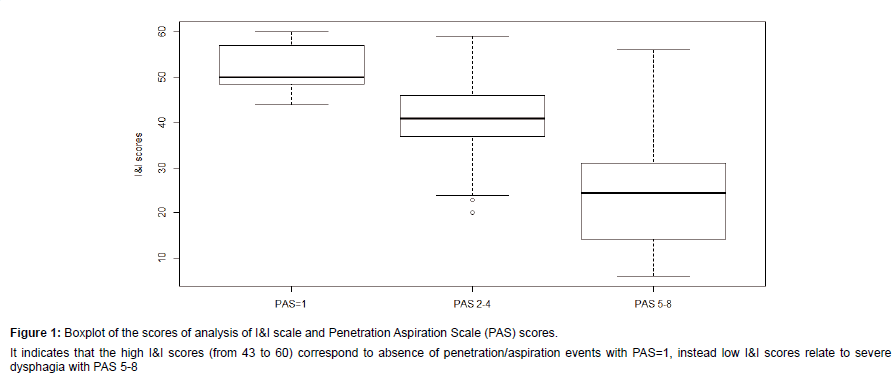
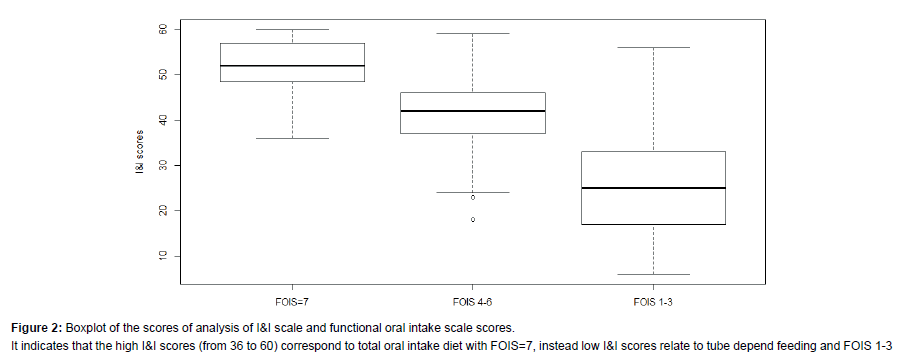
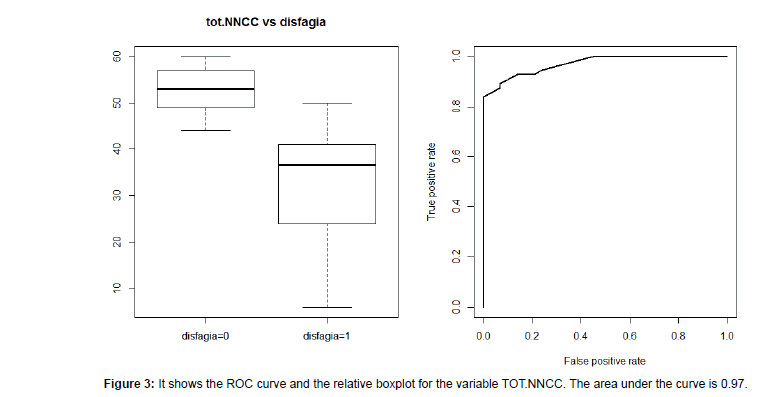
We recruited 85 persons (F: 37-56%; M: 48-44%). The majority presented with a diagnosis of stroke (n=34, 40%); followed by Amyotrophic Lateral Sclerosis-ALS (n=17, 20%); Parkinson’s disease (n=14, 16%); Traumatic Brain Injury (n=10, 12%); Head and Neck Cancer (n=10, 12%). The age varied from 18 to 83 years (mean 56.3 ± 18.2) and did not influence dysphagia severity (Test Kruskal-Wallis KWoss=5:258, p-value=0.072). Descriptive information are summarised in Table 1. The total scores of I&I ranged from 6 to 60 (mean 39.1 ± 14.). Nineteen patients (22%) did not show dysphagia at fibro-endoscopic evaluation reporting PAS=1 (Table 1). The I&I score ranged from 44 to 59. Among the 66 (88%) persons with dysphagia: 42 (50%) of them presented mild-moderate dysphagia (PAS from 2 to 5) with I&I scores from to 22 to 44 (mean 40 ± 5.31) and 24 (28%) of them presented severe dysphagia (PAS from 6 to 8) with I&I scores from 22 to 40 (mean 32 ± 5.14). We found a significant difference between PAS values and the total I&I scores (KWoss=38.07, p-value<0.001). Figure 1 shows that high PAS scores correspond to low I&I scores, revealing that persons with dysphagia performed poorly at the I&I. A significant difference was found also between FOIS and I&I scores (KWoss=42.43, p-value<0.001), indicating that persons with severe difficulties in oral intake had low I&I scores. The ROC curves (Figure 2) showed that the area under the curve (AUC) was (0.97) and the interval of confidence 0.841-1. The total scores of I&I presented good sensitivity (89%) and specificity (93%) at the cutoff value 43,5. The cut-off was obtained with the minimum criterion of Youden Index. In addition, the cranial nerve with higher sensitivity and specificity are trigeminus, glossopharyngeal and hypoglossum nerve; Table 2 summarized all the information for each nerve.Finally, the analysis of inter-rater reliability showed high values of agreement between scores of two examiners. (ICC=0.85) (Figure 3 and Table 3).
| Estimation Criteria | Cut-offs | Correct classification of ill patients | Correct classification of slightly ill patients | Correct classification of normal patients | |
|---|---|---|---|---|---|
| Tot I&I scores on Trachoestomized persons | Roc VUS=0.77 |
31-46 | 0.937 | 0.7 | 0.857 |
| Youden YI=0.62 |
28.8-43.2 | 0.789 | 0.557 | 0.888 |
Table 2: It showed the cut-offs estimated with the minimum distance between ROC surface and best point criteria and with generalized Youden Index criteria, VUS and Youden Index values and the percentage of correct classification of the patients in the subgroup of persons with tracheostomy and severe dysphagia, when compared to PAS.3LIVELLI variable.
| Estimation Criteria (Cut-off) |
AUC | Sensibility | Specificity | |
|---|---|---|---|---|
| TOT I&I |
Roc (43.5) |
0.97 | 0.89 | 0.93 |
| Youden (45.5) |
0.84 | 1 | ||
| V° CN |
Roc (9.5) |
0.9 | 0.79 | 0.83 |
| Youden (9.5) |
0.79 | 0.83 | ||
| VII° CN |
Roc (9.5) |
0.74 | 0.7 | 0.72 |
| Youden (8.5) |
0.63 | 0.83 | ||
| IX° CN |
Roc (4.5) |
0.9 | 0.87 | 0.76 |
| Youden (4.5) |
0.87 | 0.76 | ||
| X° CN |
Roc (8.5) |
0.86 | 0.68 | 0.86 |
| Youden (8.5) |
0.68 | 0.86 | ||
| XII° CN |
Roc (11.5) |
0.96 | 0.93 | 0.89 |
| Youden (10.5) |
0.87 | 0.96 |
Table 3: It showed the cut-off estimated with the minimum distance between ROC curve and best point criteria and with Youden Index criteria, AUC value and sensibility and specificity when compared to DISFAGIA variable.
Discussion
The present study aimed to create and validate a test to assess the integrity of main cranial nerves involved in swallowing process. We developed an assessment battery that included a precise scoring system to increase reliability among different examiners. The study demonstrated that the I&I is a valid and reliable scale for detecting the cranial nerves deficit related with swallowing disorders in neurological patients, showing high sensitivity and specificity in detecting dysphagia and high inter-rater reliability. It gives information on the major deficits affecting cranial nerves and defines the pathophysiology underlying dysphagia symptoms, which are fundamental for planning customised rehabilitation interventions. The scale also seems to be a versatile tool as it assesses overall severity as well as specific nerve damage, which enables precise swallowing issues to be addressed.
This is the first study to our knowledge that has designed a specific cranial nerve protocol for swallowing assessment. The I&I appears to be sensitive in detecting cranial nerve impairment associated with dysphagia. This feature is fundamental during the clinical in order to improve the understanding of which are underpinning elements the impairment in swallowing. In addition, investigating cranial nerves before instrumental examination and food/liquid bolus trials may facilitate the analysis and interpretation of the examinations. The combination of instrumental examination and bolus swallowing assessment with I&I test, can offer clinicians and researchers a standard approach to document dysphagia severity in order to design specific rehabilitation interventions. This could guide clinicians in understanding the pathophysiology in order to plan specific treatments and monitor the recovery.
In our study, the trigeminus, glossopharyngeal and hypoglossum nerves were the main nerves associated with dysphagia. This is consistent with previous evidence, as these nerves are commonly reported as the principal actors during deglutition. In particular, patients with lesions on these nerves often present severe dysphagia and are were mostly fed by enteral feeding. For this reason, it has been assumed they contribute substantially during swallowing and further studies should verify whether lesions on these nerves could be deemed a negative prognostic factor.
Collectively, these data indicate adequate consensual and criterion validity and cross-validation with other swallowing measures. In particular, three level of severity of dysphagia were individualized referring to the PAS scores and FOIS scores. The I&I scores appeared to reflex the severity levels, adding important information on potential damage of the swallowing disorders.
In addition, interrater reliability among two examiners was excellent, though the parameters were semi-quantitative. Hence, our results indicate that the I&I scale may be an appropriate tool to clinically document cranial nerve impairments.
We created the I&I for clinical purposes and for this reason included a sample of dysphagia patients with different neurologic diseases. Despite the heterogeneity of the sample, the I&I had good results among neurogenic dysphagic patients. Specific analysis of patient groups showed that the most severe patients had a diagnosis of ALS.
We are aware that this study has some limitations. First, the modality of scoring is not objective and depends on the professional expertise of the examiners. In addition, the glossopharyngeal nerve and vagus are difficult to clinically investigate. Although we tried to overcome this problem by defining specific details in the scoring system, we recognized this potential weakness. Secondly, the PAS scores during FEES do not report the presence and sites of pharyngeal residues, which are important for the understanding impairment. We chose PAS, because it is routinely used within our hospital to score dysphagia during instrumental examination. Thirdly, the sample study is not homogeneous, as we included a clinical sample of various neurological patients. Further studies should verify the efficacy of I&I against instrumental examination with a detailed analysis of pharyngeal residues. It would also be interesting to investigate the use of I&I in specific neurological pathologies, to identify potential advantages and weakness for use in specific populations.
Conclusion
In conclusion, the I&I scale was created to provide support to clinicians during clinical swallowing examination. The result showed that the I&I test is a valid test and it could guide clinicians during the clinical oropharyngeal assessment and help to plan customized treatment for patients.
References
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, et al. (2005) Dysphagia after stroke: Incidence, diagnosis and pulmonary complications. Stroke 36: 2756-2763.
- Sorenson EJ, Crum B, Stevens JC (2007) Incidence of aspiration pneumonia in ALS in Olmsted County, MN. Amyotroph Lateral Scler 8: 87-89.
- Kalf JG, de Swart BJ, Bloem BR, Munneke M (2012) Prevalence of oropharyngeal dysphagia in Parkinson's disease: A meta-analysis. Parkinsonism Relat Disord 18: 311-315.
- Edwards LL, Quigley EM, Pfeiffer RF (1992) Gastrointestinal dysfunction in Parkinson’s disease: Frequency and pathophysiology. Neurology 42: 726-732.
- Wilson RD (2012) Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis 21: 61-67.
- Pikus L, Levine MS, Yang YX, Rubesin SE, Katzka DA, et al. (2003) Video fluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol 180: 1613-1616.
- Donovan NJ, Daniels SK, Edmiaston J, Weinhardt J, Summers D, et al. (2013) Dysphagia screening: State of the art invitational conference proceeding from the state-of-the-art nursing symposium, international stroke conference 2012. Stroke 44: 24-32.
- Bours GJJW, Speyer R, Lemmens J, Limburg M, De Wit R (2009) Bedside screening tests vs. video fluoroscopy or fibreoptic endoscopic evaluation of swallowing to detect dysphagia in patients with neurological disorders: Systematic review. J Adv Nurs 65: 477-493.
- Sasaki CT, Leder SB (2007) Utility of clinical swallowing examination measures for detecting aspiration post-stroke. Dysphagia 22: 76.
- Ricci Maccarini A, Filippini A, Padovani D, Limarzi M, Loffredo M, et al. (2007) Clinical non-instrumental evaluation of dysphagia. Acta Otorhinolaryngol Ital 27: 299-305.
- Brady S, Donzelli J (2013) The modified barium swallow and the functional endoscopic evaluation of swallowing. Otolaryngol Clin N Am 46: 1009-1022.
- Rangarathnam B, McCullough GH (2016) Utility of a clinical swallowing exam for understanding swallowing physiology. Dysphagia 31: 491-497.
- Mann G (2002) MASA, the Mann assessment of swallowing ability. Singular Thomson Learning.
- O'Neil KH, Purdy M, Falk J, Gallo L (1999) The dysphagia outcome and severity scale. Dysphagia 14: 139-145.
- Martino R (2012) Screening and clinical assessment of oropharyngeal dysphagia. Nestle Nutr Inst Workshop Ser 72: 53-56.
- Shaw SM, Martino R (2013) The normal swallow: Muscular and neurophysiological control. Otolaryngol Clin North Am 46: 937-956.
- Wilson-Pauwels L (2010) Cranial nerves? Function and dysfunction. People’s Medical Pub.
- Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, et al. (2009) Predicting aspiration in patients with ischemic stroke: Comparison of clinical signs and aerodynamic measures of voluntary cough. Chest 135: 769-777.
- Miles A, Moore S, McFarlane M, Lee F, Allen J, et al. (2013) Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav 118: 25-31.
- Daniels SK, Pathak S, Rosenbek JC, Morgan RO anderson JA (2016) Rapid aspiration screening for suspected stroke: Part 1: Development and validation. Arch Phys Med Rehabil 97: 1440-1448.
- Leder SB, Murray JT (2008) Fiberoptic endoscopic evaluation of swallowing. Phys Med Rehabil Clin N Am 19: 787-801, viii-ix.
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL (1996) A penetration-aspiration scale. Dysphagia 11: 93-98.
Citation: Koch I, Ferrazzi A, Busatto C, Ventura L, Palmer K, et al. (2017) Cranial Nerve Examination for Neurogenic Dysphagia Patients. Otolaryngol (Sunnyvale) 7:319. DOI: 10.4172/2161-119X.1000319
Copyright: © 2017 Koch I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproductio`n in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12426
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 11136
- PDF downloads: 1290