COVID-19-Related Arthritis: Difference between Viral Arthritis and Reactive Arthritis
Received: 19-Dec-2022 / Manuscript No. JIDT-22-84203 / Editor assigned: 22-Dec-2022 / PreQC No. JIDT-22-84203 (PQ) / Reviewed: 05-Dec-2022 / QC No. JIDT-22-84203 / Revised: 12-Jan-2023 / Manuscript No. JIDT-22-84203 (R) / Published Date: 19-Jan-2023 DOI: 10.4172/2332-0877.1000524
Abstract
Sterile arthritis has been reported in patients with Coronavirus Disease (COVID-19). However, some patients had been inappropriately diagnosed with ‘Reactive Arthritis (ReA)’ instead of ‘viral arthritis’. ReA is a form of sterile arthritis that occur secondary to an extraarticular infection in genetically predisposed individuals. The extra articular infection is typically a bacterial infection of the gastrointestinal or genitourinary tract. The definition, diagnostic criteria, and list of causative bacteria were proposed at the Fourth international workshop on reactive arthritis held in 1999. The clinical features of patients with COVID-19-related arthritis do not only fit with the definition and diagnostic criteria. The clinical course of extraarticular infection and subsequent self-limiting arthritis is sometimes similar for both viral arthritis and ReA. However, the duration of arthritis is generally much shorter in viral arthritis than in ReA. Therefore, it should be necessary to understand that COVID-19-related arthritis falls into a distinct category of ‘viral arthritis’ that is different from ReA.
Keywords: COVID-19; Reactive arthritis; SARS-CoV-2; Spondyloarthritis; Viral arthritis
Introduction
Postinfectious arthritis is a broad group of non-suppurative arthritis induced during and after an extraarticular infection by various microorganisms. Classical ReA (formerly Reiter’s syndrome), infection-related arthritis, and viral arthritis or postviral arthritis is considered independent entities that are classified within postinfectious arthritis. However, the definition and classification of postinfectious arthritis remain misleading entities, especially concerning terminology.
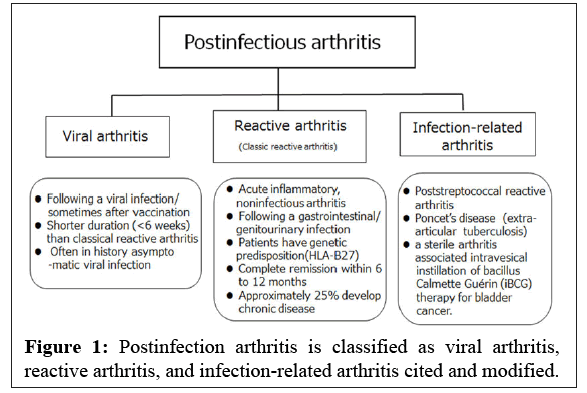
The term ReA is sometimes incorrectly used interchangeably with postinfectious arthritis, especially viral arthritis. In other words, some authors inappropriately consider postinfectious arthritis to be a large group of ReA. Viral infections are known to have both clinical features and a complex immunological relationship similar to various autoimmune diseases. However, the terms 'viral arthritis' or 'postviral arthritis' is not widely used or understood in clinical practice. Thus, viral arthritis is sometimes misdiagnosed [1] (Figure 1).
More than 30 cases of COVID-19-related arthritis have been recently reported, with more than half of them diagnosed as ReA. However, the cases do not fit with the established definition and traditional diagnostic criteria of ReA [2].
Viral arthropathy and myalgia following COVID-19 are commonly reported, and some viral infections induce clinical manifestations similar to those of well-defined rheumatic diseases [3,4]. However, there is few papers show that COVID-19-related arthritis is viral arthritis instead of ReA, leading to widespread misdiagnosis of ReA.This mini-review presents our opinions based on the original definition of ReA and the difference between viral arthritis and ReA.
Literature Review
The concept, definition, and diagnostic criteria of ReA
Ahvonen, et al. defined ReA as aseptic or non-suppurative arthritis following microbial infection of sites other than the joints. This is a well-known concept of ReA that is simply based on a pattern of developing arthritis after an extraarticular bacterial infection [5].
At the Fourth International Workshop on Reactive Arthritis, the designation of Reiter's syndrome was discarded because Hans Reiter was accused of war crimes with the Nazi Party [6]. The eponym was replaced with the term ReA which was introduced by Ahvonen, et al. The definition and diagnostic criteria for ReA were proposed by Braun and associates at the workshop (Table 1) [2,5]. This is the most commonly used definition and diagnostic criteria of ReA [7-11].
| The Berlin diagnostic criteria for reactive arthritis | ||
|---|---|---|
| Typical peripheral arthritis epredominantly lower limb, asymmetric oligoarthritis Plus | Evidence of preceding infection | |
| a) Where clear clinical diarrhoea or urethritis within the preceding four weeks, laboratory confirmation is desirable but not essential | b) Where no clear clinical infection, laboratory confirmation of infection is essential | |
| Exclusion criteria: Patients with other known causes of mono/oligoarthritis, such as other defined spondyloarthropathies, septic arthritis, crystal arthritis, Lyme disease and post-streptococcal reactive arthritis. The diagnosis of reactive arthritis does not require the presence of HLA-B27 or extra-articular features (conjunctivitis, iritis, skin lesions, non-infectious urethritis, cardiac and neurological features) or typical spondyloarthopathic features (inflammatory back pain, alternating buttock pain, enthesitis, iritis) but these should be recorded if present. | ||
Table 1: The Berlin diagnostic criteria for reactive arthritis and its exclusion criteria.
A panel of experts attending the workshop determined a specific list of possible causative gastrointestinal and urogenital pathogens. These included Chlamydia trachomatis, Yersinia, Salmonella, Shigella, Campylobacter. Escherichia coli, and Clostridioides (formerly Clostridium) difficile [10]. Although other bacteria and viruses were proposed, they are not included as causative agents of ReA by this definition [4].
Bacterial, immunological, and genetic factors including Human Leukocyte Antigen (HLA)-B27, play an important role in the pathogenesis of ReA. Therefore, ReA is not only a pattern of acute inflammatory arthritis following an infection outside the joint but also the specific clinical entity (classic ReA) defined at the workshop. Further, ReA is categorized as one of the diseases of Spondyloarthritis (SpA) that consisted of axial SpA including ankylosing spondylitis, psoriatic arthritis and SpA with inflammatory bowel diseases.
The clinical feature of ReA refers to a constellation of arthritis, enthesitis, mucocutaneous lesions, and ocular symptoms of sterile inflammation occurring after a urinary or gastrointestinal infection [7-11]. Peripheral arthritis is typically induced in ReA, found predominantly in lower limbs and large joints, and it asymmetrically develops in less than 5 joints. The typical disease duration is 3 months-5 months. Most patients either achieve complete remission or have little active disease within 6 months-12 months after presentation and 15%-20% of patients may likely experience chronic persistent arthritis [12].
Additionally, it was proposed at the workshop that, except for septic arthritis, any other post infectious non-suppurative arthritis, such as post streptococcal ReA, Poncet’s disease, and aseptic arthritis induced by intravesical instillation of Bacillus Calmette-Guérin (iBCG) therapy for bladder cancer, were proposed to be referred to as “infection-related arthritis” not but ReA [13]. Therefore, post infectious arthritis is fundamentally categorized as ReA (classic ReA), infection-related arthritis, and post infectious viral arthritis [1] (Figure 1).
Based on this established definition, and diagnostic criteria, arthritis induced by a Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, which has recently been reported, does not fit with the criteria of ReA described in the literature and textbook should be categorized as ‘viral arthritis’. Therefore, the term ReA is now widely used but often inappropriately spread [14].
Viral arthritis
Viral arthralgia and viral arthritis occur commonly in patients infected with parvovirus B19, hepatitis B virus, Human Immunodeficiency Virus (HIV), flaviviruses, and alphaviruses and some patients demonstrated arthritis that is very similar to Rheumatoid Arthritis (RA) and other rheumatic diseases such as ReA [15,16]. Among the virus-induced arthritides, ReA associated with an HIV infection is often reported and discussed in the literature. However, ReA is found to be related to other bacterial infections that patients with HIV are exposed to, not to the HIV infection itself [17,18].
Pathogenesis of viral arthritis
Various mechanisms of viral arthritis have been discussed including direct entry into joint cells or tissues, Immune Complex (IC) formation, by directly or indirectly causing immune dysregulation, and molecular mimicry. Rubella, rubella vaccine virus, parvovirus, enterovirus, and chikunga virus have tropism for synovial tissue, allowing the direct invasion to the joint [16,19]. Both parvovirus and enteroviruses have been isolated from joint fluid. Alphavirus and Ross River virus persist in synovial macrophages. Viral particles act as the antigenic component of IC and IC preferentially deposits in the joints and skin which causes arthritis and skin rash. This type of presentation commonly occurs in cases of hepatitis B, hepatitis C, and parvovirus infections [20].
Interestingly, a mechanism of molecular mimicry refers to a significant similarity between certain pathogenic molecules of a virus or viral vaccine and specific human proteins. This similarity may lead to immune cross-reactivity, wherein the reaction may harm similar human proteins, essentially causing an autoimmune disease. Many authors assume the existence of molecular mimicry between viral epitopes and synovial membranes causing local inflammation [21]. Recently, 28 human proteins harboring regions homologous to SARSCoV- 2 peptides have been studied and reported [22].
Considering the mechanisms of IC formation, immune dysregulation, and molecular mimicry, it can be understood that the development of arthritis could take several days to a few weeks from the onset of SARS-CoV-2 infection. In a patient-reported analysis, 69.1% of patients with COVID-19 reported arthralgia during a SARSCoV- 2 infection, 9.9% of patients reported arthralgia after COVID-19, and 20.9% had arthralgia both during and after COVID-19 [3]. This biphasic illness is a well-known pattern or course of ReA, but it easily confuses or misleads the diagnosis of ‘viral arthritis’ as ReA. This is another especially important feature of viral arthritis which is not widely understood in medical practice.
Recently, Kuschner, et al. [23] reported the detection of RNA of the SARS-CoV-2 in the synovial fluid in the case of COVID-19. This is the first report demonstrating the presence of SARS-CoV-2 RNA in the joint. Therefore, this evidence does not support Ahvonen, et al.’s concept of ReA [5].
Cytokine and chemokine
The exact mechanisms for joint inflammation are not fully understood, however, a SARS-CoV-2 infection leads to stimulation of macrophages which in turn causes a release of high levels of cytokines such as IL-6, IL-1, IL-8, TNF, and other chemokines, such as granulocyte-macrophage colony-stimulating factor, CXCL10 (IP10), and CCL2 (MCP1), which all leads to the development of and increase the inflammatory response [24]. Interleukin 17A has been involved in the pathogenesis of reactive arthritis and other SpA in general, and also in the hyper inflammatory state of COVID-19 [25]. Therefore, it is not surprising that COVID-19 can sometimes induce musculoskeletal and skin manifestations of SpA. In fact, up to onethird of HIV-infected persons with psoriasis develop psoriatic arthritis [26].
Clinical manifestation of COVID-19-related viral arthritis
The clinical features of COVID-19-related arthritis have been reported by Gracia-Ramos, et al. [4]; among their 32 patients, 3 had axial involvement and 29 had peripheral involvement (9 monoarticular and 20 polyarticular), and in a few patients, concomitant enthesitis (n=3), psoriatic lesion (n=2) and balanitis (n=1) were found. According to the classification criteria, patients were satisfied with RA, 3 with axial SpA, and only 6 with peripheral SpA. However, the remaining 17 patients did not fulfill the Assessment of SpondyloArthritis international Society classification criteria for SpA, as they had isolated arthritis in the context of post-COVID-19 arthritis [4]. After the arthritis was resolved, no treatments were administered in most patients, except in patients with RA. Based on this review and other reports, the clinical presentation of COVID-19-related arthritis is not similar to that of ReA. Moreover, COVID-19-related arthritis generally demonstrates a milder disease presentation than that of ReA.
Duration of arthritis
Both viral arthritis and ReA follow a self-limiting course. Viralinduced acute arthritis is generally described in the textbook as arthritis that remits within 6 weeks. However, it is not the case with ReA induced by bacteria. The natural history of ReA suggests that most patients achieve complete or near complete remission within to 12 months. Approximately 25% develop chronic disease and require on-going treatment [10,27]. We analysed the duration of arthritis in patients with COVID-19-related arthritis reported in 2021 by PubMed.
In 22 of 32 patients, the duration of arthritis could be identified. The mean and median durations were 17 and 14 days, respectively, with a range of 2 days~30 days.
The bacteria that cause ReA to invade and persist in the host cell are called intracellular bacteria. Although intracellular bacteria could not be cultivated, some molecules of the bacteria within the host cells are activated themselves to involve in persistence within the host cells with an interaction of immune responses associated with HLA-B27 molecules [28]. This is one of the explanations for the generally longer duration of arthritis in ReA [29].
Discussion and Conclusion
COVID-19-related arthritis is classified as viral arthritis or postviral arthritis rather than ReA based on the 1999 definition and diagnostic criteria of ReA. The difference between viral arthritis and ReA can be explained by several reasons. First, the causative microorganisms in ReA are bacteria rather than viruses, and urogenital or enteric bacterial infections induce ReA as defined at the workshop. In a recent review article, although other bacteria and viruses have been proposed in some reviews, they are not among the causative agents of ReA based on the classical definition. Additionally, various other bacterial and viral infections have been recently suggested of triggers of postinfectious arthritis but they are by convention not considered as ‘ReA’.
The clinical manifestations of SpA have been reported in only a few cases during or after COVID-19. No patients presented with the classic triad of reactive arthritis formerly termed ‘Reiter’s syndrome’ (conjunctivitis, arthritis, and non-infectious urethritis). In a previous study, HLA-B27 is poorly observed among 8 patients tested, and only two patients (25%) were HLA-B27 positive.
Both viral arthritis and ReA exhibit a self-limiting disease course. However, the most prominent difference is the duration of arthritis. Viral arthritis usually remits within 6 weeks, whereas complete remission of ReA usually takes 6 months~12 months. Approximately 30% of ReA patients develop a chronic form of arthritis; however, these patients do not include those with COVID-19-related viral arthritis.
Since the term ReA is sometimes inappropriately used interchangeably with viral arthritis. There is a fundamental need to understand the separate categories of viral arthritis and ReA.
References
- Plesca DA, Luminos M, Spatariu L, Stefansecu M, Cinteza E, et al. (2013) Postinfectious arthritis in pediatric practice. Maedica (Bucur) 8:164-169.
[Google Scholar] [PubMed]
- Braun J, Kingsley G, van der Heijde D, Sieper J (2000) On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3-6, 1999. J Rheumatol 27:2185-92.
[Google Scholar] [PubMed]
- Herndon CM, Nguyen V (2000) Patterns of viral arthropathy and myalgia following COVID-19: A cross-sectional national survey. J Pain Res 29:3069-3077.
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G (2021) New onset of autoimmune diseases following COVID-19 diagnosis. Cells 20:3592.
[Crossref] [Google Scholar] [PubMed]
- Ahvonen P, Sievers K, Aho K (1969) Arthritis associated with Yersinia enterocolitica infection. Acta Rheumatol Scand 15:232-253.
[Crossref] [Google Scholar] [PubMed]
- Wallace DJ, Weisman M (2000) Should a war criminal be rewarded with eponymous distinction? The double life of Hans Reiter (1881–1969). J Clin Rheumatol 6:49-54.
- Jubber A, Moorthy A (2021) Reactive arthritis: A clinical review. J R Coll Physicians Edinb 51:288-297.
[Crossref] [Google Scholar] [PubMed]
- Selmi C, Gershwin ME (2014) Diagnosis and classification of reactive arthritis. Autoimmun Rev 13:546-9.
[Crossref] [Google Scholar] [PubMed]
- Bennet JL, Sen ES (2022) Infection-induced arthritis. Paediatrics and Child Health 32:50-56.
[Crossref]
- Taniguchi Y, Nishikawa H, Yoshida T, Terada Y, Tada K, et al. (2021) Expanding the spectrum of reactive arthritis (ReA): Classic ReA and infection-related arthritis including poststreptococcal ReA, Poncet's disease, and iBCG-induced ReA. Rheumatol Int 41:1387-1398.
[Crossref] [Google Scholar] [PubMed]
- Ferreira A, Monteiro M, Vita P, Marinho A, Vasconcelos C (2015) Post-infectious arthritis and reactive arthritis: infection and autoimmunity, 2nd ed. Amsterdam, Elsevier, Netherlands.
- Yu DT, van Tubergen A, Sieper J, Curtis MR, Reactive arthritis, UpToDate, 2022.
- Sieper J, Braun J, Kingsley GH (2000) Report on the fourth international workshop on reactive arthritis. Arthritis Rheum 43:720-734.
[Crossref] [Google Scholar] [PubMed]
- Kobayashi S, Taniguchi Y, Kida I, Tamura N (2021) SARS-CoV2-triggered acute arthritis: Viral arthritis rather than reactive arthritis. J Med Virol 93:6458-6459.
[Crossref] [Google Scholar] [PubMed]
- Marks M, Marks JL (2016) Viral arthritis. Clin Med 16:129-134.
[Crossref] [Google Scholar] [PubMed]
- Sharmaa V, Sharmab A (2022) Infectious mimics of rheumatoid arthritis. Best Pract Res Clin Rheumatol 36:101736.
- Cuellar ML, Espinoza LR (2000) Rheumatic manifestations of HIV-AIDS. Best Pract Res Clin Rheumatol 14:579-593.
- Clark MR, Solinger AM, Hochberg MC (1992) Human immunodeficiency virus infection is not associated with Reiter's syndrome: Data from three large cohort studies. Rheum Dis Clin North Am 18:267-276.
[Google Scholar] [PubMed]
- Demicco EG, Kattapuram SV, Kradin RL, Rosenberg AE (2018) Infections of joints, synovium-lined structures, and Soft Tissue. 2nd ed. Amsterdam, Elsevier, Netherlands.
- Moore TL, Schur PH, Romain PL, Viral arthritis: Approach to evaluation and management, UpToDate, 2020.
- Andrade SB, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, et al. (2021) long-covid and post-covid health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 18:700.
[Crossref] [Google Scholar] [PubMed]
- Mohkhedkar M, Venigalla SSK, Janakiraman V (2021) Untangling COVID-19 and autoimmunity: Identification of plausible targets suggests multiorgan involvement. Mol Immunol 137:105-113.
[Crossref] [Google Scholar] [PubMed]
- Kuschner Z, Ortega A, Mukherji P (2021) A case of SARS-CoV-2-associated arthritis with detection of viral RNA in synovial fluid. J Am Coll Emerg Physicians Open 26:e12452.
[Crossref] [Google Scholar] [PubMed]
- Schett G, Manger B, Simon D, Caporali R (2020) COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol 16:465-470.
- Zacharias H, Dubey S, Koduri G, D'Cruz D (2021) Rheumatological complications of Covid 19. Autoimmun Rev 20:102883.
[Crossref] [Google Scholar] [PubMed]
- Madoff LC, (2022) Viral Arthritis. Harrison's principles of internal medicine, 20 ed. New York, McGraw Hill, USA.
- Schmitt SK (2017) Reactive Arthritis. Infect Dis Clin North Am 31:265-277.
[Crossref] [Google Scholar] [PubMed]
- Kriston-Vizi J, Lenart I, Iwasaki T, Gould K, Nesbeth, et al. (2022) Salmonella exhibit altered cellular localization in the presence of HLA-B27 and codistribute with endo-reticular membrane. J Immunol Res 16:9493019.
[Crossref] [Google Scholar] [PubMed]
- Generali E, Ceribelli A, Massarotti M, Cantarini L, Selmi C (2015) Seronegative reactive spondyloarthritis and the skin. Clin Dermatol 33:531-537.
[Crossref] [Google Scholar] [PubMed]
Citation: Kobayashi S, Kida I, Taniguchi Y, Tada K, Tamura N (2023) COVID-19-Related Arthritis: Difference between Viral Arthritis and Reactive Arthritis. J Infect Dis Ther 11:524. DOI: 10.4172/2332-0877.1000524
Copyright: © 2023 Kobayashi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3770
- [From(publication date): 0-2023 - Jul 08, 2025]
- Breakdown by view type
- HTML page views: 3332
- PDF downloads: 438