Research Article Open Access
Counterfeit Tablet Investigations: Can ATR FT/IR Provide Rapid Targeted Quantitative Analyses?
Graham Lawson*, John Ogwu and Sangeeta TannaLeicester School of Pharmacy, Faculty of Health and Life Sciences, De Montfort University, UK
- *Corresponding Author:
- Graham Lawson
Leicester School of Pharmacy
Faculty of Health and Life Sciences
De Montfort University, Leicester, LE1 9BH, UK
Tel: 44(0) 1162577129
E-mail: glawson@dmu.ac.uk
Received date: October 07, 2014; Accepted date: October 21 2014; Published date: October 24, 2014
Citation: Lawson G, Ogwu J, Tanna S (2014) Counterfeit Tablet Investigations: Can ATR FT/IR Provide Rapid Targeted Quantitative Analyses? J Anal Bioanal Tech 5:214. doi: 10.4172/2155-9872.1000214
Copyright: © 2014 Lawson G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Counterfeit medicines are now a global public health problem. In developed countries up to 1% of medicines are reported to be counterfeit whilst in developing countries the level is ~30-40%. In this research the potential of Attenuated Total Reflection (ATR) FT/IR techniques to provide rapid quantitative analyses of suspect tablet formulations is reported. Unlike conventional tablet analyses where several tablets are crushed and solvent extracted, ATR FT/IR methods require that only a single tablet be crushed prior to analysis. This provides a considerable time saving over the solvent extraction protocols cited in the British Pharmacopoeia. Reference ATR FT/IR spectra of the active pharmaceutical ingredient (API) and excipients, from crushed tablets, were recorded for identification purposes. Quantitative data was obtained from ATR FT/IR spectra of calibrated mixtures of the API in the excipients. Tablet samples from various countries, India, Africa, China, Belgium and the UK were examined. Initial results showed the API could be identified down to ca 5% w/w of the tablet. Quantification was linear with selected characteristic peak areas for each API/excipient mixture. The analysis of the tablet samples generally showed good agreement with expectation. This was confirmed by conventional extractive analyses followed by UV quantification. ATR FT/IR can therefore identify counterfeit tablets rapidly without the need for solvents. Whilst LCMS/ MS and NMR techniques may be the ‘gold standards’ of the analytical world they are of much reduced value in sub-Saharan African countries whereas a portable ATR FT/IR may prevent the use of counterfeit antimalarial tablets and contribute to improvements in patient health.
Keywords
Counterfeit medicines; ATR FT/IR; Paracetamol; Quantitative analysis
Introduction
The existence of counterfeit designer clothes, handbags and watches is well known and there is a growing public awareness of the existence of counterfeit medicines arising from the ‘lifestyle’ drugs Viagra and Cialis. Counterfeiters are now also focusing on prescription medicines including ‘lifesaving’ medicines for cancer and heart disease [1-3]. For patients the use of counterfeit medicines can result in unexpected side effects, treatment failure or even death. Counterfeit medicines are in fact a vast global problem impacting not only on the developing countries but also on the developed countries with effective regulatory systems and market control. In the last decade conservative estimates suggest that, taken globally, 10% of all medicines are counterfeit [3,4]. The World Health Organisation defines a counterfeit medicine as ‘a medicine which is deliberately and fraudulently mislabelled with respect to its identity and/or source’ and may include but not limited to: medicines containing no active ingredient, the wrong amount of active ingredient, the wrong active ingredient, high levels of contaminants and fake packaging. These statements apply to both branded and generic products and to traditional medicines [5].
Controlling the quality of medicines available to the public is challenging in both developed countries and those countries with limited resources. Access to the internet increases the opportunities for possible exposure to counterfeits such as Viagra where counterfeit levels have been estimated to be as high as 70% of the products available [6]. Less well-resourced countries struggle to provide the necessary quality control and quality assurance (QC/QA) procedures to protect the public from exposure to counterfeit medicines. In these countries, where poor quality medicines continue to be a burden, there is neither the analytical capability to identify a problem nor a sufficiently strong regulatory framework to take corrective action when counterfeit medicines are identified [7].
The following examples of identified counterfeit medicines reinforce the global nature of the problem:
• Belgium: fake traditional Chinese medicines resulting in several deaths and excess cancer cases [8] and major skin problems associated with counterfeit skin whitening creams [9].
• Sub-Sahara Africa: Up to 40% of anti-malarial medication and anti-HIV medication thought to be counterfeit [10] resulting in an unknown number of deaths and the potential for disease resistance to develop
• Pakistan: in 2011/12 counterfeit cardiovascular medication resulted in some 400+ deaths [11].
India: both high and low levels of the active ingredient in simple over the counter medicines have been identified [12].
• China: problems have been identified with both anti-cancer and diabetes drugs [13].
• USA: the lifestyle medications provide the major examples [2,3] and counterfeit anti-cancer drugs are also a concern [14] possibly due to the high cost of the genuine products.
• South America: Steroids and contraceptives are the target materials in these countries [15].
• UK: in the National Health Service (NHS) possibly the most rigorously controlled medicines supply system it is estimated that around 1% of all medicines are counterfeit. Furthermore it is estimated that circa 50% of medicines from the internet are counterfeit [16,17].
In terms of the types of counterfeit medicines the products reported by WHO were grouped into the following categories [18]:
• No active ingredient 32%
• Wrong active ingredient 22%
• Wrong content 20%
• Counterfeit packaging 16%
• Contaminated 9%
• Copies of original product 1%
This pervasive spread of counterfeit medicines demands that efforts be made to find rapid detection methods to control the penetration of the market in order to protect the patient, to protect profits for the pharmaceutical industries and very importantly to prevent drug resistance developing as a result of incomplete dosing regimes [19,20]. As the counterfeiters become more skilled well-resourced countries can apply more sophisticated analytical techniques such as LC-MS/ MS and NMR to detect these more subtle changes [21]. With increased scan speeds and lower levels of detection these techniques are capable of identifying more compounds present in a larger number of samples leading to what is known in the UK as BIG DATA. Whilst this approach is becoming mandatory for metabolomic studies, in drug development work for example, it cannot be used by a poorly resourced country to deliver on a vital part of its healthcare requirements i.e. is this a good quality medicine or not? Such countries need simplified screening methods which are rugged, portable and do not need sophisticated laboratories or costly reagents [22]. Screening will reduce the number of samples that must be processed by a central QC laboratory which will in turn reduce costs and reduce the time delays for the patient’s treatment. There have been several approaches to develop suitable screening systems including the Minilab™ based on TLC test methods [16], a handheld Raman Spectroscopy device [5] and systems based on Near Infrared Spectroscopy (NIR) [17]. The Minilab™ requires training in handling chemicals and the test sample has to be solvent extracted prior to analysis which increases both the time and complexity of each test. Spectroscopic tests require little or no sample preparation and the ease of operation of the equipment and rapid generation of results make them potentially ideal for screening purposes.
Attenuated Total Reflection Infra-Red (ATR FT/IR) analysis (Figure 1) offers a similar capability in terms of simple sample treatment. Paste or powder samples can be applied directly to the sensing head and tablet samples need only to be crushed prior to analysis. Once analysed the sample is available to the patient, albeit in powder form and in this respect the technique can be classed as non-destructive. Applications of ATR techniques to identify possible fakes based on principal component analysis have been reported [9] but this is the first report of quantitative estimation of the active pharmaceutical ingredient (API) level in a series of formulations. This paper reports on the evaluation of an ATR FT/IR instrument for the qualitative and quantitative analysis of commercial pharmaceutical tablet products. The ability of the system to identify the active pharmaceutical ingredient, paracetamol, in the presence of the excipients was determined. Finally the potential of the system to measure the actual level of this active pharmaceutical ingredient again in the presence of the excipients was assessed.
Materials and Methods
Reference chemicals: Paracetamol (Acetaminophen) was used for this investigation as representative of a common OTC medicine. Furthermore Paracetamol is often combined with other active ingredients, in the same tablet, providing an opportunity to assess the presence of this material with other active ingredient/s present.
A reference samples of Paracetamol (Acetaminophen) was obtained from Sigma-Aldrich Company, Dorset UK. The materials used to produce simulated excipient formulations: magnesium stearate, calcium carbonate, titanium dioxide and carboxymethyl cellulose were obtained from Fisher Scientific Ltd. Loughborough, UK.
Test tablet samples: The paracetamol tablets collected for analysis from the UK, Belgium, India, China and Rwanda were coded as follows:
• Europe EU UK 1 / EU UK 2 /EU B1 /EU B2
• Africa OYEM 27 / OYEM 28 / OYEM 42(2) / OYEM 49 (2)
• China China 2#1 / China 1 / HK 1 / HK 2
• India India 1#2 / India 1#3 / India 2#1 / India 3#1 / India 4#2 / India 5#2 / India 6#1
A total of 47 tablets were analysed. The expected amount of Paracetamol in each of the tablets was 500 mg as indicated on the packet except for Paracetamol Belgium and a Paracetamol India sample which were expected to contain 1000 mg and 325 mg respectively.
Instrumental
ATR FT/IR: ATR FT/IR data was generated using the FTIR 2 Bruker Alpha spectrometer with its accompanying platinum diamond ATR sampling accessory. The background spectrum within the instrument was recorded prior to the start of the analysis and subsequently after every fifth sample. This spectrum, notably from water vapour, was subtracted from the acquired sample spectra. The system scanned each sample 20 times over the range 4000-400 cm-1 measured at a spectral resolution of 2 cm-1. Spectral data were processed using OPUS software 7.2 (Bruker Corporation) and recorded as absorbance data. In quantitative mode measurements were made using the different integration modes provided by the software:-
• Mode A – integrates the area between the band, abscissa and the frequency limits defined
• Mode B – integrates the area above a straight line drawn between the peaks of the two frequency limits defined.
• Mode J-measures the highest absolute peak intensity
• Mode K-measures the peak intensity relative to the local baseline.
UV – Vis Instrument: UV spectra were collected using UV-Visible spectrophotometer, Helios Gamma (Thermo Electron Corporation England). The studied spectral range was 190-400 nm with a scan interval of 0.5 nm. The UV-Visible spectrophotometer was controlled using the Vision Lite software 2.2 (Ueberlingen, Germany).
Methods
Individual finely ground samples of the reference materials and the crushed tablets were placed on the diamond sampling head and 5 separate spectra recorded for each sample. This was repeated for 3 different powder portions from each sample. Comparison of these results was used to identify characteristic fingerprint data for the target active ingredient and also to assess the reproducibility of the data. Opus 7.2 integration methods were used in conjunction with the fingerprint peaks to assess the potential to quantify the level of Paracetamol present. Measured levels of API correspond to the percentage level present in the final tablet formulation and were related to the actual dosage through the mass of the tablet. Calibration powder mixtures of Paracetamol in magnesium stearate were prepared at 20, 30, 40, 60, 70, 80 and 90% of Paracetamol in the tablet, covering the different dose levels in common UK OTC medicines. The spectra of these mixtures were recorded as detailed above. Calibration graphs were prepared for individual fingerprint peaks using each of the integration routines. The data obtained for each fingerprint peak, using a particular integration mode was then combined to give a mean result. This quantification approach was used to determine the level of the stated API in crushed Paracetamol tablets obtained from eclectic sources around the world. As a final check on the validity of the data obtained the level of Paracetamol in each tablet was determined by conventional solvent extraction analysis in a method analogous to that cited by the British Pharmacopoeia. The protocol used by Behera [23] was adopted for this part of the study.
Results and Discussion
Spectral reproducibility
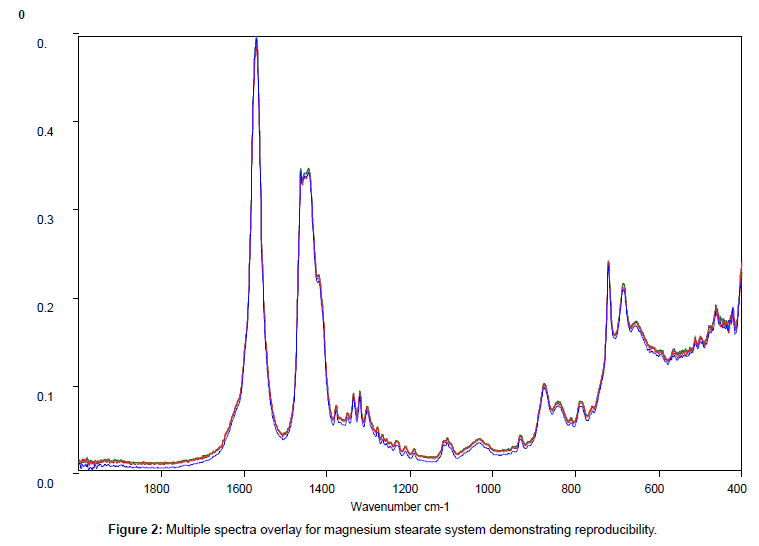
Replicate samples of the reference materials were run and providing the instrumental conditions remained constant there were no detectable differences in peak positions in the fingerprint region (1900-400 cm-1) between individual samples of the same material. Some variations in signal intensity, between replicate samples were initially noted but the cause was traced to either incomplete coverage of the sampling area or to poor sample homogenisation. Once a standard procedure was adopted reproducible spectral data was obtained across the complete scan range (4000-400 cm-1).
Fingerprint data
Characteristic fingerprint spectra for the selected excipients and Paracetamol, recorded from the pure reference compounds, indicated both the characteristic peaks for a particular compound and the regions of spectral overlap.
Figure 2 shows the relatively simple spectrum obtained from magnesium stearate and clearly indicates the presence of potentially interfering peaks between 1700 and 1400 cm-1which should be avoided.
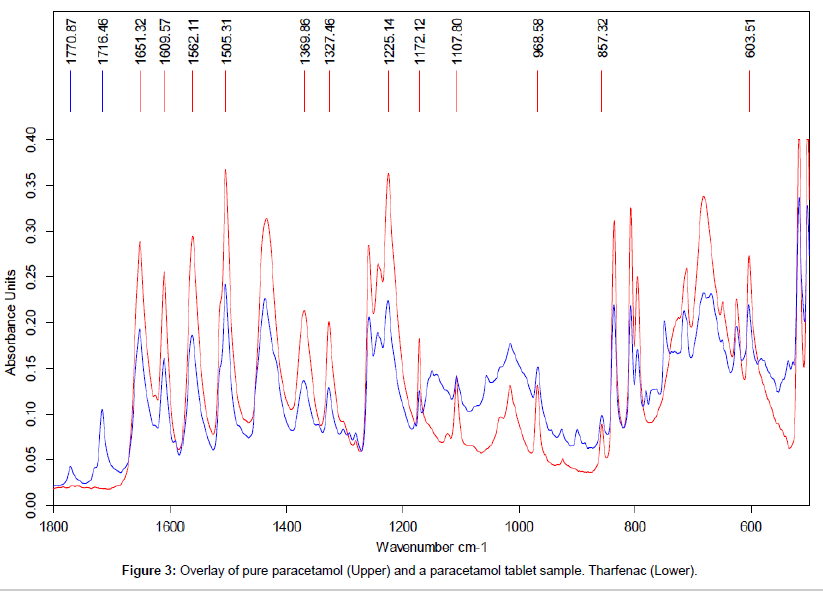
The data shown in Figure 3 demonstrates the close agreement between the reference spectra for pure Paracetamol (upper) and the data obtained the analysis of the powder from a crushed Paracetamol tablet (lower trace). This close agreement between the spectra confirms the presence of Paracetamol in the tablet formulation. The peaks at 1770 and 1716 cm-1were only seen in this sample and were presumed to be from the excipient mix in the tablet formulation.
In order to avoid major interference from the excipient peaks the characteristic peaks selected for paracetamol for this investigation were: 1225 cm-1 ,1172 cm-1, 1108 cm-1, 603 cm-1.
Quantification
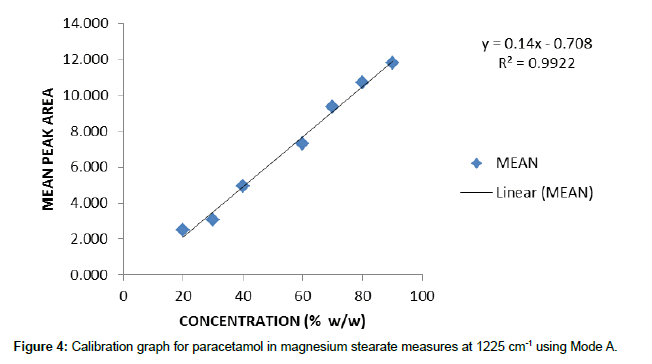
Individual calibration graphs, using each of the integration modes, were plotted for the characteristic peaks selected. Data was recorded from 5 replicate samples for each concentration. For brevity only one example of these graphs is reproduced here (Figure 4) and the other data is given as the calibration equation of best fit and the associated r2 value to give an indication of the quality of the data.
Mode A
y=0.14x – 0.708 (1225cm-1) r2=0.9922
y=0.0301x+0.3367 (1172cm-1) r2=0.9952
y=0.02x+0.662 (1108cm-1) r2=0.9879
Mode B
y=0.0387x – 0.5437 (1225cm-1) r2=0.9743
y=0.0071x-0.0486 (1172cm-1) r2=0.9855
y=0.0067x+0.0316 (1108cm-1) r2=0.9877
y=0.0277x-0.3385 (603cm-1) r2=0.9927
Mode J
y=0.0043x – 0.0211 (1225cm-1) r2=0.9837
y=0.002x+0.0056 (1172cm-1) r2=0.9905
y=0.0014x+0.0307 (1108cm-1) r2=0.9864
Mode K
y=0.0018x – 0.0323 (1225cm-1) r2=0.9734
y=0.0011x-0.0094 (1172cm-1) r2=0.9807
y=0.0009x+0.0005 (1108cm-1) r2=0.9862
y=0.0018x+0.0157 (603cm-1) r2=0.9969
During this phase of the investigation the critical dependence on good mixing was noted as indicated by r2 values improving with increased mixing time.
Qualitative results confirm that all the tablets tested contained paracetamol. Quantitative assessment using the ATR approach gave values around that expected for all the samples except for India 4#2 (Table 1).Not only was the level of API too high but there was also a large SD. The trace for this sample is shown in Figure 3 and as can be seen the top of the peak at 1108 cm-1 is in the same position for both this sample and pure paracetamol. This was not the case for the other peaks selected for this work. Measurements using modes A and J would therefore produce an incorrect high API level for this sample. The combination of correct (1225, 1172 and 603 cm-1) and artificially high data will result in the high standard deviation data observed. This observation suggests that the discriminatory nature of the peak at 1108 cm-1 is less good in this particular sample possibly due to the presence of a second API in the tablet which contributes to the signal at this wavelength. There may also be a contribution from the baseline correction prior to data collection particularly for lower levels of paracetamol. The limit of quantification was shown to be around 15% based on the tablet mass and for a real tablet this would be around 60 mg based on a 400 mg tablet. The very low SDs for the first three Indian samples is probably indicative of no contribution to any of the marker peaks due to a fortuitous choice of the excipients in the tablets. Given that no sample clean-up is used the other SD levels observed are considered to be acceptable.
| Tablet | UV Measured Content (mg) | Mode A MEAN ± SD (mg) |
Mode B MEAN ± SD (mg) |
Mode J MEAN ± SD (mg) |
Mode K MEAN ± SD (mg) |
Expected Amount (mg) |
|---|---|---|---|---|---|---|
| EU UK 1 | 532 ± 4 | 419 ± 18 | 429 ± 28 | 450 ± 26 | 461 ± 53 | 500 |
| EU UK 2 | 479 ± 3 | 417 ± 22 | 442 ± 32 | 456 ± 31 | 476 ± 58 | 500 |
| EU B 1#1 | 1031 ± 8 | 974 ± 11 | 977 ± 78 | 1036 ± 37 | 1050 ± 127 | 1000 |
| EU B 2#1 | 1090 ± 8 | 981 ± 12 | 986 ± 79 | 1049 ± 44 | 1065 ± 134 | 1000 |
| OYEM 27 | 543 ± 4 | 426 ± 10 | 422 ± 33 | 447 ± 21 | 446 ± 56 | 500 |
| OYEM 28 | 476 ± 3 | 436 ± 13 | 440 ± 32 | 466 ± 25 | 475 ± 60 | 500 |
| OYEM 42 (2) | 581 ± 4 | 537 ± 53 | 448 ± 38 | 524 ± 21 | 472 ± 63 | 500 |
| OYEM 49 (2) | 519 ± 4 | 445 ± 15 | 422 ± 37 | 456 ± 23 | 440 ± 57 | 500 |
| China 2#1 | 495 ± 3 | 432 ± 12 | 419 ± 39 | 455 ± 21 | 456 ± 66 | 500 |
| China 1 | 541 ± 4 | 412 ± 10 | 410 ± 36 | 441 ± 23 | 450 ± 62 | 500 |
| HK 1 | 488 ± 3 | 475 ± 24 | 431 ± 24 | 492 ± 32 | 468 ± 54 | 500 |
| HK 2 | 548 ± 4 | 512 ± 21 | 457 ± 24 | 529 ± 29 | 494 ± 56 | 500 |
| India 1#2 | 499 ± 4 | 460 ± 25 | 407 ± 40 | 464 ± 7 | 439 ± 63 | 500 |
| India 1#3 | 528 ± 4 | 447 ± 20 | 405 ± 40 | 455 ± 8 | 435 ± 62 | 500 |
| India 2#1 | 478 ± 3 | 462 ± 35 | 412 ± 37 | 462 ± 7 | 435 ± 61 | 500 |
| India 3#1 | 504 ± 3 | 438 ± 15 | 434 ± 39 | 465 ± 30 | 469 ± 68 | 500 |
| India 4#2 | 358 ± 3 | 487 ± 283 | 253 ± 77 | 510 ± 88 | 327 ± 87 | 325 |
| India 5#2 | 545 ± 5 | 447 ± 22 | 445 ± 41 | 480 ± 38 | 487 ± 73 | 500 |
| India 6#1 | 521 ± 5 | 499 ± 67 | 392 ± 40 | 472 ± 30 | 400 ± 58 | 500 |
Table 1: Summary of the quantitative results from the analyses of the Paracetamol tablets
It was interesting to note that the UV measurements tended towards higher than expected API levels. There was general agreement within the data from the ATR FT/IR measurements with a trend towards lower estimated values of the API level than from the UV method.
Conclusions
The main objective of this study was to find a simple, economic rapid and robust analytical method being suitable for the qualitative and quantitative analysis of APIs in tablet dosage forms. The simple ATR FT/IR approach employed in this study provides a rapid identification of the API in the presence of excipients. Identification of Paracetamol in the presence of excipients was possible at >10% w/w in the tablet and is viable within 5 minutes. Quantification of the Paracetamol can be obtained from the same sample within the same time frame. The results confirmed that the ATR FT/IR method was able to both identify and quantify the presence of the target API without the use of solvents and in timescales appropriate to development as a screening technique. Conventional FTIR samples would produce similar results but extra equipment, for example a KBr disc press would be required along with skilled staff to produce useable quality discs. Mulls would also require extra chemicals and equipment and both these approaches would detract from the simplicity and robustness of the ATR based system. There would also be an additional time constraint of between 10 and 30 minutes per sample depending on the skill of the staff preparing the KBr disc or mull samples. UV analyses took around 60 minutes per sample versus only a few minutes for ATR FT/IR which also provided unique identification information in the form of the spectral data collected. This method provides a quick and simple way to identify counterfeit Paracetamol tablets and has the potential to be applied to tablet formulations containing other APIs.
Acknowledgements
The authors thank Professor Larry Goodyer from the Leicester School of Pharmacy for kindly supplying paracetamol tablet samples from India. Thanks are also due to Dr Roland Marini and Mr Vedaste Habyalimana of the University of Liege, CIRM, Laboratory of Analytical Chemistry, Leige, Belgium / Rwanda Biomedical Center/Medical Procurement and Production Division for the donation of the tablets from Africa (OYEM samples) and for discussions about the feasibility of using the simple ATR/FTIR method in Rwanda.
References
- Reggi V (2007) Counterfeit medicines: An intent to deceive. WHO News. Int J Risk Safety Med 19: 105-108.
- Dégardin K, Roggo Y, Margot P (2014) Understanding and fighting the medicine counterfeit market. J Pharm Biomed Anal 87: 167-175.
- Anzanello MJ, Ortiz RS, Limberger R, Mariotti K (2014) A framework for selecting analytical techniques in profiling authentic and counterfeit Viagra and Cialis. Forensic Sci Int 235: 1-7.
- Martino R, Malet-Martino M, Gilard V, Balayssac S (2010) Counterfeit drugs: analytical techniques for their identification. Anal Bioanal Chem 398: 77-92.
- WHO (2003) Substandard and counterfeit medicines. Fact sheet No 275. World Health Organisation.
- Haiken M (2013) Up to 77 percent of Viagra bought online may be fake, and possibly dangerous, research shows. Forbes.
- Hajjou M, Qin Y, Bradby S, Bempong D, Lukulay P (2013) Assessment of the performance of a handheld Raman device for potential use as a screening tool in evaluating medicines quality. J Pharm Biomed Anal 74: 47-55.
- Gottlieb S (2000) Chinese herb may cause cancer BMJ 320: 1623A.
- Deconinck E, Bothy JL, Desmedt B, Courselle P, De Beer JO (2014) Detection of whitening agents in illegal cosmetics using attenuated total reflectance-infrared spectroscopy. J Pharm Biomed Anal 98: 178-185.
- Harris J, Stevens P, Morris J (2009) Keeping it real: Combating the spread of fake drugs in poor countries. International Policy Network.
- BBC News Asia (2012) Pakistan heart drugs: Faulty batch kills 70 in Lahore.
- Lawson G, Turay E, Armitage R, Goodyer, Tanna S (2014) Is it what it says on the packet? ATR FTIR provides a rapid answer to counterfeit tablet formulations. British Global and Travel Health Association Journal 23: 55-57.
- Yao J, Shi YQ, Li ZR, Jin SH (2007) Development of a RP-HPLC method for screening potentially counterfeit anti-diabetic drugs. J Chromatogr B Analyt Technol Biomed Life Sci 853: 254-259.
- U.S. Food and Drug Administration (2012) Counterfeit version of Avastin in U.S. Distribution. FDA.
- da Justa Neves DB, Marcheti RG, Caldas ED (2013) Incidence of anabolic steroid counterfeiting in Brazil. Forensic Sci Int 228: e81-83.
- Monge ME, Dwivedi P, Zhou M, Payne M, Harris C, et al. (2014) A Tiered Analytical Approach for Investigating Poor Quality Emergency Contraceptives. PLoS ONE 9: e95353.
- World Health Organisation (2006) Counterfeit medicines. Fact sheet Revised.
- World Health Organisation (2014) General information on counterfeit medicines.
- Newton PN, Green MD, Fernández FM, Day NP, White NJ (2006) Counterfeit anti-infective drugs. Lancet Infect Dis 6: 602-613.
- Chika A, Bello SO, Jimoh AO, Umar MT (2011) The Menace of Fake Drugs: Consequences, Causes and Possible Solutions. Research Journal of Medical Sciences 5: 257-261.
- Lu F, Weng X, Chai Y, Yang Y, Yu Y, et al. (2013) A novel identification system for counterfeit drugs based on portable Raman spectroscopy. Chemometrics and Intelligent Laboratory Systems 127: 63-69.
- Lon CT, Tsuyuoka R, Phanouvong S, Nivanna N, Socheat D, et al. (2006) Counterfeit and substandard antimalarial drugs in Cambodia. Trans R Soc Trop Med Hyg 100: 1019-1024.
- Behera S, Ghanty S, Ahmad F, Santra S, Banerjee S (2012) UV-Visible Spectrophotometric Method Development and Validation of Assay of Paracetamol Tablet Formulation. J Anal Bioanal Tech 3:151.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18315
- [From(publication date):
November-2014 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 13761
- PDF downloads : 4554