Research Article Open Access
Could Smokers’ Socio-Demographic and Housing Factors Affect and Influence the Choice Between Smoking Cessation Therapies?
| Silvia Leone3*, Roberto Carrozzino1,2, Monica Tassistro1,2, Luigi Robbiano3, Maria Laura Zuccoli3, Francesca Romani2, Antonietta Martelli3 and Francesca Mattioli3 | |
| 1“Ambulatorio per la terapia del tabagismo” of the Department of Mental Health and Dependences, San Paolo Hospital, Savona (Italy) | |
| 2Department of Mental Health and Dependences, San Paolo Hospital, Savona (Italy) | |
| 3Department of Internal Medicine, Pharmacology and Toxicology Unit, University of Genoa, Viale Benedetto XV,2 Italy-16132, Genoa | |
| Corresponding Author : | Silvia Leone Department of Internal Medicine, Pharmacology and Toxicology Unit University of Genoa, Viale Benedetto XV, 2 I-16132, Genoa, Italy Tel: +39 010 5554804 E-mail: drsilvialeone@gmail.com |
| Received: December 11, 2015; Accepted: January 25, 2016; Published: February 02, 2016 | |
| Citation: Leone S, Carrozzino R, Tassistro M, Robbiano L, Zuccoli ML, et al. (2016) Could Smokers’ Socio-Demographic and Housing Factors Affect and Influence the Choice Between Smoking Cessation Therapies? Clin Pharmacol Biopharm 5:152. doi:10.4172/2167-065X.1000152 | |
| Copyright: © 2016 Leone S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Introduction: The published data suggest that interventions which combine pharmacotherapy and behavioural support increase success rates of smoking cessation compared to minimal intervention or usual care; however, a standardized behavioural psychotherapy programme has not been assessed yet. Our main aim was to assess if socio-demographic and housing characteristics of smokers attending an Italian smoking cessation centre, could have influenced the choice between varenicline therapy and psychological support only. Our secondary aims were: i) to evaluate the 6-month abstinence rates (ARs), confirmed by comparing exhaled air carbon monoxide concentrations, in smokers according to whether they took varenicline or received only psychological support; ii) to assess the most frequently reported adverse drug reactions (ADRs) by the varenicline group, mainly focusing on psychiatric events; iii) to evaluate the differences between men and women with regard to specific varenicline-related ADRs
Method: 142 smokers were enrolled; all of them received the same psychological support programme. They were evaluated by a team of clinical experts, who advised them to opt for either one quitting method or the other; then the smokers chose themselves a treatment option of either varenicline plus psychotherapy (VAR: 78 patients) or psychotherapy alone (PSY: 64 patients).
Results: Socio-demographic and psychological characteristics of patients have significantly influenced the treatment choice; the 6-month ARs were 35.9% versus 10.9% (p<0.01) in those using varenicline versus psychotherapy, respectively; 57.7% of the patients reported at least one adverse event.
Conclusion: The analysis of socio-demographic factors and psychological characteristics of patients seems to be necessary to offer them the most effective therapy in order to achieve good abstinence rates. Therefore, this study confirms the data about the efficacy and safety of varenicline. Our screening methods and exclusion criteria seem to be valid aids to achieving good therapeutic outcomes with a low risk of occurrence of severe psychiatric events.
| Keywords |
| Varenicline; Socio-demographic features; Smoking cessation; Cognitive behavioural psychotherapy; Adverse events |
| Introduction |
| According to the WHO, tobacco is one of the leading causes of deaths in the world. It is regarded as a significant risk factor for: ischemic heart diseases, cerebrovascular events, lower and upper respiratory infections, chronic obstructive pulmonary disease (COPD), and lung cancer. Tobacco must be considered one of the most preventable causes of death out of all the various diseases in the world (the World Health Organization, 2008). Smoking has a high prevalence worldwide; a survey conducted in the USA in 2008 revealed that current smokers in the adult population accounted for 20.8% [1]. In Italy there are 10.5 million smokers (20.6%) with a higher prevalence of males (6.4 million vs. 4.1 millions) [2]. |
| Identifying optimal prevention campaigns to promote smoking cessation and to prevent smoking addiction is of substantial clinical and public health importance. In Italy, the passing of the “Sirchia Act,” in force since 2005, has been a turning-point in the battle against tobacco; the Law prohibits smoking in closed public places unless they are equipped with appropriate air-conditioning systems. The Italian Superior Institute for Health (ISS) has reported a decrease of 15% in the number of smokers after passing this Law [3]. |
| In addition to this legislation, a lot of substances are now available in Italy to aid smoking cessation. Out of these, vareniclineis the most recent drug to be developed for this specific use. Varenicline was approved by the FDA in 2006. In 2007, after obtaining regulatory approval by the Italian Drug Agency (AIFA), the drug also came onto the market in Italy. |
| Varenicline is a partial agonist for the α4β2 nicotinic acetylcholine receptor subtypes, and it has also been shown to be a full agonist for the homomeric α7 nicotinic acetylcholine receptors [4]. It should thus provide relief from craving and withdrawal and concomitantly reduce the reinforcing effects of smoking, offering benefits over currently available smoking cessation agents [5]. |
| Partial agonists aim to provide a low-to-moderate level of dopamine stimulation to reduce craving and withdrawal symptoms. The lower level of dopamine release may be less dependence forming than the intermittent spikes in dopamine release produced by inhaled nicotine. The antagonist effect blocks the reinforcing effects of nicotine and potentially reduces the risk that a lapse to smoking would turn into a full-blown relapse. Considerable evidence suggests that repeated nicotine exposure results in an increase in functional nicotinic receptors in the brain and, specifically, a sensitisation of the mesolimbic dopamine response to nicotine. This dopamine response (i.e., an increase in extra-synaptic dopamine in the extracellular space between fibres in the accumbens) appears to be associated with the reinforcing and addictive properties not only of nicotine but also of other psychostimulant drugs of abuse (e.g. amphetamine, cocaine). This response confers hedonic properties on the behaviours associated with the dopamine activation [6]. |
| Despite the availability of effective treatments for smoking cessation, such as nicotine replacement therapy and bupropion sustained-release, abstinence rates remain less than optimal. As a nAChR partial agonist, varenicline attenuates the craving and withdrawal symptoms that occur with abstinence from nicotine and also reduces the rewarding effects of nicotine obtained from smoking in patients who lapse [7]. |
| The efficacies of varenicline have been tested in comparison to placebo and other smoking cessation pharmacotherapies, i.e., sustained-release bupropion (bupropion SR) and nicotine transdermal patch. Varenicline has higher abstinence rates than placebo and the alternative active treatments at the end of standard regimen treatment periods. Significantly higher abstinence rates were also found with varenicline in comparison to both placebo and bupropion SR at the end of a 40-week non-treatment follow-up period. Varenicline typically tripled the abstinence rates compared with placebo. In addition, varenicline reduced craving and withdrawal symptoms as well as some of the positive experiences associated with smoking to a greater extent than placebo, bupropion SR, and nicotine replacement therapy (NRT). These findings are consistent with the proposed agonist/antagonist effects of varenicline [8]. |
| A recent meta-analysis [9], which included 267 studies and involved 101,804 subjects, claimed that varenicline is superior to a placebo, to single forms of nicotine replacement therapies (NRTs), and to bupropion, but not to combination NRTs. |
| Initiation, maintenance and cessation of smoking appear to be strongly influenced by the social environment. Smokers are more inclined to consider a cessation attempt if they figure out that people, whose opinion is valued, feel that they should quit smoking and if they comprehend that smoking is unacceptable behaviour in a number of social situations. In a recent British study [10] the strongest sociodemographic predictors of quitting smoking in a large group of adult smokers were: occupational social class, social support, and the number of smokers in the household. In particular, marital status and the level of support by family members appear to be important predictor of smoking cessation. These findings are similar to those published by West et al. [11], who found that smokers whose partners objected to smoking were more likely to quit, and by Gourlay et al. [12], who found that marital status and the presence of smokers in the household were the strongest predictors of quitting smoking. Higher success rates have been commonly reported for older subjects. |
| The work environment is another important social determinant of smoking cessation. |
| Factors in the environment that potentially influence initiation and maintenance of smoking by adolescents have been the focus of many investigations since early studies demonstrated the importance of peer and parental smoking as risk factors. Suzanne L Tyas et al. [13] performed an analysis of psychosocial risk factors for smoking among adolescents. The broad categories that have been studied were: smoking among parents, siblings and peers; attitudes and norms about smoking (including parental reactions to smoking by their children); family environment; and attachment to family and friends. |
| In 2005, a cross-sectional study was carried out [14] in order to study associations with smoking initiation and smoking cessation within the general population, obtaining data from 11967 people. They claimed out that smoking initiation was associated with adverse childhood events and personality predispositions, while smoking cessation was associated mainly with socio-demographic factors and factors related to tobacco use: older age, being married, and higher socio-economic status (SES). Older people, married persons, and people with a medium or high SES have higher odds for quitting, both for men and women. Regarding tobacco-related factors, associations with smoking cessation were less consistent. The number of cigarettes smoked per day was positively associated with smoking cessation in men, whereas the age people started to smoke was inversely related with smoking cessation in women. Regarding psychosocial factors, only depressive symptoms were significantly associated with smoking cessation; loss of interest was inversely associated with smoking cessation among men, whereas sustained depressed mood was inversely related with smoking cessation among women. They found no significant associations between personality characteristics or psychosocial job characteristics and smoking cessation. |
| Pasquale Caponnetto et al. [15] have identified a number of common predictors that can be grouped into the following domains: personal-demographic and social/familial context (e.g. sex, age, age at smoking initiation, previous quit attempts, living as a couple, smokers in the household and in the workplace), psycho (patho) logical/ physio (patho)-logical, (e.g. depression, anxiety, nicotine dependence, alcoholism), and cognitive (e.g. motivation). |
| Many studies have suggested that men have a better long-term outcome than women. Women appear to be less motivated to quit smoking. Although women smoke fewer cigarettes and attempt to quit smoking at the same rate as men, they appear to be less likely to succeed at quitting smoking than men. That women have usually a worse long-term outcome than men has been generally attributed to women greater concerns about weight gain as a precipitant for relapse. As a matter of fact, cigarette smoking for many women is an effective aid used to control weight. [15] |
| The association between nicotine dependence and affective disorders, particularly major depressive disorder (MDD), is well known with high prevalence rates being reported for smokers. The reason for this association is not clear, but it has been argued that smoking may help individuals to cope with stress or medicate depressed mood. Smoking is highly prevalent across most anxiety disorders and varies widely, depending on the specific diagnosis and the sample selected. The highest prevalence estimates of smoking have been found among those with panic disorder with or without agoraphobia and among those with post-traumatic stress disorder [15]. |
| A high prevalence of smoking has also been identified among individuals with social anxiety disorder, generalized anxiety disorder, and specific phobias. Smokers with anxiety disorders have more severe withdrawal symptoms during smoking cessation than smokers without anxiety disorders. Moreover, smokers commonly implicate anxiety as a risk factor for relapse to smoking. In these cases, psychological treatments that incorporate cognitive restructuring of automatic thoughts may also have considerable utility. |
| To date and to our knowledge, clinical trials that analyse the association between socio-demographic and housing characteristics of smokers and their tendency to opt for a therapy or another are limited/ absent. Thus, we decided to analyse this issue and describe the results we obtained, stressing the freedom of choice of patients to decide whether or not to take varenicline. |
| This study’s main aim is to assess if socio-demographic and housing characteristics of smokers attending an Italian smoking cessation Centre, could influence the choice between smoking cessation therapies or strategies. Patients chose their treatment option by themselves, therefore this study can be considered a picture of the “real” daily clinical practice of an Italian Clinical Centre for smoking cessation by comparing the smoking cessation rates between varenicline plus psychotherapy treated patients (VAR), versus patients treated with only psychotherapy (PSY). |
| Then, we evaluated the 6-month abstinence rates (ARs) confirmed by comparing exhaled air carbon monoxide concentrations in smokers according to whether they took varenicline or received only psychological support. |
| Our secondary aims were: to assess the most frequent adverse drug reactions reported by VAR patients during the treatment period, and to compare our data with those from the literature with specific regard to psychiatric events. |
| This study was carried out at the smoking cessation centre “Ambulatorio per la terapia del tabagismo” (San Paolo Hospital, Savona, Liguria Region, Italy), which provides a choice of smoking-cessation therapies. The daily clinical practice of the “Ambulatorio per la terapia del tabagismo” (San Paolo Hospital) consists of an initial evaluation by a multi-disciplinary team, following which patients are commonly advised to opt for one treatment from: varenicline, bupropion, NRTs, or psychotherapy alone. All the patients attending the centre also usually receive psychological support following a regimen adapted from the Maudsley model, so all the smokers receive the same personal behavioral support programme. The enrolment period of this study was 1 year. Drop-out and smoking cessation rates among patients who chose either varenicline or psychotherapy alone have been compared. |
| Method |
| Study Design |
| The daily clinical practice of the “Ambulatorio per la terapia del tabagismo” (San Paolo Hospital) consists of an initial evaluation by a multi-disciplinary team, following which patients are commonly advised to opt for one treatment from: varenicline, bupropion, NRTs, or psychotherapy alone. |
| In this study we compared patients taking varenicline plus psychotherapy versus psychotherapy alone. The varenicline was administered in accordance with the European Summary of Product Characteristics, which states that the recommended dose is 1 mg of varenicline twice daily following a 1-week titration. The treatment should start 1-2 weeks before the cessation date. Patients who cannot tolerate adverse effects may have their dose lowered temporarily or permanently to 0.5 mg twice daily. Patients should be treated with varenicline for 12 weeks (3 months). Psychological support following a regimen adapted from the Maudsley model [16] was provided by an expert team and offered to both VAR and PSY patients for a 6-month period. The Maudsley model is an evidence-based approach to treating dependent smokers. This approach entails regular meetings (group or one to one) with a trained adviser using structured, withdrawaloriented behavioural therapy sometimes combined with smoking cessation medications such as nicotine replacement therapy (NRT), bupropion or varenicline [17]. The centre collected data for 1 year (2012), with a follow-up period of 6 months for the last enrolled patient. The inclusion/exclusion criteria for our study were: males and females ≥ 18 years of age who scored 5 or more on the Fagerström Test for Nicotine Dependence [18,19] with HADS score <21 (Hospital Anxiety and Depression Scale) [20]. Recent studies have reported good reliability for both anxiety and depression subscales [21,22]. There were no restrictions on prior or concomitant medications or comorbidities, apart from the usual prescribing information on the Summary of Product Characteristics. |
| The psychiatrists, after a complete and structured psychiatric evaluation, excluded patients who were taking psychotropic medication and who had been diagnosed with psychiatric disorders; patients who did not agree to sign the informed consent form were excluded. The primary outcome measure was self-reported abstinence from the cessation date to the 6-month follow up, confirmed by an expired CO<10 ppm, as specified in the Russell Standard [23]. As assessed by the Russell Standard, patients not attending the follow-up were considered to be smoking relapsers. |
| Variables measured at the baseline were: age, gender, years of addiction, number of cigarettes smoked per day, BMI, the HADS test, their Fagerström score, exhaled CO (ppm) [Smokerlyzer monitor Bedfont Scientific Ltd., Rochester, England; cut-off 10 ppm], previous attempts to quit smoking, and their housing situation. Other data collected by the physicians included: blood pressure, heart rate, and a physical general examination. During the 6-month period of treatment all the adverse events reported by patients were collected. |
| Interventions |
| Written informed consent was obtained from all the subjects. Both the study protocol and the informed consent were approved by the Ethical Committee at “San Paolo” Hospital. The study was conducted in compliance with the ethical principles of the Helsinki Declaration. |
| The first screening visit was carried out by a physician who collected medical histories and socio-demographic and anthropometric data from the patients. During this screening visit Fagerström and HADS test were administered, and inclusion/exclusion criteria were evaluated. Every patient attending the centre was asked to complete a HADS form in order to verify the possible correlation with psychiatric adverse events, before being referred to an expert team of psychiatrists and psychologists. |
| A second visit (V0) was conducted by an expert team of psychiatrists and psychologists. The enrolled patients met with a psychologist for a clinical interview that focused on behaviour and personality. The patients were asked how they had come to the decision to stop smoking. After a brief interview and, also on the basis of their Fagerström score, psychologists assessed the grade of nicotine dependence and the motivation to quit smoking. The patients then underwent a structured psychiatric interview. A primary function of this psychiatric evaluation was to determine the presence of any psychiatric conditions. Patients were assessed with regard to the symptoms of depression, anxiety, mania, psychosis, suicidal ideation, substance abuse, history of abuse, and family history of mental health issues. After a complete evaluation, and taking into account HADS score, housing situation, and previous attempts to quit smoking, psychiatrists advised the patients to opt for one treatment, explaining the pros and cons of each therapy. Finally, barring medical contraindications, a choice was made by the smokers themselves. If the smokers chose varenicline, they were prescribed the standard dose of 1 mg twice daily, and they started cognitive behavioural psychotherapy with a personal psychologist; those who chose just the psychotherapy treatment also started the same supportive psychological programme. |
| The smokers who opted for varenicline were advised to use their medication for at least 3 months. The cessation date for all the patients (VAR and non-VAR) was set during the V0, usually for a date within the next 2 weeks. The first psychological visit occurred within 2 weeks and then monthly thereafter (although the patients could attend the centre at their discretion). Individual psychological therapy was generally organized on the basis of the patients’ availability and on their needs, and was initiated in both groups. During each visit, the patients’ compliance with the treatment was assessed, as well as the occurrence of any possible adverse events. Their exhaled CO was also measured. |
| This study focused on: the first baseline visit (V0), the visit 1 and 3 months after the V0, and the follow-up visit 6 months after the V0. |
| Results |
| A pool of 872 smokers attended the “Ambulatorio per la terapia del tabagismo” during the study period: 233 of them chose bupropion, while 394 opted for NRTs; 103 patients never started any kind of treatment. |
| There were 142 patients enrolled in our trial, of whom 78 used varenicline plus psychotherapy (group: VAR), and 64 only psychotherapy (group: PSY). Table 1 shows the subjects’ characteristics at the baseline and demonstrates that the groups were homogenous in terms of: age, years of addiction to smoking, BMI, their Fagerström score, exhaled CO, and previous attempts to quit smoking. |
| PSY patients showed a higher than average number of cigarettes smoked per day (p<0.01 t Student test); no statistical significant difference was evidenced between the groups in terms of the number of subjects who used to smoke fewer than 20 cigarettes/day, between 20 and 30 cigarettes/day, and more than 30 cigarettes/day. |
| With regard to their HADS scores, the PSY group evidenced statistically significantly higher scores (p<0.001 χ square test); 46.9% of the PSY patients (57.1% males and 38.9% females) had a score of ≥ 16, whereas only 7.7% of the VAR patients had a score of ≥ 16. Being the choice of treatment free, this datum about lower HADS score in patients choosing the pharmacological aid could be of interest, indicating perhaps a stronger intention on smoking cessation. |
| Concerning their socio-demographical situation, VAR patients were significantly more likely than PSY patients to live with other smokers (44.9% vs. 6.2%, p<0,001 χ square test), and less likely to live with non-smokers (p<0.05). |
| The rates of tobacco abstinence were compared between both groups at 1, 3, and 6 months. The efficacy of both treatments was evaluated on the basis of the overall abstinence rates after 3 and 6 months. |
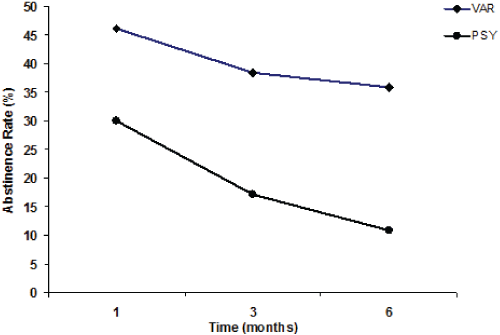
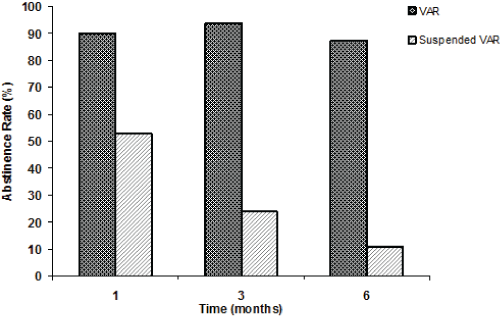
| Figure 1, Panel A shows the overall tobacco abstinence rates (%) of the VAR and PSY groups after 1, 3 (end of the varenicline therapy), and 6 months (follow-up); Panel B (regarding the VAR group only) shows the abstinence rates (%) of those patients who completed their varenicline therapy (VAR), and of those who prematurely suspended their varenicline therapy (Suspended VAR) at 1, 3, and 6 months. |
| After 1 month of therapy with varenicline, only 40 subjects were continuing with their treatment. Out of them, 36 were abstinent (90% of those patients who were continuing with varenicline, and 46.2% of the total). However, out of the patients who had prematurely suspended varenicline (38), 20 subjects were found to be abstinent (53%). |
| Patients who completed their 3-month therapy with varenicline were 32 (41% of the total); 17 females (F: 38.6%) and 15 males (M: 44.1%). Thirty patients out of these 32 (100% F and 86.7% M) were found to be completely abstinent, as confirmed by the exhaled-CO evaluation (93.8% of those who completed the treatment, and 38.5% of the total). Out of the patients that had prematurely stopped varenicline therapy before the 3-month visit (46 patients) 11 (8 F and 3 M) were found to be abstinent after 3 months (23.9%). |
| Also taking into account these 11 patients who had prematurely suspended their varenicline treatment and who were found to be abstinent at the 3-month visit, the overall 3-month abstinence rate was 52.8% (11+30)/78 for the VAR group. |
| The 3-month abstinence rate was 38.5% (30 patients: 17 F and 13 M) for the VAR group (for those who had completed their treatment) compared with 17.2% (7 F and 4 M) for the PSY group (Figure 1, Panel A), and the difference was statistically significant (p<0.01). |
| Twenty-eight (16 F and 11 M) of the thirty abstinent VAR patients (87.5%) had continued being abstinent at the 6-month follow-up visit. Out of the 46 patients who had stopped their varenicline treatment before the 3-month period of therapy, 3 F and 2 M were found to be abstinent after 6 months (5/46=10.9%). |
| Twenty-eight subjects completed their 3-month therapy with varenicline and were found to be abstinent; these 28 patients were 35.9% of the total (VAR group: 78 patients). Subjects who discontinued their therapy were assumed to be smokers from the point of discontinuation. VAR patients discontinuing their therapy due to adverse events related to the therapy were 3 (6 %), and no longer willing to participate were 47 (94%). |
| There are no specific reasons why almost 94% of the patients discontinued varenicline treatment. |
| After 1 month the PSY group showed an abstinence rate of 30% (19 out of 64 patients quit smoking). After 3 months the PSY group showed an abstinence rate of 17.2% (11 patients: 7 F and 4 M). The rate dramatically decreased after 6 months to 10.9% (7 patients: 4 F and 3 M). Only 11 PSY group patients completed their cognitive behavioural counselling treatment. |
| A comparison between the VAR and PSY groups (Figure 1, Panel A) demonstrates that both the 3-month and the 6-month abstinence rates were significantly lower (p<0.01) in the PSY group (17.2% vs. 38.5%; and 10.9% vs. 35.9%, respectively). |
| Data regarding reported adverse drug reactions (ADRs) during the treatment period with varenicline are shown in Table 2. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 13.1). |
| Forty-five (57.7%) out of the seventy-eight VAR patients reported at least one ADR. The most common adverse drug reactions were: nausea (26.7%), insomnia or sleep disorders (22.7%), weight gain (12%), and epigastralgia (8%). Nausea, sleep disorders and epigastralgia were more frequent in men than in women (respectively: 50% vs. 40.7%; 44.4% vs. 33%; 27.8% vs. 3.7%); on the other hand, weight gain and nervousness were more common in women (respectively: 25.9% vs. 11.1% and 11.1% vs. 5.6%). |
| It seems important to emphasize that only women complained of constipation (4 females) and mood swings/depression (3 females), accounting for 14.8% and 11.1% respectively of all the patients who reported ADRs. |
| The most frequent psychiatric disorders were insomnia and abnormal dreams (22.7%), nervousness (5.3%), and mood swings (4%). No serious adverse events were observed. No severe psychiatric events occurred. Side-effects were also recorded in the PSY group; the most common reactions were: weight gain (47%), nervousness (61%), anxiety (33%), depression (12%), and sleep disorders (8%). |
| Discussion |
| Varenicline has not been tested by itself as it is well known that its use may be associated with neuropsychiatric disorders. Attendance at individual or group psychotherapy could help the staff to strictly follow the patients’ attitude and to promptly detect the eventual onset of ADRs. On the other hand, the study did not aim to verify the effectiveness of varenicline, which is already documented in many pivotal clinical trials. Moreover, the drug had been tested (in almost all the previous studies) in association with psychotherapy. |
| The SmPC affirms that changes in behavior or thinking, anxiety, psychosis, mood swings, aggressive behavior, depression, suicidal ideation and behavior and suicide attempts have been reported in patients attempting to quit smoking with CHAMPIX in the postmarketing experience. Not all patients had stopped smoking at the time of onset of symptoms and not all patients had known pre-existing psychiatric illness. |
| Clinicians should be aware of the possible emergence of significant depressive symptomatology in patients undergoing a smoking cessation attempt, and should advise patients accordingly. |
| CHAMPIX® should be discontinued immediately if agitation, depressed mood or changes in behavior or thinking that are of concern for the doctor, the patient, family or caregivers are observed, or if the patient develops suicidal ideation or suicidal behavior. In many post-marketing cases, resolution of symptoms after discontinuation of varenicline was reported although in some cases the symptoms persisted; therefore, ongoing follow up should be provided until symptoms resolve. Depressed mood, rarely including suicidal ideation and suicide attempt, may be a symptom of nicotine withdrawal. In addition, smoking cessation, with or without pharmacotherapy, has been associated with exacerbation of underlying psychiatric illness (e.g. depression). |
| The Italian SmPC recommends the use of varenicline in association with psychotherapy. |
| The first consideration to be done is that we strongly deem that patients’ active involvement in treatment decisions for smoking cessation may positively affect their motivation, and thus the final outcome. |
| The “Ambulatorio per la terapia del tabagismo” has a specific health policy: the free choice of the patient; we think that the choice is driven by many different factors on the basis of socio-demographic, housing, and psychological characteristics. The Psychiatrist could optimize the decision obviously taking into account the possible medical contraindications and adverse events. Thus, the therapy is always highly customized for each patient in order to offer the highest chance of smoking cessation to anyone. |
| Since the treatment choice were completely free it’s scientifically interesting to underline and note that patients with high HADS score (≥ 8, with a precise cut-off of 21) who might be at higher risk of psychiatric events, are inclined to choose the psychological therapy alone. This datum could be confirmed by literature. Alex J Mitchell and Thomas Selmes [24] argued that there has been little research on reluctance to start medication among psychiatric patients. They found some predictors of intentional non-adherence: less severe disease (and feeling well), the desire to manage independently of the medical profession (self-efficacy), disagreement with or low trust in clinicians, and receipt of low levels of medical information [24]. Our smokers were not psychiatric patients; we can consider those with a high HADS score as affected by a “less severe disease”. A condition of anxiety and a tendency towards depression may lead to a refusal of a pharmacological therapy: this may be because of the impact of stigma which can involve people’s own responses to depression and help-seeking (self-stigma) as well as their perceptions of others’ negative responses (perceived stigma) [25]. |
| On the other hand, patients who live with smokers generally ask for a pharmacotherapy in combination with cognitive behavioral therapy to help them quit smoking; probably because they deem that the use of a drug in combination with a psychological supportive therapy can help most to quit smoking even in a “difficult” environment (e.g. living with other smokers). This finding is supported by literature [26] and seems to be rational and obvious for several reasons: smokers living with other smokers (SLSs) are probably afraid to relapse or never quit smoking. In many cases SLSs show a high level of awareness of the dangers of smoking: thus, they opt for pharmacotherapy to be more certain to achieve their aim. |
| An Australian survey [27] declares that, among those who had made a previous quit attempt, the most commonly reported reason for relapse were: craving and ‘socializing with friends who smoke’. Surveyed smokers were asked whether they agreed or disagreed with a number of statements relating to negative as well as positive beliefs about smoking; respondents tended to agree with negative statements such as ‘children are more likely to smoke if their parents smoke’. |
| In our opinion, the analysis of patients’ socio-demographical and psychological characteristics is necessary and inescapable to offer them the most effective therapy. In fact, the free choice of patients, associated with the presence of an expert team of psychologists and psychiatrists, has allowed us to achieve good results with the use of varenicline, comparable to those described in the literature. |
| Our study reveals that smokers using varenicline to help them quit smoking in association with a cognitive behavioural support programme showed higher abstinence rates at the end of their therapy (3 months), and after 6 months than those receiving psychotherapy alone. The VAR group maintained smoking abstinence, while the PSY group gradually started smoking again after their treatment. |
| The success rates among the patients using varenicline were similar to previous community trials with varenicline. Our 3-month abstinence rate is comparable to that defined by the Summary of Product Characteristics (SmPC) of Champix®, which declared a 3-month continuous abstinence rate of about 44%. Between 2007 and 2009 a prospective, observational, non-comparative trial (CHOICES study) was conducted in four European countries (Belgium, Greece, Hungary and Slovenia) to investigate varenicline as a smoking cessation aid in routine clinical practice: a self-reported abstinence rate (without objective controls of abstinence) of 64.4% after a 12-week treatment period was reported [28]. |
| Grassi MC et al. [29] examined the rate of smoking cessation associated with 6 weeks of counselling therapy either given alone, or as a combination of a 12-week course of therapy along with varenicline in 112 smokers. The authors found out that: only 33.3% completed their varenicline treatment; abstinence rates at 26 and 52 weeks of follow up were respectively 62.5% and 56.3% in the combination treatment group, and 39.6% and 33.3% in the counselling therapy group. It seems very important to emphasize that patients enrolled on this study contributed to the cost of the treatment, paying a fee of 100 €. |
| The effectiveness data about varenicline plus psychotherapy and psychotherapy alone, reported and published by Grassi et al. [29], are quite astonishing. The authors conducted an observational study comparing 2 treatment groups: varenicline plus group counselling therapy (VAR+GCT), and GCT alone. At 3 months subjects treated with VAR+GCT showed higher smoking abstinence rates compared with the GCT-group (68.8% vs. 56.3%), with no statistically significant difference. The difference between the groups became significant at both the 6-month and 1-year follow ups (62.5% vs. 39.6% and 56.3% vs. 33.3%). The abstinence rates achieved by Grassi et al. [29] are completely satisfying, but seem to be in conflict with the results reported by the SmPC. We may suppose that their cognitive behavioural programme was well-structured enough to achieve such high rates of abstention and to aid smoking cessation, also in the absence of varenicline (GCT group). |
| Since no other research report with psychotherapy (alone)- controlled group has been carried out, we are not able to make any other comparisons. The effectiveness of varenicline is well-known as stated by the SmPC and many other trials, and our abstinence rates are similar to those reported by the literature; for this reason, we have tried to call into question and analyse our model of psychotherapy. Individual sessions may be efficient at diagnosing and treating psychological/ psychiatric conditions, but probably, in cases of addiction (i.e., alcohol dependence), a group programme is definitely the best way to increase motivation, and to achieve better results. |
| Our type of cognitive behavioural programme, based on single smokers’ needs and availability to attend the centre, could also have affected their adherence to varenicline treatment. The idea of holding group sessions seemed to be efficient. It is possible that motivation to quit smoking increases when a subject is surrounded by other smokers who share the same aim. |
| The most common ADRs reported by our patients are already wellknown and listed in the SmPC: nausea (26.7% vs. 28.6% reported in the SmPC), sleep disorders/insomnia/abnormal dreams and nightmares (22.7%), weight gain (12%) and epigastralgia/upper abdominal pain (8%), and led to a small number of discontinuations (6%). ADRs that led to discontinuation were: nausea and weight gain. The incidence of AEs was similar to that of other observational or randomized controlled trials. |
| Also psychiatric-like events (i.e., mood swings and depression; nervousness and sleep disturbances) reported by our patients are listed in the SmPC. The collected data show that women have a higher propensity for reporting than men. |
| Sex differences in pharmacokinetics and pharmacodynamics, as a function of multiple physiological and body composition characteristics, may contribute to individual differences in drug efficacy and toxicity [30]. However, how these differences result in an increased risk of ADRs is not clear [31]. |
| The strong aspects of our study include: the free choice of the patients, the evaluation of socio-demographical characteristics of the patients and the correlation with the treatment choice, the evaluation of exhaled CO at every visit (in some other studies [32] abstinence rates are based on “self-reported abstinence”), and the fact that, contrary to other research studies [29,33], in our study patients did not have to pay a fee to participate or to buy the therapy. This must be seen as a crucial motivational issue. |
| Further trials and studies have to be carried out to confirm our data and to assess other significant associations between sociodemographical characteristics and smoking habits. |
| We strongly deem that a link between patients’ features and their attitude towards smoking initiation or cessation should be analysed in depth in order to grant better quitting outcomes. |
| Acknowledgements |
| We are grateful to the staff of the “Ambulatorio per la terapia del tabagismo” at San Paolo Hospital, Savona. |
| The material has not been published in whole or in part elsewhere. The paper is not currently being considered for publication elsewhere. All authors have been personally and actively involved in substantive work leading to the report, and will hold themselves jointly and individually responsible for its content. All relevant ethical safeguards have been met in relation to patients’ protection. |
| Declaration of Interest |
| The authors declare any financial conflict of interest arising from involvement with organisations that seek to provide help with or promote recovery from tobacco addiction. The authors declare no conflicts of interest regarding the content of this manuscript. |
References
- Center for Disease Control and Prevention (2008) Smoking attributable mortality, years of potential life lost, and productivity losses United States, 2000-2004. Morbidity and Mortality Weekly Report 57: 1226-1228.
- Pacifici R (2013). Rapporto sul Fumo in Italia 2012, Osservatorio OSSFAD Istituto Superiore di Sanità.
- Mariano ME, Elia D, Scarascia A, Tzani P, Giucastro G, et al. (2009) Smoking cessation: aspects of prevention and therapy The activity of the Smoking Cessation Center of Parma - Italy. ActaBiomedica 80: 42-46.
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O’Brien CP, et al. (2013) Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug and Alcohol Dependence 133:754-758.
- Rollema H, Chambersa LK, Coe JW, Glowa J, Hurst RS, et al. (2007) Pharmacological profile of the a4ß2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985-994.
- Foulds J (2006)The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J ClinPract60:571-576.
- Rao J, Shankar PK (2009)Varenicline: for smoking cessation. Kathmandu Univ Med J (KUMJ) 7:162-164.
- Fagerström K, Hughes J (2008)Varenicline in the treatment of tobacco dependence. Neuropsychiatr Dis Treat 4:353-63.
- Cahill K, Stevens S, Perera R, Lancaster T (2013) Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 5:CD009329.
- Chandola T, Head J, Bartley M (2004) Socio-demographic predictors of quitting smoking: how important are household factors? Addiction 99:770-777.
- West R, McEwen A, Bolling K, Owen L (2001) Smoking cessation and smoking patterns in the general population: a 1-year follow-up. Addiction 96:891-902.
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ (1994) Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ 309:842-846.
- Tyas SL, Pederson LL (1998)Psychosocial factors related to adolescent smoking: a critical review of the literature. Tob Control 7: 409-420.
- van Loon Jeanne M,Tijhuis M, Surtees PG, Ormel J (2005) Determinants of smoking status: cross-sectional data on smoking initiation and cessation. Eur J Public Health 15: 256-261.
- Caponnetto P, Polosa R (2008) Common predictors of smoking cessation in clinical practice. Respir Med102: 1182-1192.
- Hajek P (1989) Withdrawal-oriented therapy for smokers. Br J Addict 84:591-598.
- Bauld L, Bell K, McCullough L, Richardson L, Greaves L (2010)The effectiveness of NHS smoking cessation services: a systematic review. J Public Health (Oxf) 32:71-82.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K (1991) The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 86:1119-1127.
- Fagerström K (2012) Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research 14:75-78.
- Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, et al. (2012) Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry 24:12-16.
- Olssøn I, Mykletun A, Dahl AA (2005) The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry 14:5-46.
- Yang Y, Ding R, Hu D, Zhang F, Sheng L (2014) Reliability and validity of a Chinese version of the HADS for screening depression and anxiety in psycho-cardiological outpatients. Compr Psychiatry 55:215-220.
- West R, Hajek P, Stead L, Stapleton J (2005) Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 100:299-303.
- Mitchell AJ, Selmes T (2007)Why don’t patients take their medicine? Reasons and solutions in psychiatry. Advances in Psychiatric Treatment 13: 336-346.
- Barney LJ, Griffiths KM, Jorm AF, Christensen H (2006) Stigma about depression and its impact on help-seeking intentions. Aust N Z J Psychiatry 40:51-54.
- Statistics on Smoking: England, 2013.
- White V, Webster B, Wakefield M (2008) Do graphic health warning labels have an impact on adolescents' smoking-related beliefs and behaviours? Addiction 103:1562-1571.
- Boudrez H, Gratziou C, Messig M, Metcalfe M (2011) Effectiveness of varenicline as an aid to smoking cessation: results of an inter-European observational study. Curr Med Res Opin27:769-775.
- Grassi MC, Enea D, Ferketich AK, Lu B, Pasquariello S, et al. (2011)Effectiveness of varenicline for smoking cessation: a 1-year follow-up study. Journal of Substance Abuse Treatment 41:64-70.
- Waxman DJ, Holloway MG (2009) Sex differences in the expression of hepatic drug metabolizing enzymes. MolPharmacol76:215-228.
- Rademaker M (2001) Do women have more adverse drug reactions? American Journal of Clinical Dermatology 2:349-351.
- Jain R, Jhanjee S, Jain V, Gupta T, Mittal S, et al. (2014) A double-blind placebo-controlled randomized trial of varenicline for smokeless tobacco dependence in India. Nicotine Tob Res 16:50-57.
- Kralikova E, Kmetova A, Stepankova L, Zvolska K, Davis R, et al. (2013) Fifty-two-week continuous abstinence rates of smokers being treated with varenicline versus nicotine replacement therapy. Addiction 108:1497-1502.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1a | Figure 1b |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 10347
- [From(publication date):
February-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9457
- PDF downloads : 890
