Cosmetic Benefits of Natural Components Extracted from Garcinia Indica (Kokum) Dried Fruit Rinds
Received: 01-Mar-2023 / Manuscript No. jbtbm-23-88694 / Editor assigned: 03-Mar-2023 / PreQC No. jbtbm-23-88694 (PQ) / Reviewed: 17-Mar-2023 / QC No. jbtbm-23-88694 / Revised: 22-Mar-2023 / Manuscript No. jbtbm-23-88694 (R) / Published Date: 28-Mar-2023 DOI: 10.4172/2155-952X.1000319
Abstract
The benzophenones present in Garcinia indica, are known for their anti-proliferative activities against cancerous cells. However, little literature is available on the cosmetic utilization of Garcinia indica benzophenones hence it is a mandate to check the safety of these extracts as raw materials and in the finished products. In the current study, two different solvents methanol (ME) and ethyl acetate (EAE) were used to extract benzophenones from G. indica fruit rinds. A third extract was made by fractionating EAE with water to give a garcinol-enriched extract (TEAE). All three extracts were tested for their toxicity on mice fibroblast cell lines (NIH/3T3). Our previous research data reveals that TEAE extract showed the highest garcinol content and antioxidant and SPF activity, thus a standardized SPF formulation was prepared using TEAE. The IC50 value of ME, EAE, and TEAE was 400μg/ml, 80μg/ml, and 63.32μg/ ml respectively. TEAE showed the highest toxicity in cosmetic formulations as well. Hence, kokum butter with cell proliferative and migratory potential was used to ameliorate these toxic effects. Additionally, in our previous findings, the cream formulations were also tested for SPF activity with 3% OMC and 2.5% TEAE and as per the COLIPA guidelines it showed a synergistic SPF of 14 in comparison when OMC and TEAE were used alone i.e. 8.05 and 3.68 respectively. The cytotoxicity induced by OMC and/or TEAE was also controlled using Kokum butter. The formulations were tested for irritation on the mice fibroblast cells and found to be practically non-Irritant according to the Japanese Ministry of Healthcare and welfare guidelines for manufacturing cosmetics and quasi-drugs. To conclude, the ethyl acetate extract and kokum butter-containing formulations can pose severe skin benefits when put together and can be further explored for their non-SPF potentials such as MMPase inhibition and cell-based antioxidant potential.
Keywords: Garcinia indica; Fruit rinds; Toxicity; Scratch test; Ethyl acetate; SPF; Non-SPF; Kokum butter
Keywords
Garcinia indica; Fruit rinds; Toxicity; Scratch test; Ethyl acetate; SPF; Non-SPF; Kokum butter
Introduction
Cosmetic preparation using natural extracts requires scientific backup concerning toxicity evaluation and efficacy determination. With increasing awareness, it has become extremely important to access the cytotoxicity of such cosmetic actives before their utilization in cosmetic formulations. In our institution work has been undertaken to study the ability of natural benzophenones in UV absorption. The research work is focused on dried fruit rinds of the indigenous species, Garcinia indica; commonly known as Kokum. In the purview of this, different extracts have been screened for UV-absorbing ability [1,2] and it was found that the fruit rinds when treated with non-polar solvents like butanol or ethyl acetate were much more effective in UV absorption. When the extracts were incorporated into cosmetic cream, they showed excellent sun protective factor (SPF). The Garcinia Benzophenones also behaved synergistically very well with commercial UV protectants like Uvinul A plus, and TiO2 [2]. In our previous study, the authors attempted to develop a sunscreen formulation that is based on natural benzophenones, bioactive extracted from fruit rinds of Garcinia indica, a 62.6% garcinol-enriched fraction. The SPF of a cream formulation containing 3% Octyl methyl cinnamate (OMC) was found to be 8.05 and it was increased to 14 in the presence of 2.5% Garcinia extracts (3.68) with an improvement in the UVA protection [3]. OMC and the Garcinia indica extract acted synergistically.
Polyisoprenylated benzophenones extract from Garcinia is known to possess anti-proliferative activity against cancerous cells such as lung cancer [4, 5], hepatic cancer [6], prostate cancer [7], oral cancer [8], breast cancer [9], colon cancer [10], pancreatic cancer [11], and leukemic cells [12]. Garcinol is one such poly-isoprenylated benzophenones that has exhibited high anticancer potential at extremely low concentrations i.e., the IC50 value on HT29 was found to be10 μm [13], for the human leukemia cell line, HL60, it was 9.42 μm [14], gall bladder carcinoma cells, GBD SD, 10.14 μg, and for NOZ cells it was 10.77 μm [15]. In a particular study, it was documented that garcinol showed toxic effects against tumorigenic MCF-7 breast cancer cells but exhibited no effect on non-tumorigenic MCF-10A cells suggesting specificity against cancerous cells versus normal cells [16].
However, little information is available on the effect of G. indicia on normal cells. Hence the current study was undertaken to analyze the toxicity of Garcinia benzophenones on mice fibroblast cells to determine safe non-toxic concentrations suitable for cosmetic application. The creams formulated with the treated ethyl acetate extract with the highest garcinol percentage and in-vitro activities such as anti-oxidant, and UV-absorption [3] were then tested for toxicity. Since the extract alone and along with OMC exhibited toxic effects, the cell rejuvenating ability of kokum butter or cocoa butter was exploited to ameliorate the toxicity. Kokum butter showed better wound healing potential than cocoa butter. Thus, the resulting formulation containing OMC, TEAE, and kokum thus exhibits enhanced SPF potential, antioxidant activity, and skin rejuvenating potential.
Methods and Materials
Mice embryo fibroblast cell line NIH 3T3 ATCC 1640 was procured from NCCS, Pune. Dulbecco's Modified Eagle's Media DMEM was purchased from Himedia Laboratories, Mumbai, India, Fetal Bovine Serum, FBS was procured from Gibco, Thermo Fischer, USA, 100 IU/ mL Penicillin and 100μg/mL Streptomycin, Sodium Lauryl Sulfate, Sulforhodamine B Dye were purchased from Himedia Laboratories, Mumbai, India. T25 Flask was taken from Nunc, Nalgene Nunc, International, Rochester, NY, USA) and 1X trypsin-EDTA 0.25% from Gibco, Thermo Fischer, USA. The dried kokum rinds were obtained as a gift from Yojak Foods and beverages Pvt. Ltd., Ratnagiri, Maharashtra, India. The samples were crushed using a Philips mechanical blender to give a particle size of 0.5 - 0.7 mm.
Extraction of Garcinia benzophenones in different polar and nonpolar solvents
The extraction was done via maceration of dried fruit rinds in the respective solvents in the system of 200g of rinds each in 1L of methanol (ME) and ethyl acetate (EAE) respectively in a 2.5L flask. The flasks were given intermittent shaking for 2 days at room temperature and the extracts were collected, concentrated in a rotary evaporator, kept in an oven at 60ºC for drying, weighed, and stored at RT for further use.
Extractions of high purity Benzophenone fraction from Ethyl acetate extract TEAE
The ethyl acetate extraction was carried out as described in the previous section. EAE showed a pH of 1-2, and hence it was washed with water till the pH of water-treated ethyl acetate extract (TEAE) reached to 6. The TEAE extracts were further concentrated in a rotary evaporator, kept in an oven at 60ºC for drying, weighed, and stored at RT for further use.
Cell Culture and Maintenance
The cells were maintained in a T25 flask in DMEM with 10% FBS and 1% PenStrep. The media was changed thrice a week. The cells were sub cultured using 1.5ml of 1X Trypsin for 2 mins. The cells were then centrifuged at 1500 rpm for 5 min in 5mL DMEM and 10% FBS. The cells were sub cultured in a ratio of 1:3 per T-25 flask. The cells were used for 10 passages.
Sample Preparation
Different concentrations ranging from 20, 30, 40, 50, 60, 70, and 80 μg/mL were prepared in DMEM after preparing 10 mg/ml of TEAE and EAE in DMSO. The sample ME was prepared in DMEM at dilutions 25, 50, 100, 200, 300, and 400 μg/mL.
Cell Viability and Proliferation Assay
The methodology for performing cytotoxicity was adopted from by Kakodkar et al., 2019, to study the cytotoxicity of ME, EAE, and TEAE extracts. Cells were trypsinized as mentioned earlier and seeded with a density of 0.5 X 104 cells/ well in a 96-well plate with DMEM with 10% FBS and 1 % PenStrep. The cells were incubated at 37°C for 24 hours in a CO2 incubator with 5% CO2. After 24 hours, the media was removed and 100 μL extract at varying concentrations in media without serum was added to the well. 1% IPM was used as solvent control and 1% SLS was used as a negative control. Media alone was used as 100% cell control. The cells were again incubated for 22-24 hours. Following incubation, SRB staining mentioned by Orellana and Kasinski 2016 was utilized to estimate the cell viability. Cell viability was calculated using the formula Abs (sample)*100/Abs (control), where Abs (sample) is the absorbance at 530 nm of cells treated with samples and Abs (control) is media control [17, 18].
Synergistic Effect of Kokum Butter and G. indica extract (TEAE) on Cell viability
The synergistic effect of Kokum butter and TEAE was evaluated on the 3T3/NIH fibroblast cell line. As mentioned above the cell cytotoxicity assay was set up. To the wells, 100 μL of 50 μg/mL of TEAE and 100, 200, 300, and 400 μg/mL of Kokum butter alone and in combination were added and cell cytotoxicity and proliferative effect of kokum butter were estimated in absence of the growth factors.
Formulation of oil-in-water SPF creams
All the materials used for cosmetic formulation were of analytical Grade. The oil phase and oil phase mentioned in table 1 were weighed in different crucibles. Both phases were heated at 65ºC in a boiling water bath. The water phase was kept for stirring in a mechanical top stirrer at 100rpm. When the temperature of both phases came down to 55 ºC, the oil phase was added to the water phase to give an oil-inwater emulsion. The cream was stirred for another 20 mins to ensure complete mixing. The final weight was measured to be 10.0 - 0.5g. The creams were stored at RT until further use.
Toxicity of Cosmetic Formulations
The cosmetic formulations mentioned in (Table 1) were tested for toxicity on 3T3/NIH fibroblast cells. The cream formulations were dissolved in IPM at 100mg/ml and dilutions were prepared in DMEM with 10% FBS and 1% PenStrep. The concentrations prepared were 0.0625, 0.125, 0.25, 0.5, and 1.0 mg/ml in DMEM media with 10% FBS. The toxicity analysis was done as mentioned before [17, 18].
| Component | Base | 1% Kokum Butter (KB) | 1% Cocoa Butter (CB) | 1% TEAE | 1% TEAE + 1% KB | 2.5% TEAE | 2.5% TEAE + 1% KB | 3% OMC | 3% OMC + 2.5% TEAE | 3% OMC + 2.5% TEAE + 1% KB |
|---|---|---|---|---|---|---|---|---|---|---|
| Oil Phase (g) | ||||||||||
| CCTG | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| IPM | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Cetostearyl Alcohol | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Stearic Acid | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Propyl Paraben | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| Kokum Butter | - | 0.1 | - | - | 0.1 | - | 0.1 | - | - | 0.1 |
| Cocoa Butter | - | - | 0.1 | - | - | - | - | - | - | - |
| TEAE | - | - | - | 0.1 | 0.1 | 0.25 | 0.25 | - | 0.25 | 0.25 |
| OMC | - | - | - | - | - | - | - | 0.3 | 0.3 | 0.3 |
| Water Phase (g) | ||||||||||
| EDTA | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Glycerin | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| TEA | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 | 0.042 |
| Methyl Paraben | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 |
Cell proliferation and Migration Assay (Scratch test)
The wound healing assay was performed using a scratch test method on the 3T3/HIH fibroblast cell line. 4 x 104 cells /well were seeded into a 24-well plate in DMEM with 10% FBS and 1% PenStrep. The cells were allowed to adhere and proliferate till the well was 80% confluent. A scratch was made using a micropipette tip (200μL) and the dead cells were removed using media DMEM with 10% FBS and1% PenStrep. Samples (0.1mg/ml of the base alone, base with 1% Kokum Butter, base with 1% Cocoa Butter, base with 2.5% TEAE, and base with1% KB with 2.5% TEAE) were added to the wells and further incubated for 24 hours. The area of the scratch at 0 hours and 24 hours was calculated using Image Software. Percent wound healing activity was calculated [19].
Statistical Analysis
All the measurements are expressed as mean ± standard deviations. Analysis of Variance, ANOVA, was calculated using Turkey’s multiple comparison tests to analyze differences, and p<0.05 was an indication of statistical significance.
Results and Discussions
Toxicity of ME, EAE, and TEAE
In our previous research study, the garcinol content increased as the non-polar components of the extracts increased. It was found that ME showed the least amount of garcinol followed by EAE, and TEAE showed the highest garcinol content up to 62.6% when tested by HPLC [3]. The effect of garcinol on in-vitro cytotoxicity was evaluated in the current study. A correlation was obtained between garcinol content and the cytotoxicity of the extracts.
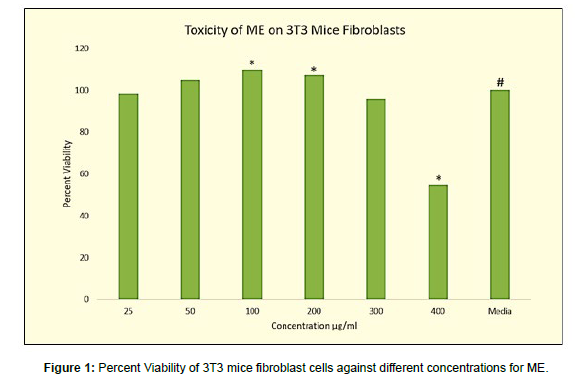
Toxicity of ME on 3T3 Mice Fibroblast Cell line
As depicted in (Figure 1), at 25 μg/ml of methanol extract the cell viability slightly decreased (98.92%) to control cells in which viability was regarded as 100%. But this decrease was insignificant when analyzed by one-way ANOVA at p-value <0.05. With supplementation of 50 μg/ml of methanolic extract, the viability increased significantly to 105.13%. When the concentration of ME was increased from 50 μg/ ml to 100 μg/ml also, there was a significant increase in cell viability. It was 109.85 ± 0.006%. Further increase in ME concentration to 200 μg/ ml did not cause much change in cell viability. It was 107.22 ± 0.006%. Concentrations of ME beyond 200μg/ml caused significant reduction in cell viability i.e., at 300 μg/ml the cell viability was found to be 95.85 ± 0.008. At 400 μg/ml the viability was close to 50% beyond which the extract was toxic. SLS at 1% showed almost complete cell death with cell viability 15.01 ± 0.006. IPM showed no effect on cell viability with 96.62 ± 0.036%.
Toxicity of EAE and TEAE on 3T3 Mice Fibroblast Cell line
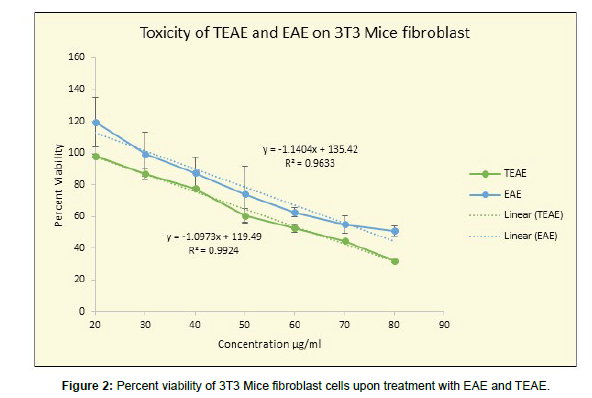
For ethyl acetate extract EAE and fractionated extract (TEAE), various concentrations from 20 to 80 μg/ml were tested for cytotoxicity to mice fibroblasts. A standard curve (Figure 2) was plotted for EAE and TEAE by using values of percent viability obtained on the Y-axis versus concentrations in μg/ml on the X-axis. A dose-dependent decrease in percent viability was observed when cells were treated with EAE and TEAE. The R2 value revealed a very strong negative correlation between the increasing concentrations of EAE (R2= 0.9633) and TEAE (R2= 0.9924) in μg/ml and the percent viability.
As seen in fig 2, the ethyl acetate extract EAE at lower concentration of 20 μg/ml was not at all cytotoxic but rather proliferative. At 20 μg/ml the increase in viability of cells was significantly higher over control when tested by One-Way ANOVA (P< 0.01). Further increase in concentration from 20 μg/ml to 30 μg/ml and 40 μg/ml, brought about a decrease in the cell viability i.e., 99.28% ± 0.023 at 30 μg/ml and 87.42% ± 0.026 at 40 μg/ml. At 60 μg/ml and 70 μg/ml the viability was above 50% suggesting the non-toxic concentrations of the EAE i.e., 62.69 ± 0.015% and 54.94 ± 0.008 % respectively (P<0.01). The IC50 was at achieved at 80 μg/ml of EAE (Figure 2).
The ethyl acetate extract was also containing HCA and was very acidic with pH as low as 1-2. So, it was not suitable for cosmetic formulations and to remove acidic HCA the extracts was fractionated with water. TEAE was also tested for cytotoxicity. The concentrations 20 and 30 μg/ml showed no statistically significant effect on the cell viability with percent viabilities as 98.50 ± 0.050 (p>0.01) and 86.90 ± 0.045 (p>0.01). However, there was a significant difference observed at concentrations 40, 50 and 60 μg/ml, showing cell viability above 50%, 77.57 ± 0.033, 60.47 ± 0.050 and 52.73 ± 0.010 (p <0.01 using One-Way ANOVA). At 70 μg/ml the cell viability dropped below 50 i.e., 42.50 ± 0.018 (p<0.01), at and above which the extract was toxic.
Standard curve was plotted for TEAE (Figure 2) by using values of percent viability obtained on the Y-axis versus concentrations in μg/ml on the X-axis. The regression line equation thus obtained was used to detect the IC50 value of the extracts by substituting 50 in the place of Y in the equation y = -1.0973x + 119.49. The IC50 value was found to be 63.32 μg/ml for TEAE which was lower in comparison to EAE for which IC50 was achieved at 80 μg/ml. And for methanolic extract IC 50 was beyond 400μg/ml
Many of the studies conducted on the Polyisoprenylated Benzophenone, Garcinol, isolated from Garcinia indica, are concerning the anti-proliferative and very little literature is available on the effect of G. indica fruit rinds extract on normal cells.
Recently Ramachandran used ethanolic extracts of Garcinia indica on the proliferation of 3T3 preadipocytes. Their study intended to check whether HCA containing ethyl acetate extract could inhibit the differentiation of preadipocytes to adipocytes. The 50% cell death of adipocytes was observed at 141 μg/ml concentration of ethyl acetate extract of G. indica. The extract could inhibit the adipocyte differentiation significantly and dose-dependent inhibition was quite visible with the extract. Differentiation was inhibited to an extent of 20- 25% in 50- 100 μg extract-treated cells compared to untreated control cells. In the present study also for mouse embryo 3T3 fibroblast, with methanolic extracts the 50% cell death was not achieved up to 400 μg/ ml suggesting for normal fibroblast cells the extracts are not cytotoxic up to 400 μg/ml [20]. For EAE and TEAE, the IC50 values were found to be 80μg/ml and 63.3 μg/ml respectively (Figure 2).
As compared to G. indica, more data of toxicity evaluation is present for benzophenones extracted from G. mangostana. In a research study conducted by Ngawhirunpat el al., 2010, two extracts of G. mangostana, aqueous methanolic and hexane, was tested for their cytotoxic effect on human keratinocytes. The methanolic extract displayed less cytotoxic effects i.e., IC50 was more than 200 μg/ml in contrast to the hexane extract (IC50 = 30 μg/ml). A correlation was studied between the toxicity of extracts and the presence of the Garcinia benzophenone, alpha mangosteen. It was evident that higher toxicity was related to a higher amount of alpha mangosteen present in the hexane extract up to 28.7%, in contrast to methanolic extract with 15.5% of alpha mangosteen [21]. Similar results were obtained in the current study.
A patent published by Deodhar et al., 2022, and the previous work done revealed the percentage of garcinol present in each of these three extracts (ME, EAE, and TEAE) [3]. It was evidently seen that the garcinol content increases in decreases in polarity of the solvents. The correlation between garcinol content and solvents used is also reflected in the toxicity analysis. There was a negative correlation between garcinol content and IC50 value for toxicity of the extracts i.e., -0.652 using Pearson’s Correlation statistic method (Figure 3).
Toxicity of cream formulations containing TEAE
Before testing the products for their efficiency, it is crucial to determine the safety of the cosmetic products involving a new chemical entity (NCE). Since water-treated ethyl acetate extract of G. indica fruit rind is not been used as a cosmetic ingredient before, it was important to achieve its toxic profile in-vitro. The use of animal models for testing the safety of cosmetics is banned and hence cell culture tests have become vital in testing the safety of these products. In order to determine the irritation potential and toxicity of the cosmetic products on skin cells, 3T3/NIH mice fibroblast cells were utilized. The toxic dose of the cosmetic formulation is the concentration above which the cell viability is below 50%, also called as IC50 value.
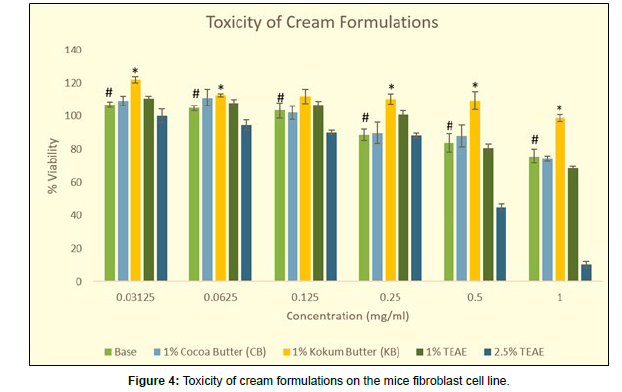
As seen in fig 4, the base formulation without any active ingredient showed cell proliferation at concentrations of 0.03125, 0.0625 and 0.125 mg/ml with percentage viabilities of 106.99, 104.78, and 103.32%. The cell viability declined below 100% after concentrations of 0.25mg/ ml. At 0.25mg/ml the cell viability was found to be 88.85%, at 0.5mg/ ml it was 83.91% and 1mg/ml the cell viability was 75.72%. As the cell viability at 1mg/ml was higher than 50%, the IC50 value of the base formulation can be considered to be above 1mg/ml.
The formulation prepared by adding the active ingredient TEAE at 1% and 2.5% was also tested for safety profiling. The Formulation containing 1 % TEAE at the lower range of concentration up to 0.25 mg/ml looks to be more effective compared to the usual cream base. At 0.0625mg/ml the cell viability was 108.00% whereas the viability of cream base at the same concentration was 104.78%. At 0.25 mg/ml the viability of TEAE-containing cream was 100.87% whereas for the cream base it was only 88.85%. But as the concentration of TEAE was increased to 1mg/ml the cell viability drastically reduced to 68.41% whereas for the cream base it was 75.72%. But for both creams, it was above 50% cell death. When the formulation contained 2.5 % TEAE 1 mg/ml concentration the cell viability drastically decreased to 10.44%.
Toxicity of Cream Formulations containing Natural Butters
The viability of the base formulation at 1mg/ml concentration was itself showing only 75.72% cells. To increase the cell viability of base cream formulations rejuvenating adjuvants like cocoa butter or kokum butter were screened (Figure 4).
When 1% cocoa butter cream formulation was also tested for its proliferative ability, it was observed that both the formulation containing cocoa butter and the base formulation showed the same pattern for cell viability up to 1mg/ml. At lower concentrations such as 0.03125 and 0.0625 mg/ml in the cocoa butter formulation cell viabilities were slightly higher in comparison to the base formulation. At 0.0625mg/ml, the cell viability in cocoa butter was 111.11% whereas in cream base it was 104.78%. However, from 0.125mg/ml onwards the cell viability was almost the same indicating no effect of the butter added on cell viability. At 0.5mg/ml the cell viability in the cocoa butter formulation was 88.13% and that of the base formulation it was 83.91%. And at 1mg/ml concentration, viability in cocoa butter formulation was 74.27% and in base cream formulation it was 75.72%.
However, when the base was enriched with 1% kokum butter showed a statistically significant increase in cell viabilities at all the tested concentrations in comparison to the base formulation. The 1% kokum butter-fortified formulation was found to be proliferating till 0.5mg/ml. Also, at 1.0 mg/ml, the cell viability was found to be 98.53%. This formulation can be considered to be highly rejuvenating in nature due to its cell-proliferating capacity. At 0.03125mg/ml the cell viability was found to be 121.82mg/ml, followed by 112.68% at 0.0625mg/ml, 111.88% at 0.125mg/ml, 110.36 at 0.25mg/ml and 109.39% at 0.5mg/ ml.
Fortification of TEAE with kokum butter
Kokum butter is known in Ayurveda as vrukshamala Beeja Taila and is used in the management of cracked heels [22]. Traditionally it is used to treat dry and dead skin stretch marks sores stomach ulcers. But until now there are no systematic reports validating the regenerative ability of kokum butter. Hence this study was undertaken.
Cell proliferative ability of Kokum butter
Kokum butter was tested at 4 different concentrations (100, 200, 300, and 400 μg/ml) on the 3T3 cell line. The entire experiment was conducted in serum-deprived conditions. In Media with FBS, the viability was regarded as 100% while serum-deprived medium viability was reduced to 90.47%, kokum butter was replaced by serum and cell viability was assessed. Though 100 μg /ml of kokum butter was not enough to nullify serum deprivation where the viability was reduced to 88.9% the higher concentrations of kokum butter replenished the cells from serum deprivation
The concentrations of 200 μg/mL, 300 μg/mL, and 400 μg/mL gave cell viability as 102.77%, 102.47, and 110. 961% respectively. Since the cell viability was above 90% the concentrations of 200 μg/mL, 300 μg/ mL, and 400 μg/mL of kokum butter were considered proliferative.
Synergistic Effect of TEAE and Kokum Butter
The concentration of TEAE extract used was 50μg/ml and the cell viability found at the concentration of TEAE was 63.39%. TEAE at 50 μg/ml was tested with different concentrations of Kokum Butter (100-400 μg/ml) (Table 2). The TEAE was incubated along with these concentrations there was an increase in cell number in comparison with plant extract used alone. TEAE along with 100 μg/mL did not give any increase in cell number. TEAE along with 100 μg/mL of Kokum butter gave 67.34% cell viability similar to TEAE when used alone i.e. 69.39%. However, when the concentration of kokum butter was increased, it could decrease the toxic effect of TEAE and there was an increase in cell numbers. The 50 μg/mL of TEAE along with 200 μg/mL, 300 μg/mL, and 400 μg/mL gave results as 97.44%, 95.15% and 102.02% cell viability with p<0.01 (Students T-Test).
| Sample | Percent viability | Effect of KB on PE |
|---|---|---|
| PE 50 µg /mL | 60.36# | N/Ap |
| KB 100 µg/mL | 88.96 | |
| KB 200 µg/mL | 102.77 | |
| KB 300 µg/mL | 102.47 | |
| KB 400 µg/mL | 110.96 | |
| PE 50 µg/mL and KB 100 µg/mL | 67.34 | 6.88 |
| PE 50 µg/mL and KB 200 µg/mL | 97.44 | 37.07** |
| PE 50 µg/mL and KB 300 µg/mL | 95.19 | 34.82** |
| PE 50 µg/mL and KB 400 µg/mL | 102.02 | 41.65** |
| Media only | 100 | N/Ap |
| Media 10% FBS | 109.53 |
Incorporation of various concentrations of TEAE in cosmetic formulation fortified with kokum butter
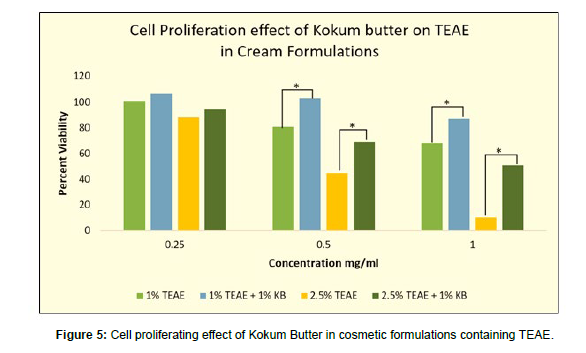
The cell toxicity of the formulations containing TEAE (1% and 2.5%) alone and in combination with 1% kokum butter were also tested for cell proliferation and rejuvenating capacity. The tested concentrations were 0.25, 0.5 and 1 mg/ml as these were non-proliferative and/or cytotoxic concentration of 1% and 2.5% TEAE cream formulations. It was found that at all the tested concentrations, the presence of kokum butter effectively increased the cell viability of TEAE formulation in comparison to when TEAE was present alone (p<0.01).
The 1% TEAE formulation at 0.25mg/ml showed cell viability of 100.87%. When the 1% TEAE cream sample was enriched with 1% kokum butter, the cream sample showed a cell viability of 106.56% at 0.25 mg/ml. Similarly, at 0.5mg/ml, the cell viability of 1% TEAE cream alone was found to be 80.92, whereas, in presence of 1% kokum butter, the viability was found to be 103.37%. At 1mg/ml as well, the cell viability of the 1% TEAE cream formulation was found to be 68.41%. However, cell viability at 1mg/ml of 1% TEAE went up 87.17% in presence of 1% kokum butter.
A similar proliferating effect of kokum butter was evidently seen when the concentration of TEAE was increased from 1% to 2.5%. At 0.25mg/ml the cell viability was 88.30% in presence of only 2.5% TEAE and viability were increased to 94.48% in the formulation fortified with 1% kokum butter along with 2.5% TEAE. The same effect was seen at concentrations higher concentrations. At 0.5 and 1 mg/ml of 2.5% TEAE as the viability increased by 24.29% and 40.79% respectively. There was an increase in cell viability from 10.44% to 51.23% at a concentration of 1mg/ml of 2.5% TEAE in the absence and presence of 1% kokum butter respectively (Figure 5).
Cell proliferation and cell migration capacity of Kokum Butter
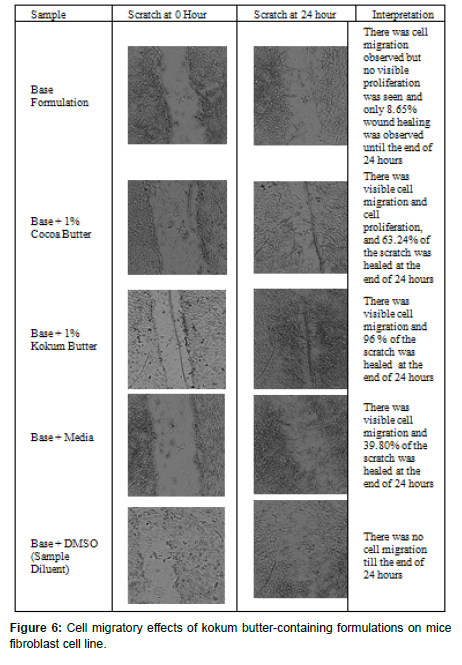
The in-vitro cell migration assay involves creating a “scratch” in a monolayer of cells and capturing images before and after the application of the product at regular time intervals. This test is conducted to mimic wound healing or cell rejuvenation in-vivo. Since kokum butter had shown cell proliferation capacity, the formulations made include a base without any actives, a base with Kokum butter, a base with Cocoa butter, a base with TEAE, and a TEAE cream fortified with Kokum butter were tested for cell migration capacity. The cell rejuvenating capacity of the cream formulations can be attributed to their cell migration capacity in-vitro.
In the current study, the decrease in the area of the scratch due to cell movement was considered to be cell migration, and an increase in cell numbers was considered to be cell proliferation. Both these actions are equally important for healing wounds.
For media control without any samples added, migration was observed. The area of the scratch decreased at the end of 24 hours. However, the cell proliferation was not evidently seen as the in-vitro wound healing ability of the control was found to be 39.80%. The samples were dissolved in DMSO and the wound healing and/or cell migration was completely absent in DMSO control as the area at 0 hours and 24 hours was found to be 1343154 and 1473026 units respectively. The base formulation was also tested for wound healing ability. There was cell migration observed however, no effect on wound healing was documented. At the end of 0 hours and 24 hours, the area of the scratch reduced from 1815878 units to 1658787 to give 8.65% scratch healing. Also, at 0.125 mg/ml the base formulation had cell viability of 103% explaining no effect of base formulation i.e., neither proliferating nor toxic, the absence of cell migration, or inability of base formulation to heal the scratch may be attributed to the presence of DMSO.
However, both the butter Cocoa and Kokum showed a significant (p<0.01) cell migration and scratch healing capacity at 0.1mg/ml as compared to media control. The area of the scratch at 0 hours and 24 hours in presence of 1% Cocoa butter significantly reduced from 1958082 to 718584 to give a percent scratch healing of 63.24%. There was visible cell migration and cell proliferation that was seen due to the presence of cocoa butter in the base formulation.
When the base was supplemented with 1% Kokum butter, complete wound healing was found at the end of 24 hours. The scratch area was reduced to 83565 units at 24 hours from 2090754 at 0 hours. The percent scratch healing ability of 0.1mg/ml of 1% Kokum butter in the base formulation was found to be 96.00%. From the pictures, the density of cells was also found to be highest when treated with Kokum butter in comparison to Cocoa butter. 1% Kokum butter in the base formulation was found to be 1.5X more significantly effective (p<0.01, Independent T-Test) than 1% Cocoa butter (Figure 6).
Since the Kokum butter effectively increased cell proliferation, showed cell migration, and was capable of fully healing the scratch, it was selected to increase the cell rejuvenation capacity of formulation with 2.5% TEAE. Further, both the formulations, base with 2.5% TEAE and base with 2.5% TEAE fortified with 1% Kokum butter were tested for cell migration and scratch healing ability in-vitro on 3T3/NIH mice fibroblast cell line.
In order to increase the cell rejuvenating potential of 2.5% TEAE skin cream, kokum butter was added at a 1% concentration. From the cell proliferation assay, it was found that kokum butter was able to pacify the detrimental effect of TEAE extract in-vitro on 3T3 mice fibroblast cell lines. This cell proliferating ability was extended to check the cell rejuvenation ability of Kokum butter in presence of TEAE extract.
The TEAE extract when added to a cream formulation was unable to heal the scratch. Cell migration was found to be completely absent; cell proliferation was seen though was not effective enough to heal the wound. The area of the scratch at the end of 24 hours reduced to 1682282 units from 2090754 units at 0 hours. The area of scratch at the end of 24 hours was only reduced by 19% which was significantly lower than the media control (p<0.01, Students T-test) explaining the absence of cell rejuvenation capacity of TEAE extract.
However, in presence of Kokum butter, it was found that when the TEAE formulation was fortified with 1% Kokum butter cell migration improved and cell proliferation was evident and led to scratch healing ability. The area of the scratch was 2162972 and 1035099 units at the end of 0 hours and 24 hours. This resulted in scratch closure higher than media control from 39.80% to 52.14% at the end of 24 hours (Figure 7).
Effect of OMC and TEAE cream formulation fortified with Kokum butter on 3T3 cell lines
As stated earlier the authors attempted to boost the SPF of the formulation containing octyl methoxycinnamate (OMC) with TEAE. The SPF of the formulation with 3% OMC was found to be 8.05. However, supplementation with TEAE boosted the SPF to 13.93 and there was also an improvement in UVA PF which improved from 1.09 to 1.94 [3]. The development of sunscreens containing a reduced concentration of chemical UV filters, even though, possesses broad-spectrum effectiveness with the use of natural raw materials that improve and infer UV absorption is of great interest. Due to the structural similarities between polyphenolic compounds and organic UV filters, they might exert photo protection activity [23].
However, when the SPF formulations containing OMC were tested for cytotoxicity, it was found that there was a significant decrease in the viability of the fibroblast cells at higher concentrations (Figure 8). At 1mg/ml concentration the viable cell count of 3T3 dermal fibroblasts of the base formulation was 75.72%. When 3% OMC was added to the formulation the cell viability was decreased to 52.48%. An addition of 2.5 % TEAE extract to the above formulation decreased the viability further to 15.94% at 1 mg/ml. To overcome this difficulty the adjuvants like algal polysaccharides or kokum butter were added to the formulation (Patent). As seen in Fig 6 when 1% Kokum butter was added to the cream containing 3% OMC and 2.5% TEAE the cell viability increased to 74.80 % i.e., the cream is not at all toxic but proliferative. The addition of 1.5 % algal polysaccharides not only improved the cell viability to 68.51% but there was also an improvement in SPF which was increased to 14.19 and UVA-PF was improved to 2.52 [24].
Skin irritation test for cream formulation
Certain ingredients such as tween 20, sodium lauryl sulfate, and triton X-100 are used to classify cosmetic formulations as mild, moderate, or extreme irritants. If the IC50 value of the formulation is above tween 20, then the product is a non-irritant. If the product shows an IC50 value between the IC50 values of Tween 20 and SLS, then the product is a mild irritant. The formulation is a moderate irritant is the IC50 value of the product falls between SLS and triton X-100. And finally, the product is an extreme irritant if the IC50 value of the product is less than the IC50 value of triton X-100 [25, 26].
The toxicity profile and irritation of the cream formulations in the current study were tested on 3T3/NIH fibroblast cell line using standard reference materials known to cause mild (tween 20), moderate (SLS), and extreme irritation (triton X-100). The IC50 value of each of the formulations was calculated using a graph plotted for cell viability of 3T3/NIH fibroblast cells on the Y-axis versus the concentration of individual products on the X-axis. The IC50 value of tween 20 was found to be 0.342 mg/ml, SLS was 0.07mg/m and triton X-100 was 002mg/ml. As mentioned earlier, since the IC50 value of all the formulations was above the IC50 values of the irritants used in the study, all the samples were considered to be practically safe with p-value <0.01 (Table 3).
| Formulations | Toxicity Index IC 50 Value | Irritation Profile |
|---|---|---|
| Base | >1 mg/ml | Practically NOT an Irritant |
| 1% Cocoa Butter (CB) | >1 mg/ml | Practically NOT an Irritant |
| 1% Kokum Butter (KB) | >1 mg/ml | Practically NOT an Irritant |
| 1% TEAE | >1 mg/ml | Practically NOT an Irritant |
| 1% TEAE + 1% KB | >1 mg/ml | Practically NOT an Irritant |
| 2.5% TEAE | 0.42 mg/ml | Practically NOT an Irritant |
| 2.5% TEAE + 1% KB | >1 mg/ml | Practically NOT an Irritant |
| 3% OMC | >1 mg/ml | Practically NOT an Irritant |
| 3% OMC + 2.5% TEAE | 0.6 mg/ml | Practically NOT an Irritant |
| 3% OMC + 2.5% TEAE + 1% KB | >1 mg/ml | Practically NOT an Irritant |
As per the Japanese Ministry of Healthcare and welfare guidelines for manufacturing cosmetics and quasi-drugs, if the IC50 value of the cream formulation is higher than the reference standards (Tween 20, SLS, and Triton X 100), the cream formulation can be concluded to be a practically non-irritant sample [27]. The data also suggests that incorporating the formulation containing 2.5% TEAE with Kokum butter in the absence or presence of OMC has increased the IC50 value from 0.42 mg/ml and 0.6 mg/ml respectively, to 1 mg/ml explaining the regenerative effect of Kokum butter (Figure 9).
Conclusion
As per our previous research, sunscreen formulation with 2.5% TEAE and 3% OMC showed higher SPF and increased critical wavelength offering protection in the UVA region as compared to OMC cream alone. However, the formulation was found to show detrimental effects on the cells. Hence in the current study, the fortification of Kokum butter in the cream was done to substantially reduce the toxicity making it regenerative.
Acknowledgments
The authors would like to thank Dr. S. S. Barve, Director of KET’s Scientific Research Centre, Vaze college campus, for his support in conducting the experiments at this facility.
References
- Dike S, Deodhar M (2015) Sun protective activity of water-immiscible pigments of fruit extract of Garcinia indica. Int J Pharm Sci Res 6(6):2518–2524.
- Dike M, Thergoankar R, Deodhar M (2019) Screening of various extracts of Garcinia indica viz., leaf, seed, stem, root and fruit for UV protective activity and incorporation of extracts in sun protective formulations. Acta Hortic 1241: 639–646.
- SPF boosting potential, UVA protection, antioxidant, and skin rejuvenating multifunctional formulation of Garcinia extract fortified with Kokum butter. Conference: 9th World Ayurveda congress
- Wang J, Wang L, Ho CT, Zhang K, Liu Q, et al. (2017) Garcinol from Garcinia indica Downregulates Cancer Stem-like Cell Biomarker ALDH1A1 in Nonsmall Cell Lung Cancer A549 Cells through DDIT3 Activation. J Agric Food Chem 65:3675–3683.
- Huang CC, Lin CM, Huang YJ, Wei L, Ting LL, et al. (2017) Garcinol downregulates Notch1 signaling via modulating miR-200c and suppresses oncogenic properties of PANC-1 cancer stem-like cells. Biotechnol Appl Biochem 64: 165–173.
- Wang JJ, Sanderson BJ, Zhang W (2011) Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (Garcinia mangostana Linn.) on human melanoma cells. Food Chem Toxicol 49: 2385–2391.
- Sethi G, Chatterjee S, Rajendran P, Li F, Shanmugam MK, et al. (2014) Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Molecular Cancer 13: 66.
- Aggarwal S, DAS SN (2016) Garcinol inhibits tumor cell proliferation, angiogenesis, cell cycle progression and induces apoptosis via NF-κB inhibition in oral cancer.Tumor Biology 37: 7175-7184.
- Ye X, Yuan L, Zhang L, Zhao J, Zhang CM, et al. (2014) Garcinol, an Acetyltransferase Inhibitor, Suppresses Proliferation of Breast Cancer Cell Line MCF-7 Promoted by 17β-Estradiol. APJCP 15: 5001–5007.
- Ranjbarnejad T, Saidijam M, Tafakh MS, Pourjafar M, Talebzadeh F, et al. (2016) Garcinol exhibits anti-proliferative activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Hum Exp Toxicol 36: 692–700.
- Parasramka M, Gupta S (2011) Garcinol Inhibits Cell Proliferation and Promotes Apoptosis in Pancreatic Adenocarcinoma Cells. Nutr Cancer 63:456–465.
- Pan MH, Chang WL, Lin-Shiau SY, Ho CT, Lin JK (2011) Induction of Apoptosis by Garcinol and Curcumin through Cytochrome c Release and Activation of Caspases in Human Leukemia HL-60 Cells. J Agric Fod Chem 49: 1464–1474.
- Liao CH, Sang S, Ho CT, Lin JK (2005) Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem 96: 155–169.
- Hong J, Kwon SJ, Sang S, Ju J, Zhou JN, et al. (2007) Effects of garcinol and its derivatives on intestinal cell growth: Inhibitory effects and autoxidation-dependent growth-stimulatory effects. Free Radic Biol Med 42: 1211–1221.
- Duan YT, Yang XA, Fang LY, Wang JH, Liu Q (2018) Anti-proliferative and anti-invasive effects of garcinol from Garcinia indica on gallbladder carcinoma cells.Int J Pharm Sci 73: 413-417.
- Ahmad A, Wang Z, Ali R, Maitah MY, Kong D, et al. (2010) The apoptosis-inducing effect of garcinol is mediated by NF-κB signaling in breast cancer cells. J Cell Biochem109: 1134-1141.
- Kakodkar SA, Kshirsagar SN, Kelkar AS, Nair AM, Dhawal PP, et al. (2019) Evaluation of phytochemical constituents, antioxidant property, DNA damage inhibition activity and cytotoxicity of aster (Callistephus chinensis) flower waste.World J Pharm Res 8: 977-991.
- Orellana E, Kasinski A (2016) Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio-Protocol 6: 1-9
- Rodriguez-Menocal L, Salgado M, Ford D, van Badiavas E (2012) Stimulation of Skin and Wound Fibroblast Migration by Mesenchymal Stem Cells Derived from Normal Donors and Chronic Wound Patients. Stem Cells Transl Med 1: 221–229.
- Ramachandran C, Quirin KW, Cawelius A, Escalon E, Melnick SJ (2021) Anti-obesity effects of Garcinia indica high pressure ethanolic extract in vitro. Int J Herb Med 9: 1-08.
- Ngawhirunpat T, Opanasopi P, Sukma M, Sittisombut C, Kat A, et al. (2009) Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana). Pharm Biol 48: 55–62.
- Mohammed F, Joshi SV, Tantrady SB (2017) Clinical efficacy of Vrukshamla Beeja Taila (Kokum Butter) in the Management of Padadari (Cracked Heels). J Ayurveda Med Sci 2: 209–213.
- Majeed M, Majeed S, Nagabhushanam K, Lawrence L, Mundkur L (2020) Garcinia indica extract standardized for 20% Garcinol reduces adipogenesis and high fat diet-induced obesity in mice by alleviating endoplasmic reticulum stress.JFF 67: 103863.
- Deodhar MA, Thergaonkar RS, Tribhuvan AN, Pophali KH, Dhawal PP (Inventors) Cosmetic Composition with Skin Protecting Bioactives and Method of Preparation Thereof. India Patent Application 202021040383. September 2020, Published March 2022.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, et al. (1990) New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI 82:1107–1112.
- Morimitsu K (1995) Safety Division, Pharmaceutical and Medical safety Bureau, Ministry of Health and Welfare. Jpn J Radiol 55: 257–259.
- Sawant SS, Mane VK (2017) Correlating the Anti–Aging Activity with the Bioactive Profile of Chlorella emersonii KJ725233; Its Toxicological Studies for a Potential use in Cosmeceuticals. Phcog Commn 4: 152–157.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Deodhar M, Dhawal P (2023) Cosmetic Benefits of Natural Components Extracted from Garcinia Indica (Kokum) Dried Fruit Rinds. J Biotechnol Biomater, 13: 319. DOI: 10.4172/2155-952X.1000319
Copyright: © 2023 Deodhar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1857
- [From(publication date): 0-2023 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1579
- PDF downloads: 278