Short Communication Open Access
Corrosion Resistant Titanium Alloys for Medical Tools and Implants
Corby Anderson1*, Manana Mikaberidze2, George Gordeziani2, Eteri Gozalishvili2, Lia Akhvlediani2 and Dali Ramazashvili2
1Kroll Institute for Extractive Metallurgy, Colorado School of Mines, Golden Colorado, USA
2Tavadze Institute of Metallurgy and Materials Science, Tbilisi, Georgia, USA
- *Corresponding Author:
- Corby Anderson
Kroll Institute for Extractive Metallurgy
Colorado School of Mines, Golden Colorado, USA
E-mail:cgandersmines@gmail.com
Received Date: June 20, 2013; Accepted Date: August 01, 2013; Published Date: August 08, 2013
Citation: Anderson C, Mikaberidze M, Gordeziani G, Gozalishvili E, Akhvlediani L, et al. (2013) Corrosion Resistant Titanium Alloys for Medical Tools and Implants. J Powder Metall Min 2:110. doi: 10.4172/2168-9806.1000110
Copyright: © 2013 Anderson C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
New corrosion resistant Ti-8Ni-Cr system alloys with increased hardness and strength have been developed for medical tools and implants. The development was made based on study of chromium influence on the phase constituents, microstructure, mechanical properties, corrosion resistance and electrochemical characteristics of Ti- 8Ni alloy. Optimum condition of thermal treatment, providing high strength, hardness and corrosion resistance of alloys has been defined – quenching from 950°C with content of chromium in alloys 1 – 3% (by addition of Yttrium in quantity 0,001 – 0,01 mass%). The cause is that alloys after quenching from 950°C have structure of transformed β-solid solution and contain ω-phase, which is micro dispersive during concentration of chromium up to 1-3%. On the base of studying of the phase equilibrium by microstructural, x-ray investigations and differential thermal analysis of Ti-8Ni-Cr system alloys polythermal sections of phase diagram of this system have been constructed, which is in complete accordance with the phase diagram formed by thermodynamic calculations. Study of mechanical properties of Ti-8Ni-(0-3) Cr quenching alloys show, that chromium increases the tensile strength (1000MPa) and hardness (48 HRC) and slightly influences on plastic properties of the Ti-8Ni alloy. Tensile strength of titanium commercial alloys: Ti-6Al-4V and Ti-5Al-3Sn do not exceed 900 MPA. Corrosion tests in medium containing human body: blood, physiological solution, gastric juice, tissue liquid and also corrosion testing according to the following regime: cleaning+disinfection+sterilization in aggressive solutions with addition of hydrogen peroxide reveal high corrosion resistance of Ti-8Ni-(0-3)Cr alloys, without the changing of surface. After 20 cycles corrosion losses of the commercial titanium alloys are ~ one order more than the losses of new alloys. Study of toxic properties of alloys Ti- 8Ni-(1-3)Cr during their implantation in muscles and abdominal cavity of animals show, that they do not cause local irritative actions on different tissues, they do not suppress local tissue reactions and do not have any toxic effects during short or long term implantation conditions. High corrosion resistance of alloy Ti-8Ni-1Cr is established also by the modeling and prediction of corrosion behavior in the physiological solution at the temperature 37oC with using of electrochemical investigations, spectral analysis, regression and variance analysis of the mathematical statistics. Alloys Ti-8Ni-(1-3)% Cr are recommended for manufacturing high-strength medical tools of multiply usage and surgical implants. Application of new alloys will allow improving functional properties and increase quality, reliability, service life of medical tools and implants.
Keywords
Corrosion; Resistant; Alloys; Manufacturing; Strength
Introduction
The purpose of the present paper is the development of new corrosion resistant Ti-Ni-Cr system alloys with increased strength and hardness by investigating phase equilibrium and structural transformations, mechanical properties and corrosion resistance in medical solutions.
Last years are characterized by fast development of medical engineering. For maintenance of the newest methods of operations in vascular and neural surgery, ophthalmology, traumatology and other areas of medicine the new instruments, modern sets of microsurgical tools and implants are created. The most important question in creation of medical tools is not only their constructive design, but also selection of such materials, which considerably will raise their quality, reliability, service life and will improve their functional properties. For the increase of wear and corrosion resistance, and also functional properties of medical tools and implants, the microsurgical tools with hardened working surfaces are developed [1-4]. The high strength, low weight, outstanding corrosion resistance possessed by titanium and titanium alloys have led to a wide and diversified range of successful applications which demand high levels of reliable performance in surgery and medicine as well as in aerospace, automotive, chemical plant, power generation, oil and gas extraction, sports, and other major industries. More than 1000 tones of titanium devices of every description and function are implanted in patients worldwide every year. Requirements for joint replacement continue to grow. Light, strong and totally biocompatible, titanium is one of few materials that naturally match the requirements for implantation in the human body.
However, most of commercial titanium alloys have low hardness and insufficient corrosion resistance in aggressive washing and sterilizing media. In this connection, the development of new titanium alloys with high mechanical properties together with corrosion resistance represents significant interest, both for manufacturing medical tools and implants and for coating them with the purpose of their hardening.
The basic way of creation of high-strength, corrosion resistant titanium alloys at the present stage is a complex alloying of solid solutions. The choice of rational structures of alloys is based on theoretical assessment, phase diagram and “structure - property” investigations for various alloying elements.
The most cost saving elements raising hardness of titanium based binary alloys are iron, chrome, manganese, copper, aluminum, silicon etc., however they considerably lower corrosion resistance of titanium. At alloying with molybdenum, tantalum, cobalt, nickel, germanium, niobium the high corrosion resistance is achieved only at rather high concentration of these elements.
For medical tools, where the high-strength, hardness and corrosion resistance is required, the development of metastable β titanium alloys can be expedient. They can be created by complex alloying by elements, such, as: chrome, nickel, manganese, molybdenum, vanadium etc., which form with titanium substitutional solid solutions, stabilize β phase and expand its area on phase diagrams. Simultaneously, the chosen elements should raise or not influence corrosion resistance of titanium. The alloys with metastable β structure can be strengthened with the help of dispersion hardening by submicroscopic precipitates.
Titanium alloys with α and (α+β) containing up to 2% β stabilizing elements are used for tools, details and designs, which are not requiring high durability and hardness. Wider application finds titanium alloys with (α+β) structure. They have much greater strength than the α-alloys, have more bend plasticity, can be forged, rolled and stamped more easily than alloys with α and β structure. Mass manufacture of these alloys is rather simple.
Titanium and its alloys such as: unalloyed titanium (ASTM F1341, F67), Ti-6Al-4V (ASTM F620), Ti-4Al-3Mo-V, Ti-5Al-3Sn, Ti-3Al- 1,5Mn etc. are used for manufacturing some medical tools like mirrors, wound wideners, nails for osseointegration, tracheametric tubes, needle holders, eye and wire fixation tweezers, heart valves and other microtools [5-14].
In the literature there are no data on the behavior of ternary titanium based Ti-Ni- Cr system alloys in medium, that contains human body: blood, physiological solution, gastric juice, tissue liquid and also washing, disinfection and sterilization solutions for medical tools; the influence of above mentioned alloying elements on corrosion and mechanical properties of titanium is not investigated; data is scarce about phase equilibrium in titanium rich multicomponent alloys.
The development of new corrosion resistant titanium alloys with increased mechanical properties and their application in medical engineering will allow improving functional properties and to increase quality, reliability, service life of medical tools and consequently it will have significant social impact.
Technical approach
Smelting of the Ti-8Ni-Cr system alloys have been carried out in arc vacuum furnace of the MIFI type with the unexpended tungsten electrode in the atmosphere of argon. Working mixture loaded into a water-cooled copper crucible. Titanium sponge, nickel, and chromium were used as working mixture materials. Regime of melting was – 200- 300 A, at 50V. For achieving the homogeneity of composition four and five times remelting was used. The control of chemical composition was carried out by comparative weighing of the received ingots. Difference in the weigh composed not more than 0,5%/ The received rods were cut on 10 mm size pieces, which were placed into the special device, intended for getting the cylindrical ingots with 4,6 and 10 mm diameters.
Thermal treatment of alloys has been carried out according to the following regimes: quenching from temperatures 950° in the water.
The structure of the received samples was studied by optical (“Noplot-21”) microscopy methods together with x-ray analyses. Mechanical properties of alloys were carried out by measurements of hardness, tensile strength, elongation and cross-section reduction by using the standard methods.
For the estimation of corrosion resistance of alloys gravimetric method was used together with visual control.
Corrosion resistance of titanium alloys in media containing the human body: blood, physiological solution (0.9% NaCl), gastric juice (1% HCl) and tissue liquid, as well as in solutions used for disinfections, washing and sterilization of medical instruments have been studied. 3 types of sterilization were used: 1. Chemical sterilization in 6% solution of hydrogen peroxide (during 3 hours, at 50°C); 2. Sterilization in airdrying chamber (at 180°C, 45 min); 3. Vapor sterilization autoclave (at 115°C, 1.5 ATM, 30 min.). Washing solution of 0.5% hydrogen peroxide was used as cleaning solution means. Disinfection was done in boiling distilled water during 45 minutes with addition of cooling to the room temperature [15].
Electrochemical investigations on potentiostats have been carried out in NaCl, HCl and NaOH solutions. Potentiodynamic curves E-lgi of alloys have been constructed.
Corrosion currents were defined graphically according to proposed by us method: stationary potential was adjusted to the electrode, anodic and cathodic polarized curves E-lgi with the amplitude of 80- 100 (mV) were got and their linear section in 26-75 mV diapason were extrapolationed on the shown potential; corrosion rate K (g/m2hr) was calculated according to the well-known formula K = Ai/zF, where A is atomic weight, g/mol (for titanium A=47.9 g/mol), z- valency, F – Faraday number – 26.8 A·hr/mol, I – current density A/m2.
Thermodynamic study and theoretical calculation of phase diagram of Ti-8Ni-Cr system alloys were carried out by using “Thermo-Calc” Software [16-25].
Investigation of toxic properties of new titanium alloys Ti – 8Ni-(1- 3) Cr was carried out during their short and long term implantations into the muscles and abdominal cavity of animals [26].
Short term implantation
After cleaning, disinfection and sterilization in 70% Ethanol solution 5 disks of test samples (Ф10 mm, thickness – 1 mm) were implanted aseptically into the muscles of rats and mice using hypodermic needle and Nembutal narcosis (for rats 40 mg/kg and 80 mg/kg for mice). Similarly 5 equal sizes of negative control rubber dicks were implanted. There were 5 animals in each experimental and controlled group. The animals were maintained for 10 days and sacrificed. Tissues around the implant site were harvested and examined macroscopically (visual control) the implants sites were also excised, placed in formalin and processed for microscopia, pathological evaluation.
Long term implantation
After cleaning, chloroform disinfection and sterilization 5 disks of test samples were implanted aseptically into the abdominal cavity of rats and mice. Similarly 5 disks of negative control rubber were implanted. The animals were maintained for 60 days and sacrificed. There were 5 animals in each experimental and controlled group. Animals were weighed for 10, 20, 40 and 60 days. During this period their general condition was controlled.
Tissues around the implant site were harvested and examined by visual control and microscopically. Visual control of abdominal cavity has been made too.
Results
Alloys of Ti-Ni-Cr system have been melted with constant content of Nickel- 8% and variable content of Chromium 0-10%, by the addition of Yttrium in Quantity 0.001-0.01%. Thermal treatment of alloys has been carried out according to regime: quenching from temperatures: 950°C, 800°C, 700°C and 600°C in the water. Microstructure of alloys has been studied is casting condition and after quenching.
Characteristic microstructure of alloys in casting condition consists of primary crystallized grains of β-solid solution with tracks of dendrite structure.
After quenching from 950°C all alloys of this section show the structure of transformed β-solid solution.
In Table 1 results of X-ray phase analysis of alloys are represented, as it is seen the solid solution on the basis of hexagonic closepacking structure of α-titanium, β-phase on the basis of cubic sizecentrid structures of titanium and intermetallic compounds Ti2Ni and TiCr2 have been received on X-ray photographs. In alloys with 0-3% of chromium there was β phase after 800°C quenching, but because of unstableness of β-solid solution during quenching on X-ray photograph of these alloys of lines has been received, answering to hexagonic closepacking structure - α′.
| No | Alloys | Phase content of alloys after quenching from temperatures | ||
|---|---|---|---|---|
| 950° | 800° | 600° | ||
| 1 | Ti-8Ni | β+ω | α′+ Ti2Ni | - |
| 2 | Ti-8Ni-0,1Cr | β+ω | α′+ Ti2Ni | α+ Ti2Ni |
| 3 | Ti-8Ni-0,5Cr | β+ω | α′+ Ti2Ni | α+ Ti2Ni |
| 4 | Ti-8Ni-1Cr | β+ω | α′+ Ti2Ni | α+ Ti2Ni |
| 5 | Ti-8Ni-3Cr | β+ω | α′+ Ti2Ni | - |
| 6 | Ti-8Ni-5Cr | β+ω | - | α+ Ti2Ni+TiCr2 |
| 7 | Ti-8Ni-8Cr | β+ω | β+ Ti2Ni | α+ Ti2Ni+TiCr2 |
| 8 | Ti-8Ni-10Cr | β+ω | β+ Ti2Ni | α+ Ti2Ni+TiCr2 |
Table 1: Results of x-ray phase analysis of Ti-Ni-Cr alloys.
After 950°C quenching all alloys showed β+ω structure.
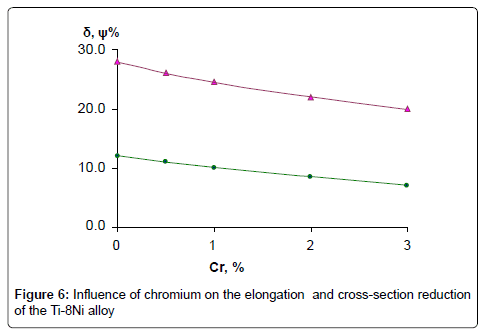
Influence of chromium on the hardness of the alloys Ti-Ni-Cr system after quenching from the temperatures: 950°, 800°, 700° and 600°C is presented in the Figure 1. As shown in diagrams, alloys of this type after quenching from 600°C is characterized with low value of hardness that is connected with dilution of matrix by alloying elements. The highest value of hardness of quenching from 950°C and 800°C alloys is connected with decomposition of β-phase in quenching conditions from these temperatures. After quenching from 950°C all alloys have β+ω structure; at the same time in alloys, containing 1-3% of chromium, formed ω-phase is microdispersive causing their high hardness.
.Corrosion resistance of Ti-Ni-Cr system alloys was studied out in 10% solutions of hydrochloric acid (HCl), sodium chloride (NaCl) and sodium hydroxide (NaOH) in cast condition and after quenching from temperatures: 950°, 800° and 600°C.
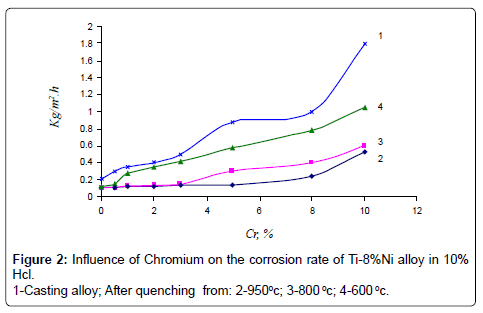
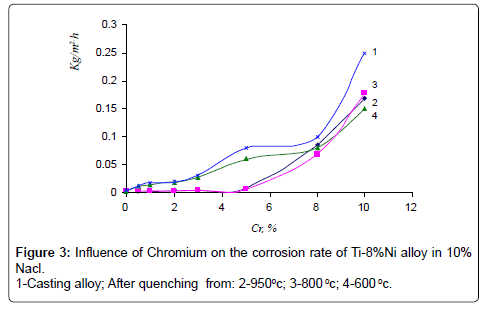
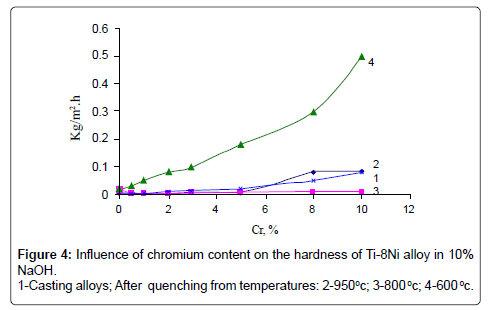
Studying of kinetics of corrosion rate of alloys showed, that maximum corrosion losses were observed in alloys after 100-hour tests. The results of corrosion tests are given in (Figures 2-4).
As it is seen in quenching from 950°C and 800°C alloys, chromium up to 3% does not influence on corrosion rate of Ti-8% Ni alloy in all examined solutions. Further increase of chromium causes insignificant rise of corrosion rate in NaCl and NaOH solutions, but it increases corrosion rate of Ti-8% Ni alloy 6 times more in solution of HCl.
It should be mentioned that pH solutions does not change after tests.
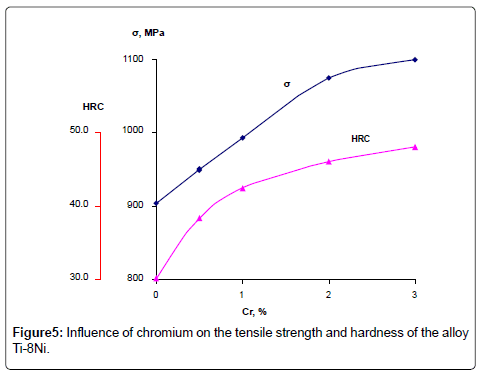
Corrosion rate of casting alloys and after quenching from 600°C is rather higher, though the character of curves is the same as in quenching from 950°C and 800°C alloys – with increasing content of chromium from 3 to 10% corrosion rate increases in all solutions, especially in solution of HCl. High corrosion resistance of quenching from 950° alloys is caused by the fact that the structure of these alloys consists of β-solid solution or contains intermetallic compound Ti2Ni. In alloys after quenching from 600°C chromium abruptly increases corrosion losses of Ti-8%Ni alloy that is caused by increasing of quantity of not stability in these solutions compound TiCr2. Results of chemical analysis after testing of alloys in all solutions are according to their corrosion resistance. In solutions NaCl and NaOH quantity of moving ions of titanium, nickel and chromium are insignificant. After tests in HCl maximum quantity of moving into solution ions is observed in quenched alloys from 600°C. With increase of chromium content, quantity of moving ions of Ni and Cr are 0.001 g/l and 0.005 g/l accordingly. Quantity of moving into solution ions of Ti increases from 0.004 to 0.03 g/l. Optimum regime of thermal treatment of alloys, guaranteeing high strength, hardness and corrosion resistance has been defined – quenching from 950°C, and content of chromium in alloys up to 1-3%. Mechanical properties of quenching from 950°C alloys Ti-8Ni-(0-3) Cr are given on Figures 5 and 6. As shown on Figures chromium increases hardness and tensile strength, and slightly influences on plastic properties of Ti-8Ni alloy. Studying of Ti-8Ni-3Cr alloy surface and distribution of alloying elements on the characteristic x-ray emission TiKα, NiKα and CrKα were carried out on the electro microanalizer, Cameca, Microsonde MS46. Surface of alloy Ti-8Ni- 3Cr and element distribution are shown on the Figure 7. As it is visible the elements – titanium, chromium and nickel are proportionally distributed on the surface of Ti-8Ni-3Cr alloy. Corrosion resistance of Ti-8Ni-(0-3)Cr alloys have been studied in medium that contains the human body: blood, physiological solution (0.9% NaCl), gastric juice (1% HCl) and tissue liquid. In conserved blood in physiological solution and tissue liquid after 100 hour tests, corrosion rate of all alloys, containing about 3% chromium did not exceed 0.0002 g/m2hr. After the researches, blood test shown that the blood formula does not change (eozinophils 0.-5%, limphocytes 20-30%, monocytes – 10%, leicocytes 5000/l, neitrophils - within norm). In the 1% solution of HCl chromium influences slightly to 1%, and further changes from the worse corrosion resistance of alloys and constitutes 0.05 g/m2hr after 100 hour tests. Phase transformation of Ti-8Ni-Cr system alloys has been determined with help of differential thermal analysis.
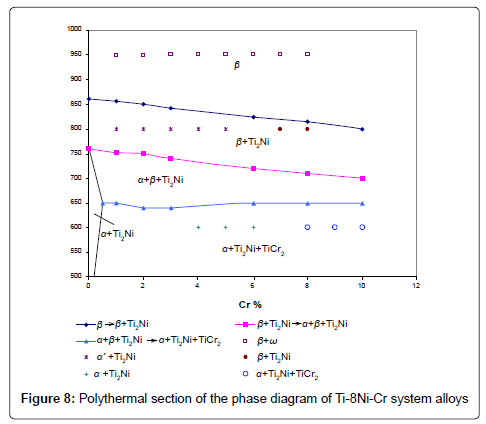
On the base of microstructural, X-ray investigations and differential thermal analysis of Ti-8Ni-Cr system alloys the polythermal sections of phase diagram of this system have been constructed Figure 8.
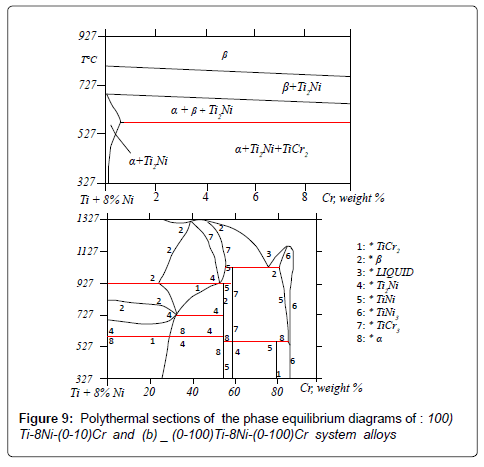
All alloys of this section are crystallized as the β solid solutions on the base of titanium. On the section there are: β, β+Ti2Ni , α+β+Ti2Ni , α+Ti2Ni and α+Ti2Ni +TiCr2 phase areas. By a method of the differential thermal analysis it is established, that alloys of the studied section undergo phase transformations in the solid state, connected with the transformations in the binary systems: Ti-Ni and Ti-Cr. Obtained temperatures of transformations are shown on the polythermal section of the Ti-8Ni-Cr system alloys phase diagram. In process of increase of chromium concentration (from 0 up to 10%) in alloys, the transition temperature β → β+Ti2Ni is reduced from 860°C to 800°C. The temperature of transition β+Ti2Ni → α+β+Ti2Ni in process of increase of concentration of chromium in alloys from 0 up to 10% decreases from 760°C up to 700°C. The alloys containing from 0,5 up to 10% chromium at the temperature 650°C undergo eutectoid transportation β → α+Ti2Ni+TiCr2. Compound TiCr2 will be in the alloys with >0,3% chromium concentration. Thermodynamic study of alloys of system Ti-8Ni-Cr was carried out. Polythermal sections of phase equilibrium diagrams of Ti-8Ni-Cr system alloys, which have been constructed by our calculations, are given on the Figure 9. For the purpose of checking the accuracy of calculated values of theoretical Figure 9 and the experimental Figure 8 diagrams of Ti-8Ni-Cr system alloys have been compared with each other.
On the diagram, that has been constructed by our calculations), all the formed phases completely coincide with phases, which are represented on the experimental diagram. Temperature -concentration sections are completely given as well and they coincide with the data determined experimentally. Hence, it can be concluded that the diagram constructed by five-member coefficients of binary interaction is sufficiently exact and coincide with the experimentally obtained diagram [27]. Corrosion testing of the Ti-8Ni-(0-3)-Cr alloys has been carried out according to the following regime: cleaning+desinfection +sterilization. Washing solution with 0,5% hydrogen peroxide was used as cleaning solution means. Desinfection was done in boiling distilled water during 45 minutes with addition of cooling to the room temperature. Three types of sterilization were used: 1.Sterilization in air-drying chamber (at 180°C, 45 min) 2. Vapor sterilization autoclave (at 115°C, 1,5 ATM, 30 min) and 3,6% H2O2 (50°C, 3hr). For comparing titanium standard alloys have been studied, they are used nowadays in medical technique. The results after 20 cycle tests according to the regime: cleaning + desinfection + sterilization are given in Table 2. As it is seen corrosion losses of alloys Ti-Ni-Cr increase insignificantly, though all alloys have high corrosion resistance. For alloy with 1% Cr Δm/s=0.0035 g/m2 after 20 cycles. Corrosion losses of the known alloys are ˜ one order more than the losses of Ti-Ni-Cr alloys. Visual control of alloys showed that known alloys withstand 10 cycle of cleaning without surface changing. Further the surface condition changes, spots of oxide tint appear; the surface of Ti-8Ni-(0-3) Cr alloys does not change after 20 cycles.
| ALLOYS | TYPES OF STERILIZATION | ||||
|---|---|---|---|---|---|
| in air drying chamber (at 180°C, 45 min) | vapor sterilization autoclave (at 115°C; 1,5 ATM, 30 min) | Chemical, in 6% H2O2; 50°C, 3 hr | |||
| Ti ��? 8Ni | 0,001 | 0,002 | 0,0202 | ||
| Ti ��? 8Ni ��? 0,5Cr | 0,002 | 0,002 | 0,0251 | ||
| Ti ��? 8Ni ��? 1Cr | 0,002 | 0,0025 | 0,0354 | ||
| Ti ��? 8Ni ��? 2Cr | 0,003 | 0,004 | 0,0541 | ||
| Ti ��? 8Ni ��? 3Cr | 0,003 | 0,005 | 0,0851 | ||
| Pure titanium | 0,17 | 0,21 | 0,2312 | ||
| Ti ��? 4Al ��?2Mn | 0,21 | 0,158 | 0,2508 | ||
| Ti ��? 6Al ��? 4V | 0,37 | 0,315 | 0,4509 | ||
| Ti ��? 5Al ��? 3Mo ��? 2V | 0,50 | 0,52 | 0,6123 | ||
| Ti ��? 5Al ��? 3Sn | 0,41 | 0,32 | 0,4822 | ||
Table 2: Corrosion loses, Δm/s (g/m2), of alloys after 20 cycle of cleaning, desinfections and sterilization
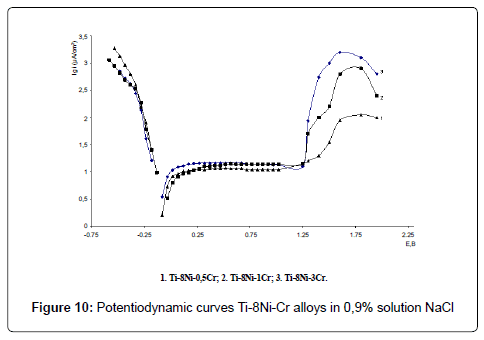
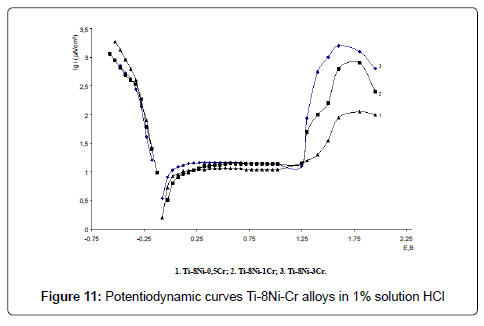
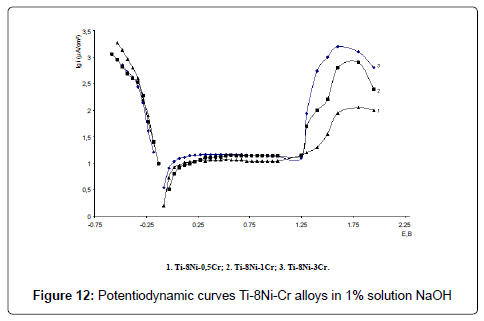
Electrochemical investigations of alloys have been carried out in 0.9% NaCl, 1% HCl and 1% NaOH solutions. Potentiodynamic curves E-lgi of Ti-Ni-Cr alloys are given in the Figures 10-12. As the analysis of the curves show, alloys in all solutions are characterized with a passive field and overpassivation; with raise of chromium content anodic currents increase in all solutions.
Corrosion currents were defined graphically, according to proposed by us method: stationary potential was adjusted to the electrode, anodic and cathodic polarized curves E-lgi with the amplitude of 80- 100 (mv) were got and their linear section in 25-75 mv diapason were extrapolationed on the shown potential; corrosion rate K (g/m2hr) was calculated according to the well – known formula K=A/ZF, where A is atomic weight, g/mol (for titanium A=47.9 g/mol), Z – valency, F – Faradey number – 26.8 Ahr/mol, i – current density A/m2.Stationary potentials, (E, V) corrosion currents (icor, µA/sm2) and corrosion rates (K, g/m2hr) of the Ti-Ni-Cr alloys have been calculated according to the formula are shown in the Table 3.
| ALLOYS | SOLUTIONS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% HCl | 0.9% NaCl | 1% NaOH | Cleaning solution +0,5%H2O2 |
||||||||
| Ecor,V | icor,µA/sm2 | K, g/m2 hr |
Ecor,V | icor, µA/sm2 | K, g/m2 hr |
Ecor,V | icor, µA/sm2 | K, g/m2 hr |
Ecor | icor | |
| Ti-8Ni | -0,4 | -0,6 | |||||||||
| Ti-8Ni-0.5Cr | 0,3 | 2,28 | 0,010 | 0,06 | 1,07 | 0,005 | -0,08 | 2,29 | 0,01 | -0,25 | -0,58 |
| Ti-8Ni-1Cr | 0,3 | 2,09 | 0,009 | 0,12 | 0,35 | 0,002 | 0,01 | 2,4 | 0,011 | -0,6 | -0,55 |
| Ti-8Ni-3Cr | 0,23 | 2,68 | 0,012 | 0,19 | 0,85 | 0,004 | -0,24 | 4,17 | 0,019 | -0,65 | -0,5 |
Table 3: Stationary potentials (E,V), Corrosion currents (icor, �?µA/sm2) and corrosion rate (K, g/m2 hr) of Ti-8Ni-Cr system quenching alloys.
Calculated values of alloy corrosion rates are in complete accordance with values of corrosion rates got by the gravimetrical tests.
Study of toxic properties of new titanium alloys
Among requirements, resulting from adopted conditions implants from the new alloys, the most significant is biocompatibility in a human body’s organism. Surgical implants closely adjoin with organism tissues during their exploitation; they are blood – washed and washed by tissue- liquids. That is why it is inadmissible that unhealthy for an organism combinations extract on the surface of these implants. Thus it is very important to study of toxicity of surgical implants during their implantation period. Investigation of toxic properties of new titanium alloys: Ti-8Ni-1Cr, Ti-8Ni-2Cr and Ti-8Ni-3Cr were carried out during their short and long term implantations into the animals muscles and abdominal cavity.
Results of short term implantation
Tissues around the implanted Ti-Ni-Cr alloys and rubber samples were harvested and examined for inflammation hemorrhage, necrosis and discoloration macroscopically the macroscopic (visual control) observation showed neither sign of inflammation no encapsulation, hemorrhage, necrosis or discoloration.
The Microscopic research of extracted tissues showed that histological picture in the case of implanted titanium alloys and rubber implantation is identical the microscopic evaluation did not reveal any increase in biological reaction as compared with the control rubber disks.
Toxicity rating for the implanted titanium alloys samples at the end of 10 days time period was – 0.18, indicating no toxicity according to ISO 10993-6. 0-indicates normal tissues and 0.5-for very slight reaction.
Thus new titanium alloys samples do not prevent the development of the normal reactions of connective tissue and consequently have not render local toxic action on the tissue elements.
Results of long term implantation
The general state of animals during all time of observation was not characterized with any symptoms of intoxication: animals were mobile, willingly ate forage, behavioral reactions – excitability, reactivity, tearfulness, spontaneous activity–did not change. That specifies absence of oppression of the central nervous system and activity vegetative ganglions. The average weight of rats bodies in the beginning of experience has made 155 g, the subsequent weighing for 60 days after operation has shown increase in weight at 15%. The same weight increasing has been observed in the group of mice. On opening the abdominal cavity investigation of tissues, harvested around the implant samples should no sign of cavity irritations, inflammation, hemorrhage, necrosis or discoloration. Only one rat had a thin purulent capsule around its heterogeneous body and its cavity appeared to be hyperemized, it might be the result of the infection obtained during the implantation process (operation). The microscopic evaluation did not reveal any increase in biological reaction. Histological picture in both cases of titanium alloys samples and rubber disks implantation are practically identical. Toxicity rating for the implant samples was 0.2, indicating no toxicity according to ISO 10993-6.
Thus, alloys Ti-8Ni-(1-3)Cr do not cause the phenomena of local irritation at contact with the peritoneum and internal organs of animals, located in abdominal cavity and they do not cause the phenomena of general intoxication, which might arise in the case of the resorptive action of the material of alloys.
New alloys do not render local irritation on different tissues of animals, do not suppress local tissue reactions and do not render any toxic action during the long implantation conditions.
Consequently using of new Ti-8Ni-(1-3) Cr alloys for manufacturing of surgical implants is rather actual nowadays.
Conclusions
New corrosion resistant Ti-8Ni-Cr system alloys with increased hardness and strength have been developed for medical tools of multiply usage and implants. The development was made based on study of chromium influence on the phase constituents, microstructure, mechanical properties, corrosion resistance and electrochemical characteristics of Ti-8Ni alloy. Chromium increases the hardness of the alloys Ti-Ni-Cr system after quenching. The highest value of hardness of quenching from 9500 and 8000 alloys is connected with decomposition of β-phase in quenching conditions from these temperatures. After quenching from 950°C all alloys have β+ω structure; at the same time in alloys, containing 1-3% of chromium, formed ω-phase is microdispersive causing their high hardness. Optimum condition of thermal treatment, providing high strength, hardness and corrosion resistance of alloys has been defined – quenching from 950°C with content of chromium in alloys 1 – 3% (by addition of Yttrium in quantity 0,001 – 0,01 mass%). On the base of studying of the phase equilibrium by microstructural, x-ray investigations and differential thermal analysis of Ti-8Ni-Cr system alloys polythermal sections of phase diagram of this system have been constructed. Based on thermodynamic study and theoretical calculations of phase equilibrium by the Maxwell rule and with using iteration procedures of Newton, phase equilibrium diagrams of Ti-8Ni- Cr system alloys have been formed too. Comparison of the theoretical and experimental diagram of Ti-8Ni-Cr system alloys showed, that all the formed phases on the diagram, that have been constructed by our calculations, completely coincide with the phases, which are represented on the experimental diagram. This fact is of definite interest as from theoretical so practical point of view. Study of mechanical properties of Ti-8Ni-(0-3)Cr quenching alloys show, that chromium increases the tensile strength (1000MPa) and hardness (48 HRC) and slightly influences on plastic properties of the Ti-8Ni alloy. Tensile strength of titanium commercial alloys: Ti-6Al-4V and Ti-5Al-3Sn do not exceed 900 MPA. Investigation of Ti-8Ni-3Cr alloy surface and distribution of alloying elements on the characteristic x-ray emission TiKα, NiKα and CrKα were carried out on the electro microanalizer, Cameca, Microsonde MS46. and showed that the elements – titanium, chromium and nickel are proportionally distributed on the surface of Ti-8Ni-3Cr alloy.
Corrosion tests in medium containing human body: blood, physiological solution, gastric juice,tissue liquid reveal high corrosion resistance, after 100 hour tests corrosion rate of all alloys, containing about 3% chromium did not exceed 0,0002 g/m2hr. Corrosion testing of the Ti-8Ni-(0-3) Cr according to the following regime: cleaning+disi nfection+sterilization in aggressive solutions with addition of hydrogen peroxide showed also high corrosion resistance of Ti-8Ni-(0-3) Cr alloys, without the changing of surface. After 20 cycles corrosion losses of the commercial titanium alloys are ˜ one order more than the losses of new alloys. Visual control of alloys showed that known alloys withstand 10 cycle of cleaning without surface changing. Further the surface condition changes, spots of oxide tint appear; the surface of Ti- 8Ni-(0-3) Cr alloys does not change after 20 cycles.
Study of toxic properties of alloys Ti-8Ni-(1-3)Cr during their implantation in muscles and abdominal cavity of animals show, that they do not cause local irritative actions on different tissues, they do not suppress local tissue reactions and do not have any toxic effects during short or long term implantation conditions.
Of the assumption of results we can give following conclusion - Alloys Ti-8Ni-(1 -3)%Cr have higher corrosion resistance, strength and hardness in comparison with commercial titanium alloys, used nowadays in medical technique.
Alloys Ti-8Ni-(1 -3 )%Cr are recommended for manufacturing high-strength medical tools of multiply usage, surgical implants and also for hardening working surface of surgical tools.
Application of new alloys will allow improving functional properties and increase quality, reliability, service life of medical tools and implants.
References
- Leyens C, Braun R, Frhlich M, Hovsepian PEH (2006) Recent progress in the coating protection of gamma titanium-aluminides. Journal of the Minerals, Metals and materials and Materials Society 58: 1.
- Leyens C, Braun R, Frhlich M, Hovsepian PEH (2006) Recent progress in the coating protection of gamma titanium aluminides. Journal JOM Journal of the Minerals, Metals and Materials Society 58: 1.
- John DK, Rivard, Craig A, Blue, David C et al. (2005) The thermomechanical processing of titanium and Ti-6Al-4V thin gage sheet and plate. Journal JOM Journal of the Minerals, Metals and Materials Society 57: 11.
- Rack HJ, Javaid Q (2005) Advanced titanium alloys and processes for minimally invasive surgery. JOM Journal of the Minerals, Metals and Materials Society 57: 11.
- Don L, Greg C (1999) Vacuum-Die casting titanium for aerospace and commercial components. JOM Journal of the Minerals, Metals and Materials Society 51: 6.
- Ivasishin OM, Markovskym PE, Matviychuk V, Sematin SL (2003) Precipitation and recrystallization behavior of beta titanium alloys during continuous heat treatment. Journal Metallurgical and Materials Transactions 34: 1.
- Ramesh KT (2002) Effects of high rates of loading on the deformation behavior and failure mechanisms of hexagonal chose-packed metals and alloys. Journal Metallurgical and Materials Transactions 33: 3.
- Recina V, Lundstrom D, Karlsson B (2002) Tensile, creep, and low-cycle fatigue behavior of a cast ?-TiAl-based alloy for gas turbine applications. Journal Metallurgical and Materials Transactions 33: 9.
- Salem AA, Kalidindi SR, Doherty RD, Semiatin SL (2006) Strain hardening due to deformation twinning in a-titanium: Mechanisms. Journal Metallurgical and Materials Transactions 37: 1.
- Sherbakov AI (2000) Kinetics of titanium dissolution, and the effect of its alloying with yttrium, lanthanum, and cerium. Protection of Metals 36: 3.
- Pohrelyuk IM, Yas kiv OI, Fedirko VM, Hrypachevs OMP, Proskurnyak RV (2006) Morphology of the subsurface layers of a Ti-Al-Mo-V after carbonitriding. Materials Science 42: 6.
- Cotton JD (2000) Anelastic deformation measurements in structural engineering alloys. Journal of Materials Engineering and performance 9: 4.
- Rangaswamy P, Bourke MAM, Von Dreele R, Bennett K, Daymond MR et al. (2000) Texture and residual strain in two SIC/Ti-6-2-4-2 titanium composites. Journal Metallurgical and Materials Transactions 31: 3.
- Habazaki H, Uozumi M, Konno H (2004) Breakdown of Anodic Films on Titanium and its Suppression by Alloying. The Journal of Corrosion Science and Engineering 6: 107.
- Instrument Care, Cleaning and Sterilization Instruction In Accordance with ISO 17664-2003, Manufacturer Zimmer, Inc.
- Thermo-Calc Software, Bo Sundman (1997) Calculating Thermodinamic Properties. Dept of Materials Sience and Engineering Royal Institute of Technology SE-100 44 Stockholm, Sweden.
- Saunders N, Miodovnik AP CALPHAD (1988) Comprehensive Guide.
- Kaufman L, Bernstein H (1970) Computer Calculation of Phase Diagrams, ManLabs, Inc. Cambridge, Massachusetts, Academic Press. New York, USA.
- Robert B (1986) Griffiths, Numerical Mathematical Analysis Global Phase Diagram For a Three-Component Model, Carnegie-Mellon University, Pittsburg, Pennsylvania.
- Sundman B (1991) User Aspects of Phase Diagrams, F.H.Hayes ed., Institute of .Metals, London.
- Christian JW (1975) The Theory of Phase Transformations in Metals and Alloys. Pergamon Press, Oxford.
- Rene T (1996) Catastrophe Theory Dinamical Systems Theory and Bifurcation Exploratorium.
- Okada K (1993) Catastrophe Theory and Phase Transitions, Scitec Publications, Zug, Switzerland.
- Kaufman L, Nesor H (1973) Titanium Science and Technology, Plenum Press, New York, USA.
- Saunders N, Chandrasekaran L (1992) Ti-data and thermodynamic database for calculation of phase equilibria in multi-component Ti-based alloys, Thermotech Ltd, Surrey Technology Centre, Guildford, U.K.
- Animesh C, Bikramjit B, Balasubramaniam (2005) Electrochemical Behavior of Ti-Based Alloys in Simulated Human Body Fluid Environment. Biomaterials & Artificial Organs. A biannual journal published by the Society for Biomaterials and Artificial Organs India 18.
- Gordeziani GA, Mikaberidze MP, Ramazashvili DR, Gozalishvili EI, Akhvlediani LA, et al. (2007) Thermodynamic study of Ti-8Ni-Cr system alloys and theoretical computation of their phase diagram. Georgian Engineering news.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 16227
- [From(publication date):
July-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11495
- PDF downloads : 4732