In this study, we are interested to study the status of insulin, HOMA-IR, leptin and oxidized LDL in a group of obese women with diabetes compared to a group of non-obese and non-diabetics women.
In our study population who are obese diabetics women (BMI=35.75 ± 3.96 kg m
-2 and blood glucose=11.04 ± 1.55 mmoles L
-1), it was found that weight and BMI are significantly higher compared to the control group (respectively 93.28 ± 13.72 kg against 72.29 ± 8.30 kg and 35.75 ± 3.96 kg m
-2 against 19.96 ± 2.17 kg m
-2). Both systolic than diastolic pressure were significantly higher in diabetic obese, respectively (140.2 ± 9.5 vs. 122.8 ± 2.5) mmHg and (88.5 ± 4.2 vs. 72.7 ± 5.3) mmHg. No relationship between age and obesity were observed in both groups (p=0.735).
Our result disagree with the results of a study by El Hazmi et al. covering 11208 individuals (4628 men and 6580 women), who found a strong relationship between age and obesity (r=0.9066 in men and r=0.9593 in women), and between age and overweight (r=0.8864 in men and r=0.8790 in women) with p<0.005 [
4].
The association of obesity and diabetes usually results in an increase in some biochemical parameters characteristic of the disturbance that occurs in the body. This disturbance mainly affects carbohydrate and lipid parameters. Among the most affected parameters by metabolic disorders caused by diabetes and obesity there’s leptin that represents a satiety signal and control of food intake. Plasma leptin values are generally a function of the degree of adiposity. Leptin is a plasma marker of energy stock that represents the adipose tissue. It is known that weight gain, related to a too high theoretical contribution and independently of the conditions of obesity, causes an increase in plasma levels of leptin. Conversely, weight loss or simply food restriction decrease plasma leptin in normal or obese humans [
5].
The results of our study supported this hypothesis: leptinemia of our diabetic obese was significantly higher than that observed in the control group (19.73 ± 4.82 ng mL
-1 against 4.73 ± 1.85 ng mL
-1). It confirms the results of Sinha who for a sample of obese type 2 diabetics found an average leptinemia 30.80 μg L
-1 against 12.00 μgL
-1 in control group [6]. Also, Edward Adeyemi team working on 70 obese subjects found results that join our results since leptin levels were higher in obese compared with the control group (35 non-obese subjects) (leptinemia: 38 against 5.6 ng mL
-1, p<0.0001) [
7].
In the same study, Edward Adeyemi found that the average leptin levels are higher in females than males (41.3 ng mL
-1 in women against 22 ng mL
-1 in men, p<0.0001). This explains in some way the high values found in our study population.
We suppose that, among others, insulin resistance is the explanation for the high levels of leptin. It seems that the decrease in insulin sensitivity, into a modest proportion in healthy individuals, is also physiologically related to age. Insulin resistance in obese subjects is associated with a secondary hyperinsulinemia present in obese subjects grade I and II, whereas grade III obese have a depletion of the secretory capacity of beta cells of the islets of langerhans [
8].
Our group of obese women with diabetes also has higher blood glucose and insulin levels compared with the control group (p<0.05). Our results showed that the value of the HOMA-IR index of insulin resistance was significantly higher in diabetic obese women and the threshold value (HOMA-IR> 2.4) is exceeded in all our diabetic obese women samples compared to controls (χ
2=64.5, p<10
-3) [
9].
To normalize the high levels of glucose, the pancreas secretes more insulin causing hyperinsulinemia. The increase in rates of glucose and insulin in obese diabetics is a consequence of insulin resistance installed in the beginning of diabetes: the decrease in the sensitivity of muscle and fat cells to the action of insulin induces the increase of blood glucose and hyper secretion of insulin by the β cells of the pancreas in order to adapt to the demand accentuated by insulin resistance.
Insulin circulates in the blood and ensures a constant supply of glucose to cells which is used as an energy source. Some of this excess glucose is stored in the short term, by liver cells (as glycogen) and muscle cells (also in the form of glycogen) which will quickly reuse the reserve of energy. The other part is stored more permanently by adipocytes. In fact, our body which is economical with its energy has selected a sustainable recovery system of the glucose molecules in excess. This is the fat cell, which is responsible for capturing them, turn them and place them in reserve.
Excess weight and obesity characterize the increased number and size of the adipocytes in proportions that do not appear to have limitations. These enormous adipocytes have a very disturbed metabolism that disrupts energy regulation that they ensure by hormones they secrete. This is one of reasons to place obesity as a risk factor for type 2 diabetes
The lipid metabolism abnormalities appear to be involved in frontline in the cardiovascular accidents [
10,
11]. It appears that insulin resistance plays a role in lipid metabolism disorders: the insulin resistance induces a defective metabolism of TG establishing a situation in which cholesterol metabolism is also strongly influenced. This implies a number of changes affecting all subclasses of lipoproteins because of metabolic links between them. Changes induced by the defective metabolism of TG make them more atherogenic and facilitate additional pathological changes caused by the diabetic environment [
12].
In our study we were interested only on quantitative disorders of carbohydrate and lipid parameters. We found high LDL-CHOL and oxidized LDL compared with the control group (p<0.05). Levels of total CHOL and TG were also increased (p=0.036 and p=0.087). HDL-CHOL was decreased in the study population compared to the control group. Our results are similar to those of a study conducted by Gharibeh and al who worked on a sample of 77 obese women with diabetes (BMI=32.39 ± 0.71 kg m
2) and found that blood glucose, insulin and TG were higher compared to the control group (64 women): (blood glucose: 10.10 ± 0.32 mmolL
-1 against 5.50 ± 0.05 mmolL
-1, insulin: 11.94 ± 0.6 mmolL
-1 against 10.38 ± 0.56 mmolmL
-1, TG: 2.7 ± 0.21 mmolL
-1 against 1.9 ± 0.11 mmolL
-1) whereas HDL-CHOL was higher in the control group (0.97 ± 0.02 mmolL
-1 against 0.9 ± 0.02 mmolL
-1 in diabetics). Also they found that LDL-CHOL was normal in the group of obese diabetic as in control group [
13] contrary to our results showing that the LDL-CHOL is higher in obese diabetic.
Although the level of LDL cholesterol in plasma is normal or slightly increased in patients with type 2 diabetes, it is observed significant changes in the kinetics of LDL, particularly a slowdown in their turn-over, so their residence time in the blood favoring the probability of their potentially deleterious oxidation. Increased oxidative stress, which translates into high levels of oxidized LDL, may precede the development of insulin resistance [
14]. Oxidized LDL high levels may play an important role in the physiopathology of onset of type 2 diabetes. In addition, the oxidative modification of LDL is considered as a major event in the initiation and progression of atherosclerosis. In adults, circulation of oxidized LDL is associated with obesity, insulin resistance, metabolic syndrome and cardiovascular diseases [
15].
As for the hyper-triglycerides observed in obese diabetic it could be related to an increase in triglyceride-rich lipoproteins (VLDL, IDL) resulting from increased hepatic VLDL production and a slowdown in their catabolism and the decrease in HDL-cholesterol is secondary to an increased catabolism of HDL [
11].
It appears that the lipid metabolism disorders are not independent of carbohydrate metabolism disorders: the two metabolisms are connected and changes of a parameter can influence the stability of others. Insulin resistance and the deficit in insulin secretion observed in type 2 diabetes, appear to play an important role because insulin performs essential functions in the control of lipid metabolism.
The study of relationships between different biochemical parameters in our control group found that BMI was significantly and positively correlated with glucose (r=0.508, p=0.019), LDL-CHOL (r=0.617, p=0.003), insulin (r=0.955, p<0.001), leptin (r=0.955, p<0.001), and oxidized LDL (r=0.857, p<0.001). Leptin was significantly and positively correlated with glucose (r=0.531, p=0.013), insulin (r=0.916, p<0.001), LDL-CHOL (r=0.569, p=0.007) and oxidized LDL (r=0.779, p=0.001).
LDL-CHOL was correlated significantly and positively with insulin (r=0.469, p=0.032) and oxidized LDL (r=0.700, p<0.001). Glucose was significantly and positively correlated with insulin (r=0.481, p=0.027). Insulin was significantly and positively correlated with oxidized LDL (r=0.796, p<0.001). CHOL was significantly and positively correlated with TG (r=0.452, p=0.04).
These correlations found in our control group agree with several studies that have found such correlations: Zimmet et al. conducted a study involving 2500 people and found a strong positive correlation between insulin and leptin. This correlation was completely independent of obesity [
16]. In the same context, the team of Brigitte Le Roy, working on a sample of 35 non-obese subjects with normal lipid, found a strong positive correlation between leptin and BMI (r=0.71, p<0.001). The team also found positive correlations between leptin and insulin (r=0.6, p<0.001) and between leptin and TG (r=0.43, p<0.02) [
6]. This correlation between leptin and TG was not observed in our study, and found inconsistently in the literature; this could be explained by the great instability of TG values which is due, among other things, to the fasting period and the quality of the meal the night before.
In the same study of Brigitte Roy, insulin, in addition to the already reported positive correlation with leptin, showed significant positive correlations with BMI (r=0.59, p<0.001) and TG (r=0.34, p<0.05) [6].
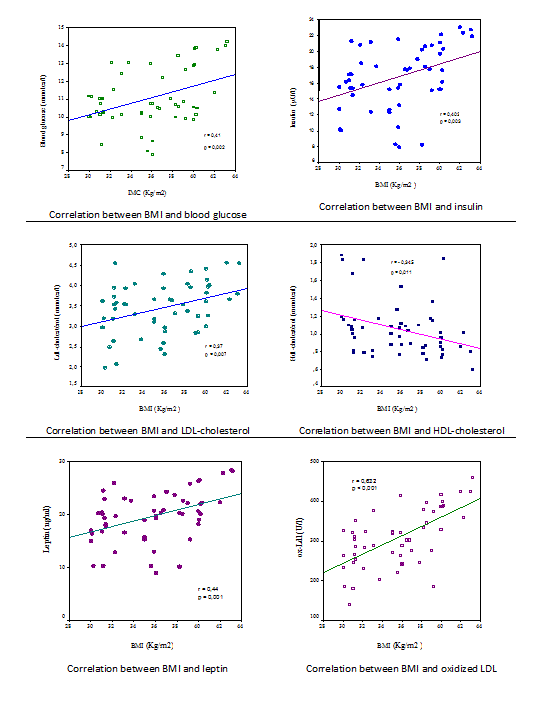
About our diabetic obese group, there is a significantly positive correlation between BMI and leptin (r=0.436, p=0.001), This result confirms those of Fischer, who worked on a sample comprised of 21 obese type 2 diabetics since found a positive and significant correlation between BMI and leptin (r=0.49, p=0.02) [
17]. Similarly, Edward Adeyemi found this positive and significant correlation between leptin and BMI (r=0.4, p<0.001) and also a positive correlation between leptin and obesity class (r=0.4, p<0.01) [
8]. Generally, the circulating leptin reflects the full fat mass which explains the increased rate of leptin associated with increased BMI [
18]. BMI was also positively and significantly correlated with blood glucose (r=0.408, p=0.002), the same result was proved by Onyesom Innocent and al in their study of 253 subjects by showing the existence of a positive correlation between BMI and blood glucose levels in all participants and that this correlation was more significant in women (p<0.05) [
19]. By against, Etukumana et al. working on 750 adults, found that there is no correlation between BMI and blood glucose [
20]. BMI is significantly and positively correlated with insulin (r=0.403, p=0.003). The hypothesis of insulin resistance increasing with obesity has been formulated to explain the association between BMI and blood glucose. Our results showed a positive significant correlation between BMI and LDL-CHOL in the group of diabetic obese women (r=0.365, p=0.007). But correlation was significantly negative with HDL-CHOL (r=-0,345, p=0.011). These results are consistent with those of Helmut Sghroder team who worked on 1577 subjects including 121 obese women and found that BMI was positively correlated with LDL-CHOL in the 2 sexes (p<0.05 in women) and negatively correlated with HDL-CHOL (p<0.001) [
21]. These correlations are the direct result of the narrow link between obesity, diabetes and dyslipidemia, also insulin resistance appears to play a role in this association.
These results contradict those of Shamai L who worked on 637 obese subjects (362 women) and found that there was no significant association between BMI and LDL-CHOL (r=0.19, p=0.07). In addition he found a direct association between BMI and TG [
22] which was absent in our results. The high instability of TG values can explain the difference between the results of two studies.
Regarding oxidized LDL, they were positively and significantly correlated with BMI (r=0.632, p<0.001), This is similar to the results of Ettore Porreca and al who found a significantly positive correlation between BMI and oxidized LDL (r=0.69, p<0.0001) in a study of 60 obese women [
23]. This suggests the existence of a link between fat mass and the oxidation of LDL-CHOL. Our results are in line with those of Holvoet P and al who found that BMI is one of the strongest predictors of circulating levels of oxidized LDL [
24]. Oxidized LDL were also positively and significantly correlated with the glucose (r=0.327, p=0.017), LDL-CHOL (r=0.753, p<0.0001), leptin (r=0.318, p=0.02), and insulin (r=0.478, p<0.0001); and correlated negatively and significantly with HDL-CHOL (r=-0,334, p= 0.015). These correlations were also observed in the study of Ettore Porreca which showed that oxidized LDL were positively and significantly correlated with LDL-CHOL (r=0.30, p=0.025), insulin (r=0.65, p<0.0001), leptin (r=0.65, p<0.0001); but no significant correlation between oxidized LDL and blood glucose nor between oxidized LDL and HDL-CHOL was found [
23]. Also, Porreca found a significant positive correlation between oxidized LDL and total cholesterol (r=0.32, p=0.017) [
22], which was not found in our results. Moreover, our results showed the existence of correlations between biochemical parameters in diabetic obese women: Leptin was significantly and positively correlated with glucose (r=0.673, p<0.001), insulin (r=0.839, p=0.003), LDL-CHOL (r=0.459, p=0.001), and LDL oxidized (r=0.318, p=0.02); and negatively and significantly correlated with HDL-CHOL (r=-0,394, p= 0.004). These results confirm those found by Wannamethee and collaborators who found a positive correlation between leptin and insulin (r=0.56, p<0.001) and a negative correlation between leptin and HDL-CHOL (r=-0.22, p<0.001) [
25]. We notice that there is a strong binding that connects leptin to BMI and different carbohydrate and lipid parameters. This can explain in some way the combination of obesity, insulin resistance and diabetes:
Obesity is at the origin of insulin resistance probably as a result of to an excess of lipid (lipotoxicity) particularly in some organs (liver, skeletal muscles).
The increase in leptin observed in obesity could explain the occurrence of this insulin resistance and hyperinsulinemia, early signs of diabetes, followed by hyperglycemia, evocative sign of non-insulin dependent diabetes (type 2) installed. We found a significant positive correlation between HOMA-IR, BMI, leptin, the LDL-oxidized and insulin. These results are agreed with those of Wang TN and Das P [
26,
27]. The mechanism of the link between leptin and oxidized LDL is not very well known, but this correlation could be based on a pro-oxidant effect of leptin [
23].
Insulin was positively and significantly correlated with blood glucose (r=0.546, p<0.001), LDL-CHOL (r=0.624, p<0.001) and oxidized LDL (r=0.476, p<0.001). The correlation is significantly positive between oxidized LDL and glucose (r=0.327, p=0.017), LDL-CHOL (r=0.753, p<0.001), leptin (r=0.459, p=0.001) and insulin (r=0.476, p<0.001); and significantly negative between oxidized LDL and HDL-CHOL (r=-0,334, p=0.015). Blood glucose was significantly and positively correlated with LDL-CHOL (r=0.277, p=0.045) and negatively and significantly correlated with HDL-CHOL (r=-396, p=0.003). CHOL was significantly correlated with TG (r=0.538, p<0.001).
So according to our results, BMI is correlated with a group of biochemical parameters: blood glucose, leptin, insulin, LDL-CHOL, oxidized LDL (positive correlations) and HDL-CHOL (negative correlations). These parameters are correlated between each other, but their relationships with BMI are not identical. This leads us to suppose that some of these parameters are not directly related to BMI but through other parameters. We are interested to look for parameters most strongly associated with BMI and obesity. We performed a multivariate logistic regression analysis with top-down approach.
We introduced BMI as the dependent variable and each of the biochemical variables correlated with BMI (glucose, HDL-cholesterol, LDL-cholesterol, leptin, insulin and oxidized LDL) as explanatory variables. We found that oxidized LDL, leptin and LDL-CHOL are most directly correlated with BMI parameters. So obesity reflects circulating levels of leptin, LDL-CHOL and oxidized LDL. BMI measured easily predicted rates of these biochemical parameters involved in diabetes and CVD and maintaining a BMI "ideal" may prevent the increase of leptin, LDL-CHOL and oxidized LDL and disturbances that follow.