Research Article Open Access
Correlation of LDL Cholesterol with Maternal and Cord Blood Heme Oxygenase 1 in Preeclampsia
| Kharb S*, Tiwari R and Nanda S | |
| Department of Biochemistry, Sharma University of Health Sciences, Rohtak, Haryana, India | |
| Corresponding Author : | Kharb S Department of Biochemistry Sharma University of Health Sciences Rohtak, Haryana, India Tel: 919812016036 E-mail: simmikh@gmail.com |
| Received: January 29, 2016 Accepted: February 19, 2016 Published: February 26, 2016 | |
| Citation: Kharb S, Tiwari R, Nanda S (2016) Correlation of LDL Cholesterol with Maternal and Cord Blood Heme Oxygenase 1 in Preeclampsia. J Preg Child Health 3:225. doi:10.4172/2376-127X.1000225 | |
| Copyright: © 2016 Kharb S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Objective Emerging evidence supports an important role for the heme oxygenase system (HO-1) in the maintenance of a healthy pregnancy, especially during pathological challenge. HO-1 is widely accepted to be a highly sensitive and reliable marker of oxidative stress. Hence the present study was planned to analyse heme oxygenase-1 and lipid profile in maternal and cord blood venous samples of normal pregnant and preeclamptic women. Methods Fifty pregnant women were selected and grouped as group 1 (control) comprising of twenty five normotensive women immediately after delivery; group 2 (study group) comprising of age-and sex-matched twenty five preeclamptic women. Study samples were drawn (maternal venous blood and umbilical cord blood) and heme oxygenase-1 was analyzed by competitive enzyme linked immunosorbent assay and lipid profile was analyzed enzymatically. Results Cord blood hemeoxygenase-1 levels in preeclamptic women were significantly higher than those of normotensive women (p<0.001). There was significant rise in serum heme oxygenase 1 levels in preeclamptic women as compared to normotensive pregnant women (p<0.001). LDL levels were positively correlated with HO 1 in preeclamptic women (r=0.236, p>0.05) and negatively correlated in normotensive women (r=-0.111, p>0.05), indicating the induction of HO 1 by LDL. Conclusion The findings of high serum heme oxygenase-1 levels in maternal and cord blood in preeclampsia supports the role of oxidative stress and excessive inflammatory response in the pathogenesis of preeclampsia.
| Keywords |
| Heme oxygenase; Oxidative stress; Cord blood; Preeclamptic; Pregnant |
| Introduction |
| Heme oxygenase (HO) is rate-limiting enzyme in heme degradation. The by-products of heme degradation have antioxidative, antiapoptotic, anti-inflammatory, and cytoprotective properties [1]. Emerging evidences support an important role for the heme oxygenase system (HO-1) in the maintenance of a healthy pregnancy, especially during pathological challenge [1,2]. During pregnancy, it may mediate the regulation of maternal blood pressure, placental development, and vascularization, and, therefore, the maintenance of a healthy pregnancy. HO-1 has been shown to play a role in the maintenance of maternal inflammatory homeostasis and normal placental vasculature development by regulating angiogenesis and matrix remodeling in early pregnancy [1]. HO-1 is widely accepted to be a highly sensitive and reliable marker of oxidative stress. Inducible isoform HO-1 is expressed at a low basal level in vascular endothelial and smooth muscle cells and induced by heavy metals, oxidative stress, inflammatory mediators and oxidized low density lipoproteins [1]. |
| NO and CO modulate intracellular cGMP levels, platelet aggregation and smooth muscle relaxation, CO has a much lower affinity for soluble guanylyl cyclase than NO. Decreased production or sensitivity to NO in atherosclerosis may be compensated for by an induction of HO-1, with bilirubin acting as a cellular antioxidant and CO as a vasodilator. Oxidized low density lipoproteins (LDL), hypoxia and pro-inflammatory cytokines induce HO-1 expression and activity in vascular endothelial and smooth muscle cells, and evaluate the antiatherogenic potential of the heme oxygenase signalling pathway [2]. |
| Together with lipid oxidation products, LDL may therefore be a vehicle for internalization of iron into the cells, a situation that could contribute to the cytotoxicity of hemin-oxidized lipoproteins, as suggested by Yuan et al. [3]. The experiments supported the hypothesis that at sites of LDL retention in the extracellular intima, heme may be a pro-oxidant of pathological significance, even in the presence of other plasma components [3]. |
| Aim of this study was to analyse heme oxygenase-1 and lipid profile in maternal and cord blood venous samples of normal pregnant and preeclamptic women. |
| Materials and Methods |
| The present study was conducted in the Department of Biochemistry in collaboration with Department of Obstetrics and Gynaecology, Pt. B.D. Sharma, PGIMS, Rohtak from July 2014 to July 2015. Heme oxygenase-1 and lipid profile were analyzed in maternal and cord blood in women with preeclampsia and compared with those of normotensive pregnant women. An informed consent was taken from all the patients and the research protocol was approved by the Institutional Review Board. Women with history of smoking, chronic hypertension, any metabolic disorder before or during pregnancy or presence of high risk factors like anemia, heart disease, diabetes, renal disease or history of any vitamin supplements or any supplements known to alter lipid profile were excluded. |
| Fifty pregnant women were selected and grouped as: Group 1 (control) comprising of twenty five normotensive women immediately after delivery. Group 2 (study group) comprising of age -and sexmatched twenty five preeclamptic women with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg with or without proteinuria immediately after delivery. Study samples were drawn (maternal venous blood and umbilical cord blood) before starting any medication. In both groups, five ml of maternal venous blood sample was collected aseptically from anticubital vein and 10 ml of cord blood was collected from placental end of umbilical cord after delivery of baby. The serum was separated by centrifugation and analyzed the same day. Routine investigation SGOT, SGPT, S. creatinine, blood urea, S. uric acid were performed as per standard enzymatic methods by autoanalyzer. Maternal and cord blood HO-1 were analysed by ELISA kits [using double-antibody sandwich enzyme-linked immunosorbent assay kits (QAYEE-BIO)]. Lipid profile was analyzed enzymatically, total cholesterol was analyzed by cholinesterase method using 4-aminoantipyrine and amount of quinoneimine dye formed was determined by its absorption at 510 nm and LDL-C was calculated by Friedwald formula [2]. SPSS ver.18 was applied for various statistical analysis and student’s t-test and regression analysis was carried out. Level of significance was 0.05. |
| Results |
| Clinical characteristics of subjects of both the groups are given in Table 1. |
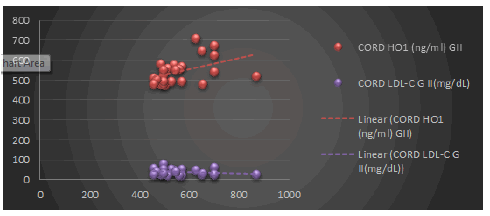
| There was significant rise in serum heme oxygenase 1 levels in preeclamptic women as compared to normotensive pregnant women (p<0.001, Figure 1). In the present study, cord blood heme oxygenase-1 levels in preeclamptic women were significantly higher than those of normotensive women (p<0.001, Figure 1). In women with preeclampsia, serum levels of low-density lipoproteins (LDL) are higher than those in normal pregnant women. LDL levels were positively correlated with HO 1 in preeclamptic women (r=0.236, p>0.05, Figure 2) and negatively correlated in normotensive women (r=-0.111, p>0.05). |
| Thus, there was inversion of correlation between LDL and HO-I levels from negative (in normotensive pregnant) to positive side (in preeclamptics), indicating the possible induction of HO 1. |
| Discussion |
| Foetus needs a considerable amount of cholesterol for development of tissues and organs, there should be no surplus cholesterol [2]. Thus, atherogenic milieu occurring during pregnancy persists into adulthood and fetal growth retardation is strongly associated with adult atherosclerosis [2]. After birth, human lipid transport system is transformed from one containing relatively low VLDL and LDL levels to adult system with a relatively high LDL level which continues to increase with age [2]. Together with lipid oxidation products, LDL may therefore be a vehicle for internalization of iron into the cells, a situation that could contribute to the cytotoxicity of hemin-oxidized lipoproteins [2,3]. |
| In women with preeclampsia, serum levels of triglycerides, lowdensity lipoproteins (LDL) are higher than those in normal pregnant women [4]. In addition, accumulating reports suggest that serum oxidized LDL (ox LDL) are higher in preeclampsia [5,6]. Ox LDL is essential in the genesis and progression of atherosclerosis [7]. It can lead to endothelial dysfunction, a key feature of preeclampsia [8], by binding to its scavenger receptors including lectin-like oxidized lowdensity lipoprotein receptor- 1 (LOX-1), CD36 and scavenger receptor A (SRISSN A) [3]. Placenta is the organ with the highest LOX-1 expression, even in a healthy state, suggesting that LOX-1 is crucial for maintaining pregnancy [9]. Recently several studies have highlighted the importance of heme oxygenase-1 (HO-1) in pregnancy [1,10-12]. |
| In the present study, maternal LDL levels were positively correlated with HO-1 in preeclamptic women (r=0.236, p>0.05) and negatively correlated in normotensive women (r=-0.111, p>0.05), but not statistically significant. And cord blood LDL levels were negatively correlated with HO-1 in preeclamptic women (r=0.204, p>0.05) and positively correlated in normotensive women (r=0.142, p>0.05), but it was not statistically significant. |
| It is well recognized that induction of heme oxygenase (HO) is a generalized response to oxidative stress. Induction of HO-1 is the protective effect against oxidative injury since HO metabolic activity leads to the formation of bile pigments that behave as antioxidants [1,12]. Also, HO-1 induction may be related to the decrease in heme iron and hemoproteins and the increased expression of iron responsive genes such as ferritin and aconitase [1]. The role of other metabolites of HO, namely, carbon monoxide (CO), have not been as well understood in the context of antioxidant defense, although recent reports indicate increased CO in inflammatory lung diseases such as asthma [12-15]. |
| Heme acts as a catalyst for oxidation of LDL. As a defense against such toxicity, normal cells upregulate heme oxygenase-1 (HO-1) and ferritin [1,14]. Free hemoglobin (Hb) in plasma, when oxidized, can provide heme to endothelium, which greatly enhances cellular susceptibility to oxidant-mediated cell injury. Oxidation of free Hb in plasma could threaten vascular endothelial cell integrity via oxidative modification of LDL. Oxidized LDL might also induce cytoprotectants such as HO-1 and ferritin [10,16,17]. HO-1 expression may protect against LDL oxidation or decrease its susceptibility to oxidation as it alters HDL protective qualities [14]. The findings of high serum heme oxygenase-1 levels in maternal and cord blood in preeclampsia in the present study supports the role of oxidative stress and excessive inflammatory response in the pathogenesis of preeclampsia. |
| Oxidized low density lipoproteins (LDL), hypoxia and proinflammatory cytokines induce HO-1 expression. An understanding of mechanisms by which HO-1 prevents various oxidative stresses may well be important for the treatment of a variety of pathophysiological conditions, including atherogenesis. The findings of high serum heme oxygenase-1 levels in maternal and cord blood in preeclampsia suggest that heme derived from free Hb in plasma may threaten vascular endothelial cell integrity via oxidative modification of LDL. This lipoprotein, in turn, induces the cytoprotectants heme oxygenase [10,12,14,15]. |
| Atherogenic milieu occurring during pregnancy persists into adulthood and fetal growth retardation is strongly associated with adult atherosclerosis. The children born of women with preeclamptic deserve a closer clinical follow-up later in life. |
References
- Schulz S, Zhao H, Wong RJ, Stevenson DK (2012) Heme Oxygenase Biology (During the Perinatal Period): Prenatal Considerations. NeoReviews 12: 13.
- Gozlan O, Gross D, Gruener N (1994) Lipoprotein levels in newborns and adolescents. ClinBiochem 27: 305-306.
- Yuan XM, Brunk UT, Olsson AG (1995) Effects of iron- and hemoglobin-loaded human monocyte-derived macrophages on oxidation and uptake of LDL. ArteriosclerThrombVascBiol 15: 1345-1351.
- Potter JM, Nestel PJ (1979) The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J ObstetGynecol 133: 165-170.
- Branch DW, Mitchell MD, Miller E, Palinski W, Witztum JL (1994) Pre-eclampsia and serum antibodies to oxidised low-density lipoprotein. Lancet 343: 645-646.
- Uzun H, Benian A, Madazli R, Topçuoğlu MA, Aydin S, et al. (2005) Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. GynecolObstet Invest 60: 195-200.
- Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL (2011) Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci 342: 135-1,
- Roberts JM, Redman CW (1993) Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 341: 1447-1451.
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, et al. (1997) An endothelial receptor for oxidized low-density lipoprotein. Nature 386: 73-77.
- Centlow M, Carninci P, Nemeth K, Mezey E, Brownstein M, et al (2007) Placental expression profiling in preeclampsia: local overproduction of hemoglobin may drive pathological changes. FertilSteril 90: 1834-1843.
- Ahmed A, Ramma W (2015) Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm? Br J Pharmacol 172: 1574-1586.
- Asatryan L, Ziouzenkova O, Duncan R, Sevanian A (2003) Heme and lipid peroxides in hemoglobin-modified low-density lipoprotein mediate cell survival and adaptation to oxidative stress. Blood 102: 1732-1739.
- Carmena R, Duriez P, Fruchart JC (2004) Atherogenic lipoprotein particles in atherosclerosis. Circulation 109: 2-7.
- Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3: 119.
- Miller YI, Shaklai N (1999) Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. BiochimBiophysActa 1454: 153-164.
- George EM, Warrington JP, Spradley FT, Palei AC, Granger JP (2014) The hemeoxygenases: important regulators of pregnancy and preeclampsia. Am J PhysiolRegulIntegr Comp Physiol 307: R69-777.
- Appleton SD, Marks GS, Nakatsu K, Brien JF, Smith GN, et al. (2002) Heme oxygenase activity in placenta: direct dependence on oxygen availability. Am J Physiol Heart CircPhysiol 282: 2055-2059.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 9904
- [From(publication date):
February-2016 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 9020
- PDF downloads : 884
