Copper Nanoparticles as Antibacterial Agents
Received: 01-Dec-2017 / Accepted Date: 04-Jan-2018 / Published Date: 07-Jan-2018 DOI: 10.4172/2329-9053.1000140
Abstract
Although antibiotics can treat most bacterial infections, development of microbial resistance restricts the advantages of the antibacterial agents in controlling infectious diseases. This is a major challenge that poses a serious threat prompting the search for alternative strategies to treat bacterial infections. Nanotechnology as an emerging field has been extensively used to overcome microbial resistance due to specific properties of nanoparticles such as increased drug uptake and high surface area to volume ratio. The metallic particles in nanoscale have demonstrated antibacterial activity against various bacterial species, including Gram-positive and Gramnegative bacteria, and fungi. Recently, copper nanoparticles have been widely investigated for use in fighting microbial infections. This article tries to briefly summarize the current studies related to the antibacterial properties of the copper nanoparticles. The reviewed papers reveal that the copper nanoparticles possess potent antimicrobial activities and can be used for controlling and treating different infectious diseases in the future.
Keywords: Metallic nanoparticles; Copper; Antibacterial activity; Infection; Microbial resistance
Introduction
Antibacterial agents are compounds that kill bacteria or slow down their growth without being generally toxic to the surrounding tissue. The word ‘‘antibiotics’’ was first introduced in 1941 by Selman Waksman to refer to antimicrobial agents produced by many microorganisms [1]. The antibacterial agents have been used in many fields, such as, textile industry, water disinfection, food packaging, and medicine [2]. Presently, the over-use of antibiotics has led to an increased occurrence of antibiotic resistant genes in various bacterial species. Many of the recognized antimicrobials have shown resistance by one species of microorganism or another [3]. Hence, a great deal of research has been performed to deal with this problem. "Nanoparticles" (NPs) has been defined by Encyclopedia of Pharmaceutical Technology as solid colloidal particles ranging in size from one to 1000 nm (one micron) [4]. Indeed, NPs exhibit a range of potentially useful applications for pharmaceutical purposes: They have the ability of targeting the drug into the site of action and consequently reducing the side effects, and increasing the drug uptake. Moreover, NPs are capable of interacting with mucosal surfaces and escaping endolysosomal compartments [5,6]. Kinetic profiles of drug release can be also modified by NPs [7]. Based on the Ostwald–Freundlich equation, the saturation solubility enhances with decreasing particle size below approximately one μm. Thus, NPs demonstrate enhanced saturation solubility and increased surface area which cause a further increase in dissolution rate according to the Noyes–Whitney equation. In contrast, the solubility of particles with normal size (above one micron) is a compound-specific constant and depends only on the temperature and the solvent [8]. The emergence of nanotechnology in the last decades has elicited immense interest in evaluating the antimicrobial activity of nano-scale metals. The use of metallic NPs results in decreased concentration as well as increased antibacterial and antifungal activity [9]. However, despite the unique set of properties of metallic NPs, there are environmental and human safety concerns regarding the release of metallic NPs, for example, release of silver causes environmental pollution [3]. The antimicrobial effects of different metallic NPs such as Alumina [10-12], silver [13,14], iron [15-19], gold [20-22], magnesium [23-25], titanium [26,27], and zinc oxide [28,29] have been widely investigated. In spite of the tremendous efforts undertaken regarding the use of metallic NPs with antibacterial effects, at present, we are far away from ideal metallic NPs with efficient activity. As a novelty discussion paper, this review will summarize the major findings for copper particles in nano-scale as antimicrobial agents.
The Privileges of NPs as Antibacterial Agents
The nano-sized particles received a great deal of attention due to their potential in biomedical and pharmaceutical applications. The particles in nano-scale are able to easily interact with bacterial membranes [30]. Based on the studies using various microscopy approaches including atomic force microscopy, transmission electron microscopy and laser confocal microscopy, after using nanoparticles, the integrity of the bacterial cell membranes changed noticeably causing bacterial cell death [31]. The advance in the preparation of metallic NPs has led to the development of a new class of antimicrobial materials. Highly ionic metallic NPs are of particular interest due to their extremely high surface areas and numerous reactive surface sites with unusual crystal morphologies [32].
Antibacterial Properties of Copper
Copper is a readily available metal and one of the essential trace elements in most living organisms. Copper particles in nano-scale have many applications in industry, including their use in gas sensors, high temperature superconductors, solar cells and wood preservatives [33]. This metal has been also used as potential antimicrobial agent since ancient times. Coppercontaining compounds such as CuSO4 and Cu(OH)2 are used as the traditional inorganic antibacterial agents [34]. Also, aqueous copper solutions, complex copper species or coppercontaining polymers are used as antifungal compounds [34]. Moreover, the control of legionella in hospital water distribution systems via the copper and silver ionization method is one of the most common applications of this metal in the modern healthcare setting [35]. Copper ions have demonstrated antimicrobial activity against a wide range of microorganisms, such as Staphylococcus aureus, Salmonella enteric, Campylobacter jejuni, Escherichia coli, and Listeria monocytogenes [36]. Currently, copper has been registered as the first and only metal with antimicrobial properties by the American Environmental Protection Agency (EPA) [37]. This material kills 99.9% of most pathogens within 2 h contact [38]. Also, in some cases, this metal possesses properties better relative to the other expensive metals with antimicrobial activity, such as, silver and gold [3]. For instance, the Cu NPs indicated higher antibacterial effect relative to the silver NPs against E. coli and Bacillus subtilis (B. subtilis ) [39,40]. The copper surfaces can be used to kill bacteria, yeasts, and viruses which are known as “contact killing” (contact-mediated killing). Contact killing by copper was reported to occur at a rate no less than seven to eight logs per hour, and in general, subsequent to the extended incubation. No live microorganisms were recovered from the copper surfaces. This leads to the idea of using copper as a self-sanitizing material [41].
Copper Bacterial Resistance
As mentioned in previous sections, copper has been utilized as an antibacterial agent in medicine. The most common copper-resistant bacteria were isolated from animals, plants and humans-associated bacteria. Different mechanisms are involved in copper homeostasis in various bacteria. For example, in E. coli , two chromosomally encoded systems, including cue and cus are responsible for copper resistance. The CueP, a periplasmic protein, is an extra component of the cue system and possesses a critical role in copper resistance. The pco (plasmid-borne copper resistance) system is also responsible for survival of some bacteria, such as E. coli in copper-rich environments. Other Gram-negative bacteria, such as Yersinia pestis, Yersinia pseudotuberculosis, Yersinia enterocolitica , Citrobacterkoseri, and Erwiniacarotovora possess CueP-like proteins [42].
The Toxicity Mechanisms of Cu NPs against Bacteria
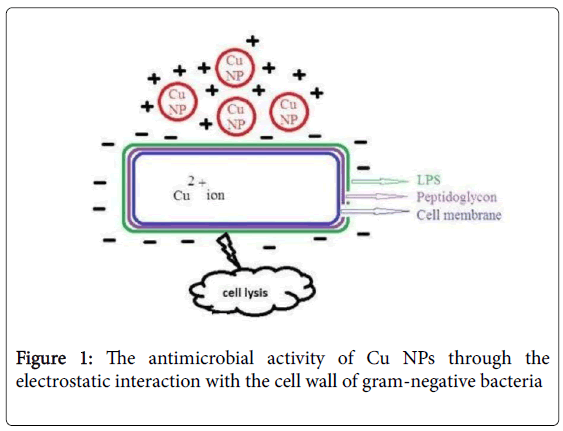
One of the most known NPs' toxicity mechanisms is the interaction between the bacterial cell membrane and NPs, which leads to the disruption of the bacterial membrane integrity and finally results in the death of the microorganism. It has been shown that several factors, including temperature, pH, concentration of bacteria and NPs, as well as aeration can promote the toxicity mechanism of Cu NPs [2,43]. Cu particles in nano-scale have been shown to have antibacterial effect on the bacterial cell functions in multiple ways, including adhesion to Gram negative bacterial cell wall due to electrostatic interaction (Figure 1), having effect on protein structure in the cell membrane, denaturation of the intracellular proteins, and interaction with phosphorus- and sulfurcontaining compounds like DNA [34]. Also, in one comprehensive study, mechanisms of antibacterial activity of Cu NPs were investigated using E. coli as a biological tool [44]. The results showed that the treatment of E. coli cells by Cu-NPs at the minimum bactericidal concentration (MBC) resulted in 2.5 times overproduction of the cellular reactive oxygen species (ROSs). Also, the NP-mediated increase in ROS level led to noticeable lipid peroxidation, protein oxidation and DNA degradation, finally killing the cells.
Synthesis of Cu-based NPs
Cu-based NPs can be synthesized using five major techniques: chemical treatment, thermal treatment, electrochemical synthesis, photochemical methods, and sonochemical techniques. The “chemical treatment” has been used as the most popular method among them and some of the more modern techniques have utilized this method for synthesis of Cu-based NPs [45]. Lately, green synthesis of environmental friendly NPs without toxic waste products during the preparation has been introduced. In this kind of preparation technique, safe biotechnological tools are used as an alternative to conventional physical and chemical synthesis and named green nanobiotechnology. In this method, NPs are prepared using biological routes such as bacteria, fungi, plants and enzymes or their byproducts, such as proteins [46].
The Copper Particles in Nano-scale as Antibacterial Agents
In 2008, Ruparelia et al. investigated antimicrobial properties of silver and Cu NPs on E. coli , B. subtilis and Staphylococcus aureus (S. aureus ) [39]. Results of minimum inhibitory concentrations (MICs), minimum bactericidal concentrations (MBCs) and disk diffusion test revealed that the Cu NPs were more efficient compared to the silver particles against B. subtilis which is suggested to be due to more affinity of the Cu NPs to surface amines and carboxyl groups of B. subtilis . In contrast, silver NPs demonstrated more antimicrobial effect against E. coli and S. aureus relative to Cu NPs.
Another application of copper oxide (CuO) NPs for antimicrobial applications was introduced by Ren et al. [47]. The metal oxide NPs were prepared using thermal plasma (Tesima TM) technology, which allows the continuous gas phase production of bulk nano-powders. The prepared CuO NPs in suspension were active against a range of bacterial pathogens, including S aureus , meticillin-resistant S. aureus , Staphylococcus epidermidis, E. coli, and Pseudomonas aeruginosa (P. aeruginosa ) with MBCs ranging from 100 mg/ml to 5000 mg/ml.
The feasibility of the use of Cu NPs as antibacterial agents against E. coli was examined by Raffi and coworkers in 2010 [34]. The antibacterial activity was assessed in liquid as well as solid growth media. On solid media, the antibacterial characterization of the prepared NPs was measured by colony forming unit (CFU). In liquid media, the antibacterial behaviour of Cu NPs was studied by determination of the optical density (OD) of the different concentrations of Cu NPs at a fixed wavelength. The growth inhibition results obtained from both studies were in good agreement. Moreover, the scanning electron microscopy (SEM) of bacterial cells treated with Cu NPs demonstrated the formation of cavities and pits in the cell walls and changes in cell morphology from normal rod-shaped to irregular appearance. The results also exhibited that the antibacterial efficacy of Cu NPs depended on the concentration of the NPs; low concentrations just led to a delay in the lag phase, showing the micronutritional role of copper for bacteria. In contrast, at higher concentrations, they showed bacterial growth inhibition.
In the same year, another research group assessed the toxicity of aggregated zero valent Cu NPs (ZVCN) against E. coli using a centroid mixture design of experiment [48]. Five environmental parameters including, temperature, pH, aeration rate, NP concentration, and bacteria concentration which were presumed to have major effects on the toxicity of the NPs against the bacteria, were assessed. According to their study, the interactive effects of the tested parameters as well as the primary effects of them are efficient on the toxicity of Cu NPs.
On the whole, ZVCN will have highest toxicity on nanoparticles under acidic conditions and higher temperature, high aeration and high concentration of NPs and bacteria. When any one of the independent variable is changed, the toxicity of NPs changes significantly.
In an effort to improve the antimicrobial properties of Cu NPs, Mohan et al utilized carbon nanotubes [49]. The prepared Cu NPs were grafted on the surface of multiwall carbon nanotubes (MWCNT). According to their research, carbon nanotubes increased the surface area of Cu NPs, and therefore the number of colonies of E. coli reduced in the Cu-MWCNT system compared to the pure Cu NPs and MWCNT. The antimicrobial efficiency (% kill) of Cu-MWCNT were found to be 75% ± 0.8 while pure Cu NPs showed a low-percent kill against E. coli (52% ± 1.8). The possible mechanism of the bactericidal effect of CuMWCNT is the release of Cu ions from Cu-MWCNT and their entrance to the bacterial cells and the subsequent disrupts of biochemical processes. Taken together, Cu-MWCNT can be used as a biocidal composite in biomedical devices and antibacterial system.
In another research, Theivasanthi and Alagar indicated that Cu NPs developed using electrolysis technique possessed more antibacterial effect against E. coli bacteria relative to those prepared through a chemical reduction process [50]. Use of electrical power in Cu NPs preparation led to an enhancement in the NPs’ antibacterial effects. As a whole, the authors proposed the feasibility of use of this material in water purification, antibacterial packaging as well as air filtration.
A novel plastic antimicrobial agent including polypropylene with embedded Cu metal or CuO NPs was examined by Delgado et al [51]. Based on their study, the ability of composites to kill bacteria depended on the type of Cu NPs. It was shown that antimicrobial effect of CuO was more than that of the Cu metallic NPs in killing E. coli since for the CuO NPs the formation of oxide layer was not required which led to high ion release rates. Besides, in terms of CuO NPs, the metal was already in the oxidation condition which resulted in the particle dissolution, while for the Cu metal the previous formation of an oxide layer was necessary.
In 2012, Chatterjee and coworkers introduced a simple robust method for synthesis of Cu NPs via reduction of CuCl2 in the presence of gelatin as a stabilizer [52]. Treatment with the NPs made E. coli cells filamentous with average filament size varied from 7 to 20 μm relative to the normal cell size of nearly 2.5 μm. The NPs were highly effective against E. coli at a much low concentration. The antibacterial effects of produced NPs were also observed in an E. coli strain resistant to multiple antibiotics as well as Gram-positive B. subtilis and S. aureus .
Aqueous solution of starch capped copper nanoparticles with bactericidal effect against both gram negative and gram positive bacteria at nano-molar concentrations were produced using starch as green capping agent [53]. Based on the in vitro studies on of the 3T3L1 cells, the capped NPs exhibited cytotoxicity at much higher concentration relative to Cu ions. Based on the results, the introduced starch capped water soluble Cu NPs are promising candidates for different applications for instance in photothermal therapy or cellular imaging.
In another interesting investigation, the antibacterial effect of CuO NP was studied on Legionella pneumophila [54]. According to a whole-genome microarray, CuO NPs significantly affected the expression of genes involved in metabolism, transcription, translation, DNA replication and repair, virulence, and unknown/hypothetical proteins.
Another research group showed that the antibacterial effect of CuO NPs was dependent on the particle size and a significant increase in antibacterial activities against both Gram-positive and -negative bacterial strains was achieved using the highly stable minimum-sized monodispersed CuO NPs [55].
Thekkae Padil et al. produced highly stable CuO NPs using gum karaya, as a naturally occurring polysaccharide component in plants via green technology [56]. The CuO NPs with smaller particle sizes demonstrated higher antibacterial activities. The authors noted that CuO NPs produced via a simple, mild, and environmentally friendly method may possess promising applications for instance in wound dressing, bed lining, active cotton bandages, and medical and food industries.
Das and coworkers fabricated CuO NPs by thermal decomposition methods and investigated their antioxidant and antibacterial effects [57]. Their produced NPs demonstrated free radical scavenging activity up to 85% in 1 h which is relatively higher compared to other metal oxide NPs. Furthermore, the CuO NPs exhibited proficient antibacterial activity against E. coli and P. aeruginosa . Bacterial growth significantly decreased with increasing NPs’ concentration.
Usman and coworkers synthesized pure Cu NPs using chitosan polymer as a stabilizer [3]. Their findings showed that the chitosanstabilized NPs were effective against Gram-negative microorganisms, including Salmonella choleraesuis and P. aeruginosa , as well as Grampositive bacteria such as methicillin-resistant S. aureus and B. subtilis , and also yeast species such as Candida albicans . Moreover, the prepared NPs indicated more antimicrobial effects against Gramnegative microorganisms (such as P. aeruginosa ) compared to Grampositive bacteria. Taken together, the researchers introduced a simple and cost-effective approach for synthesis of Cu NPs which has future potential for pharmaceutical and biomedical applications.
Another interesting preparation of Cu NPs was presented by Subhankari and Nayak who presented a novel biological technique using ginger (Zingiber officinale) extract [58]. Cu NPs prepared via green synthesis approach was found to be more effective against E. coli relative to copper sulphate solution and pure ginger extract. The researchers noted that this green method involves cheap and non-toxic materials and could be useful in water purification, air quality management, and antibacterial packaging.
In an attempt to produce highly stable CuO NPs via a green chemistry approach, aqueous extract of Acalypha indica leaf was used [59]. The resultant particles were found to be effective against E. coli , Pseudomonas fluorescens and Candida albicans . Moreover, based on the MTT assay, they possessed cytotoxicity activity against MCF-7 breast cancer cell lines.
In a research carried out by Agarwala et al., antibiofilm activity of CuO and iron oxide NPs was assessed against multidrug resistant biofilm forming uropathogens [60]. CuO NPs was found to be more toxic compared to iron oxide NPs and also possess dose dependent antibiofilm properties.
Giannousi et al produced Cu, Cu2O and Cu/Cu2O NPs using hydrothermal procedure as a costeffective and eco-friendly method [61]. The Cu-based NPs led to pDNA degradation in a dosedependent manner and extensive degradation of ds CT-DNA. Also, Cu2O NPs exhibited increased antibacterial effect against the Gram-positive strains. Hence, the possible reaction pathway was investigated. The results proved ROS production and lipid peroxidation.
The use of green nanotechnology for synthesis of Cu NPs has been also investigated by Parikh and coworkers [30]. For biosynthesis, Datura Meta leaf extract was used with the ability of metal ion reduction in NPs. The proposed method has some advantages: It is efficient, rapid, easy, inexpensive, and ecofriendly. The antibacterial behavior of Cu NPs against E. coli , Bacillus megaterium , and B. subtilis was found to be more than that of the extract.
Other nanostructured Cu was developed and investigated by Tomasz and coworkers in 2015 [62]. The copper NPs showed a high antibacterial effect against Gram positive bacteria such as clinical methicillin resistant S. aureus strains. The antibacterial effects of Cu NPs were found to be even higher than that of Ag NPs. Also, the synthesized NPs demonstrated antifungal activity against candida species. Hence, the prepared copper NPs can be used as an alternative for prevention of biofilm formation as well as reduction in bacterial or fungal adhesion at lower cost compared to use of silver.
The anti-biofilm activity of Cu NPs against P. aeruginosa was studied by Lewis Oscar et al. [63]. The authors reported that Cu NP treatments at 100 ng/ml led to a 94% reduction in biofilm formation and stated that their proposed NPs could be used as coating agents for controlling biofilm formation on surgical devices as well as medical implants.
In another study, the cytotoxicity of the synthesized CuO NPs in colon cancer cells was explored [64]. The researchers found that CuO NPs inhibited the cell proliferation in HT-29 human colon cancer cells via downregulation of Bcl-2 and Bcl-xL as the apoptosis regulatory proteins.
Taken together, in recent years researchers have paid much attention to Cu NPs due to their antibacterial activity against different microorganisms, (summarized in Table 1). This highlights the potential of the copper particles in nano-scale as effective antibacterial agents in biomedical and industrial applications.
| Targeted microorganism | Targeted microorganism | Method | References |
|---|---|---|---|
| Bacillus subtilis | 9 | Wet chemical synthesis | [39] |
| meticillin-resistant Staphylococcus aureus (MRSA) | 20-95 | Thermal plasma technology | [47] |
| Escherichia coli | 12 | Inert gas condensation method (IGC) | [34] |
| Escherichia coli | 25 | N/A | [48] |
| Escherichia coli | 22 | Wet chemical synthesis | [49] |
| Escherichia coli; Bacillus megaterium | 24 | Electrolysis method | [50] |
| Escherichia coli | 10 | N/A | [51] |
| Escherichia coli, Bacillus subtilis, Staphylococcus aureus | 50-60 | Simple reduction method | [52] |
| Staphylococcus aureus, Escherichia coli (DH5) and Salmonella typhi | 10 | Microwave irradiation | [53] |
| Legionella pneumophila | 40-80 | Heating the Cu2O NPs | [54] |
| Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus | 20 ± 1.24-28.9 ± 1.22 | Gel combustion route | [55] |
| Escherichia coli, Staphylococcus aureus | 4.8-7.8 | Colloid-thermal synthesis process | [56] |
| Eschericia coli, Pseudomonas aeruginosa | 15-30 | Thermal decomposition metho | [57] |
| Salmonella choleraesuis, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Candida albicans | 2-350 | Through chemical means in chitosan polymer medium | [3] |
| Escherichia coli | 100-200 | Green synthesis method | [58] |
| Escherichia coli, Pseudomonas fluorescens and Candida albicans | 26-30 | Green synthesis method | [59] |
| methicillin resistant Staphylococcus aureus, methicillin resistant Staphylococcus epidermidis, vancomycin resistant Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas sps., Proteus mirabilis | 25-30 | Commercial CuO NPs were used | [60] |
| Gram-positive: (Bacillus subtilis, Bacillus cereus, Staphylococcus aureus) and Gram-negative: (Xanthomonas campestris, Escherichia coli) | 10-44 | Hydrothermal synthesis method | [61] |
| Staphylococcus aureus, Candida species, Staphylococcus epidermidis | 50 | Reduction of copper salt with hydrazine in the aqueous SDS solution | [62] |
| Pseudomonas aeruginosa | 55 | One pot method | [63] |
| Gram-positive: (Staphylococcus aureus, Enterococcus faecalis, Bacillus subtilis) and Gram-negative: (Pseudomonas aeruginosa, Shigella sonnei, Escherichia coli) | 20 | Based thermal decomposition | [64] |
Table 1: The Cu NPs as antibacterial agents.
The Toxicity of Copper Nanoparticles
In spite of commercial use of NPs, their release into the environment (e.g., soil and water) is of the most important problems which affect public health, beneficial bacteria and microbial communities [2]. The information about the hazardous effects of CuO is limited. It has been shown that the toxicities of bulk and nano-sized CuO were mostly affected by soluble Cu ions. The accumulation of copper in human body leads to production of the harmful radicals such as hydroxyl radical [65].
Conclusion
There is a growing body of scientific evidence which confirms the antibacterial properties of metallic NPs against various bacterial species, including Gram-positive and Gram-negative bacteria as well as fungi. Based on the literature, in some cases Cu NPs showed higher antibacterial effect compared to the other metallic NPs. However, the in vitro investigations should be confirmed by in vivo assays using animal models prior to Cu NPs’ use in the clinical setting. Hopefully, in the not too distant future, we could probably witness using Cu NPs as effective antibacterial agents in biomedical and industrial applications to fight against pathogenic microorganisms.
Declaration of Interest
All authors declare that they have no conflict of interest.
References
- Clardy J, Fischbach MA, Currie CR (2009) The natural history of antibiotics. Curr Biol 19: 437-441.
- Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, et al. (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30: 499-511.
- Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, et al. (2013) Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomedicine 8: 4467-4479.
- Kreuter J (2007) Nanoparticles—a historical perspective. Int J Pharmaceut 331: 1-10.
- Hallaj-Nezhadi S, Valizadeh H, Baradaran B, Dobakhti F, Lotfipour F (2013) Preparation and characterization of gelatin nanoparticles containing pDNA encoding IL-12 and their expression in CT-26 carcinoma cells. Future Oncol 9: 1195-1206.
- Hallaj-Nezhadi S, Dass CR, Lotfipour F (2013) Intraperitoneal delivery of nanoparticles for cancer gene therapy. Future Oncol 9: 59-68.
- Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, et al. (2008) Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 11: 167-177.
- Hallaj-Nezhadi S, Hassan M (2015) Nanoliposome-based antibacterial drug delivery. Drug delivery 22: 581-589.
- Kon K, Rai M (2013) Metallic nanoparticles: mechanism of antibacterial action and influencing factors. J Comp Clin Path Res 2: 160-174.
- Buckley JJ, Gai PL, Lee AF, Olivi L, Wilson K (2008) Silver carbonate nanoparticles stabilised over alumina nanoneedles exhibiting potent antibacterial properties. Chem Commun 18:4013-4015.
- Chang Q, Yan L, Chen M, He H, Qu J (2007) Bactericidal mechanism of Ag/Al2O3 against Escherichia coli. Langmuir 23: 11197-11199.
- Geoprincy G, Gandhi N, Renganathan S (2012) Novel antibacterial effects of alumina nanoparticles on Bacillus cereus and Bacillus subtilis in comparison with antibiotics. Int J Pharm Pharm Sci 4: 544-548.
- Marambio-Jones C, Hoek EM (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12: 1531-1551.
- Sotiriou GA, Pratsinis SE (2010) Antibacterial activity of nanosilver ions and particles. Environ Sci Technol 44: 5649-5654.
- Tran N, Mir A, Mallik D, Sinha A, Nayar S, et al. (2010) Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. International J Nanomedicine 5: 277-283.
- Lee C, Kim JY, Lee WI, Nelson KL, Yoon J, et al. (2008) Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ Sci Technol 42: 4927-4933.
- Diao M, Yao M (2009) Use of zero-valent iron nanoparticles in inactivating microbes. Water Res 43: 5243-5151.
- Kim JY, Park HJ, Lee C, Nelson KL, Sedlak DL, et al. (2010) Inactivation of Escherichia coli by nanoparticulate zerovalent iron and ferrous ion. Appl Environ Microbiol 76:7668-7670.
- Ismail RA, Sulaiman GM, Abdulrahman SA, Marzoog TR (2015) Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mat Sci Eng C 53: 286-297.
- Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK (2011) Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol 9: 30.
- Zhao Y, Tian Y, Cui Y, Liu W, Ma W, et al. (2010) Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J Am Chem Soc 132: 12349-12356.
- Pissuwan D, Cortie CH, Valenzuela SM, Cortie MB (2010) Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol 28: 207-213.
- Jin T, He Y (2011) Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J Nanopart Res 13: 6877-6885.
- Lellouche J, Friedman A, Lellouche JP, Gedanken A, Banin E (2012) Improved antibacterial and antibiofilm activity of magnesium fluoride nanoparticles obtained by water-based ultrasound chemistry. Nanomedicine: Nanotechnology, Biology and Medicine 8: 702-711.
- Dong C, He G, Zheng W, Bian T, Li M, et al. (2014) Study on antibacterial mechanism of Mg(OH)2 nanoparticles. Mater Lett 134: 286-289.
- Besinis A, De Peralta T, Handy RD (2014) The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 8:1-6.
- Martinez-Gutierrez F, Olive PL, Banuelos A, Orrantia E, Nino N, et al. (2010) Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine 6: 681-688.
- Espitia PJ, Soares ND, dos Reis Coimbra JS, de Andrade NJ, et al. (2012) Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioproc Tech 5: 1447-1464.
- Liu Y, He L, Mustapha A, Li H, Hu ZQ, et al. (2009) Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7. J Appl Microbiol 107:1193-1201.
- Parikh P, Zala D, Makwana BA (2014) Biosynthesis of Copper Nanoparticles and Their Antimicrobial Activity. OALib 01: 1-15.
- Tiwari DK, Behari J, Sen P (2008) Application of Nanoparticles in Waste Water Treatment. World Appl Sci J 3: 417-433.
- Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18: 6679-6686.
- Salvadori MR, Ando RA, do Nascimento CA, Corrêa B (2014) Intracellular biosynthesis and removal of copper nanoparticles by dead biomass of yeast isolated from the wastewater of a mine in the Brazilian Amazonia. PLoS One 9: e87968.
- Raffi M, Mehrwan S, Bhatti TM, Akhter JI, Hameed A, et al. (2010) Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann Microbiol 60: 75-80.
- O'Gorman J, Humphreys H (2012) Application of copper to prevent and control infection. Where are we now? J Hosp Infect 81: 217-223.
- Gyawali R, Ibrahim SA, Abu Hasfa SH, Smqadri SQ, Haik Y (2011) Antimicrobial activity of copper alone and in combination with lactic acid against Escherichia coli O157: H7 in laboratory medium and on the surface of lettuce and tomatoes. J Pathog 2011: 650968.
- Prado JV, Vidal AR, Duran TC (2012) Application of copper bactericidal properties in medical practice. Rev Med Chil 140: 1325-1332.
- Hans M, Erbe A, Mathews S, Chen Y, Solioz M, et al. (2013) Role of copper oxides in contact killing of bacteria. Langmuir 29: 16160-16166.
- Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4: 707-716.
- Yoon KY, Hoon Byeon J, Park JH, Hwang J (2007) Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373: 572-575.
- Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77: 1541-1547.
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, et al. (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87: 1181-1200.
- Tiwari DK, Behari J, Sen P (2008) Application of Nanoparticles in Waste Water Treatment. World Appl Sci J 3: 417-433.
- Chatterjee AK, Chakraborty R, Basu T (2014) Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25: 135101.
- Gawande MB, Goswami A, Felpin FX, Asefa T, Huang X, et al. (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116: 3722-3811.
- Singh J, Kaur G, Rawat M (2016) A brief review on synthesis and characterization of copper oxide nanoparticles and its applications. J Bioelectron Nanotechnol 1: 9.
- Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP (2009) Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents 33: 587-590.
- Rispoli F, Angelov A, Badia D, Kumar A, Seal S, et al. (2010) Understanding the toxicity of aggregated zero valent copper nanoparticles against Escherichia coli. J Hazard Mater 180: 212-216.
- Mohan R, Shanmugharaj AM, Sung Hun R (2011) An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. J Biomed Mater Res B Appl Biomater 96: 119-126.
- Theivasanthi T, Alagar M (2011) Studies of Copper Nanoparticles Effects on Micro-organisms. Ann Biological Res 2: 368-373.
- Delgado K, Quijada R, Palma R, Palza H (2011) Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett Appl mMicrobiol 53: 50-54.
- Chatterjee AK, Sarkar RK, Chattopadhyay AP, Aich P, Chakraborty R, et al. (2012) A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology 23: 085103.
- Valodkar M, Rathore PS, Jadeja RN, Thounaojam M, Devkar RV, et al. (2012) Cytotoxicity evaluation and antimicrobial studies of starch capped water soluble copper nanoparticles. J Hazard Mater 201: 244-249.
- Lu J, Struewing I, Buse HY, Kou J, Shuman HA, Faucher SP, et al. (2013) Legionella pneumophila transcriptional response following exposure to CuO nanoparticles. Appl Environ Microbiol 79: 2713-2720.
- Azam A, Ahmed AS, Oves M, Khan M, Memic A (2012) Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. Int J Nanomedicine 7: 3527.
- Padil VVT, Cernik M (2013) Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomedicine 8: 889-898.
- Das D, Nath BC, Phukon P, Dolui SK (2013) Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf B Biointerfaces 101: 430-433.
- Subhankari I, Nayak P (2013) Antimicrobial Activity of Copper Nanoparticles Synthesised by Ginger (Zingiber officinale) Extract. World J Nano Sci Technol 2: 10-13.
- Sivaraj R, Rahman PK, Rajiv P, Narendhran S, Venckatesh R (2014) Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc129: 255-258.
- Agarwala M, Choudhury B, Yadav R (2014) Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J Microbiol 54: 365-368.
- Giannousi K, Lafazanis K, Arvanitidis J, Pantazaki A, Dendrinou-Samara C (2014) Hydrothermal synthesis of copper based nanoparticles: antimicrobial screening and interaction with DNA. J Inorg Biochem 133: 24-32.
- Kruk T, Szczepanowicz K, Stefańska J, Socha RP, Warszyński P (2015) Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf B Biointerfaces 128: 17-22.
- LewisOscar F, MubarakAli D, Nithya C, Priyanka R, Gopinath V, et al. (2015) One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling 31: 379-391.
- Khan S, Ansari AA, Khan AA, Abdulla M, Al-Obaid O, et al. (2017) In vitro evaluation of cytotoxicity, possible alteration of apoptotic regulatory proteins, and antibacterial activity of synthesized copper oxide nanoparticles. Colloids Surf B Biointerfaces 153: 320-326.
- Sani Usman M (2013) Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomedicine 8: 4467-4479.
Citation: Mahmoodi S, Elmi A, Hallaj-Nezhadi S (2018) Copper Nanoparticles as Antibacterial Agents. J Mol Pharm Org Process Res 6: 140. DOI: 10.4172/2329-9053.1000140
Copyright: © 2018 Mahmoodi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 23390
- [From(publication date): 0-2018 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 21000
- PDF downloads: 2390