Research Article Open Access
Control of Corrosion and Microbial Corrosion of Steel Pipelines in Salty Environment by Polyacrylamide
El-Shamy AM1*, Zohdy KM2 and El-Dahan HA1
1Physical Chemistry Department, Electrochemistry and Corrosion Laboratory, National Research Centre, El-Bohouth St. 33, Dokki, PO 12622, Giza, Egypt
2Higher Technological Institute, 10th of Ramadan City, Egypt
- *Corresponding Author:
- El-Shamy AM
Physical Chemistry Department Electrochemistry
and Corrosion Laboratory National Research Centre
El- Bohouth St. 33 Dokki, PO 12622, Giza, Egypt
E-mail: elshamy10@yahoo.com
Received date: April 07, 2016; Accepted date: April 27, 2016; Published date: April 29, 2016
Citation: El-Shamy AM, Zohdy KM, El-Dahan HA (2016) Control of Corrosion and Microbial Corrosion of Steel Pipelines in Salty Environment by Polyacrylamide. Ind Chem 2:120. doi: 10.4172/2469-9764.1000120
Copyright: © 2016 El-Shamy AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
The polyacrylamide was investigated for controlling of corrosion and microbial corrosion of mild steel in salty media. Both the corrosion inhibition efficiency and biocidal activity are measured at different concentrations of polyacrylamide. The electrochemical measurements are used to monitor the corrosion results and the serial dilution method is used to detect the biocidal activity. The results showed that the polyacrylamide is improved the biocidal activity corrosion resistance for mild steel with increasing the concentration. The biocidal activity was subjected to the planktonic and sessile bacteria and the obtained results proved that the killing effect of polyacrylamide is shows higher efficiency in planketoinc more than the sessile bacteria. The corrosion data proved that, the inhibitor shows acceptable effect against corrosion in chloride solution and the effect is also depends on the inhibitor concentration. The best result in both corrosion and microbial corrosion is obtained at concentration of 15 ppm.
Keywords
Biocidal activity; Mild steel; Corrosion; Electrochemical techniques; Polyacrylamide
Introduction
One of the main factors of metal deterioration is microbial corrosion and it is well identified in the most recent papers as the main reason of pipeline failure in sulfide and chloride media [1-5]. There are many types of microorganisms are involved in the microbial corrosion and responsible for corrosion failure of mild steel, stainless steel and as well as copper and its alloys. Sulfate reducing bacteria id considered one of the well-known anaerobic bacteria that have the ability to attack the metals and its alloys [5-10]. The attack of sulfate reducing bacteria (SRB) is very easy detectable by forming iron sulfide FeS with black film. The microbial corrosion starts with the formation of biofilm from the aerobic bacteria and then the underneath of biofilm the anaerobic bacteria starts to grow and in the same time the localized corrosion is start its work [11]. The galvanic corrosion is also starts due to the formation of oxygen concentration cell because the concentration of oxygen underneath biofilm is lower than that in the external part. There are many other kinds of corrosion are starts to work as a result of microbial corrosion as crevice corrosion, and selective de-alloying. The microbial corrosion not only occurs under anaerobic condition but also at aerobic conditions. The presence of aerobic bacteria initiates the general corrosion through the formation of metal oxide by the presence of metal oxidizing bacteria. The hydrogen sulfide is produced as a result of metabolism of sulfate reducing bacteria by reduction of sulfur compound [12,13]. The microbial corrosion could be controlled by using oxidizing and/or non-oxidizing biocides. By the way the oxidizing biocide became well known for all specialists in the field of microbial corrosion but the non-oxidizing still worked under license, so it is considered a very good task if we got a new available, safe and cheap compound for control the microbial growth by stopping the microbial growth of killing the anaerobic bacteria. The quaternary ammonium salts are used with higher efficiency of killing the bacteria especially anaerobic bacteria. There are many trials to investigate new organic compounds to be used as biocide for anaerobic bacteria [14]. It is very important to select biocide has considerable effect in low concentration and in the same time with environmental impact. The most abundant biocide in the field is quaternary ammonium salts because it shows highly effect with low concentration [15-17]. The killing effect of quaternary ammonium salts is occurred through its ability to diffuse through the cell wall of microorganisms and destroy the cytoplasmic membrane that causes cell death. Otherwise its toxicity is considered acceptable for a variety of uses specially in cooling water systems [18,19]. Some biocides are excluded because of its toxicity as organotin compounds, although it shows excellent killing effect in controlling the biofouling in marine environmental paints [20-23]. In the recent few years some trials are done for evaluation of some ionic liquids against microbial corrosion in cooling water [24-27]. This paper is aiming at assessment and investigation of the possibility of using polyacrylamide as biocide for general kinds of bacteria. The task is classified into three parts; the first one is concerned with the test against planketonic bacteria and the second part of research is test against sessile bacteria finally we will check the corrosion behavior at the recommended concentration of microbial activity. We predict to get promising results due to the highly reactive amide (NH2) group. Polyacrylamide can be chemically modified to produce both positively or negatively charged and this will produce cationic or anionic polymer, these properties gave polyacrylamide broad spectrum against varieties of microorganisms. There are many applications for polyacrylamide as in wastewater treatment, enhanced oil recovery, improve the economics of conventional water flooding, soil conditioner, gro-beast toys, additive in body-powder and molecular biology applications as a medium for electrophoresis of proteins and nucleic acids. Polyacrylamide used in agriculture may contaminate food with acrylamide, a known neurotoxin. While polyacrylamide itself is relatively non-toxic, it is known that commercially available [28-31].
Materials and Methods
Materials
Electrodes: Working electrode of mild steel electrode was used. The mild steel electrode was spherical shape sealed in epoxy with an exposed area of 0.785 cm2. The electrode was polished with fresh emery paper different grades till fine mirror surface, washed with acetone before immersion. The counter electrode was a platinum (Pt) wire. A saturated calomel electrode was used as reference electrode. A twocompartment glass cell holding 100 ml solution was used in the test involving mild steel. Solutions were prepared from doubly distilled water and analytical grade chemicals. Polyacrylamide was purchased from Fluka Chemicals.
Culture media: Postgate medium (B), gl−1 KH2PO4, 0.5; NH4Cl, 1.0; CaSO4, 1.0; MgSO4·7H2O, 2.0; sodium lactate, 3.5; yeast extract. 1.0; ascorbic acid. 0.1; thioglycolic acid, 0.1; FeSO4. 7H2O, 0.5. The pH 7.5. Postgate medium (E), gl−1 KH2PO4, 0.5; NH4Cl, 1.0; Na2SO4, 1.0; MgCl2·6H2O, 2.0; CaCl2·H2O, 1.0; sodium lactate, 3.5; yeast extract. 1.0; ascorbic acid. 0.1; thioglycolic acid, 0.1; FeSO4. 7H2O, 0.5; agar, 15.0 [32].
Methods
Antibacterial activity: Certain amount of salt water was subjected to circulation and the volume was established by adding distilled water to feed the vaporized water. Samples of circulated water were taken with time to monitor the microbial growth and this experiment is considered as the blank experiment. The recommended doses were added individuals to check the best one. These experiments are carried out for planketonc bacteria and in the same time we put coupon from mild steel in the circulated water to form the biofilm on its surface for the experiments of sessile bacteria. The water samples were detected for microbial growth by serial dilution method and turbidity in test tubes is used as a function of bacterial growth. A sterile safety razor is used for collecting the sessile bacteria included anaerobic and aerobic bacteria were developed from the surface of mild steel coupon which immersed in the circulating water. In a Postgate medium B inoculated with sessile bacteria for 7 days at 30°C.
Antibacterial effect of PAA: Gradual concentrations from 5 to 15 ppm of PAA were injected in cooling water and then samples were taken with period of time. After incubation time the data were collected and the bacterial growth inhibition has been estimated [33]. The sessile bacteria is scraped by sterile razor and collected in sterile test tubes containing 9 ml Postgate medium B to be treated as Planktonic ones to detect minimum inhibitory concentration (mic) of (PAA) against sessile and Planktonic bacteria.
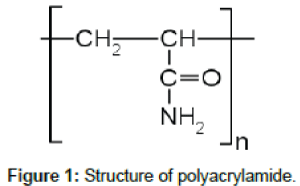
Electrochemical measurements: Potentiodynamic polarization curves were measured on the mild steel electrode in 3.5% NaCl and PAA at a voltage scan rate of 1 mVs-1. Corrosion rates and inhibition efficiencies were determined. Electrochemical measurements were performed using Gamry Instrument potentiostat/galvanostat/ZRA, which included Gamry framework system based on the Ref 600. Gamry applications that include potentiodynamic scan and EIS are DC105 and EIS300 software. Measurements were performed at 25 ± 1°C while the electrolyte was stirred using a magnetic stirrer. The potentiodynamic current voltage characterization was recorded. A Gamry water-jacketed glass cell of capacity 175 ml was used which contained three electrodes for working, counter and reference electrodes. The electrode was immersed for 30 min in the test solution in free corrosion conditions before recording the polarization curves. After attaining steady open circuit potential (OCP) the polarization was started from about 100 mV more negative than OCP to about 200mV more positive than OCP, at a scan rate of 1 mVs-1. After this forward half cycle, the scan was reversed to record the backward run. The inhibitor solutions of concentration 5, 10 and 15 ppm were prepared in analytical grade chemical reagents using distilled water. For each experiment, a freshly prepared solution was used. The polyacrylamide is formed through the action of free radical initiators, the dissolved monomers are induced to polymerize to form the resultant polymer and the structure of acrylamide repeating unit has been listed in Figure 1.
Results and Discussion
Monitoring of bacterial growth
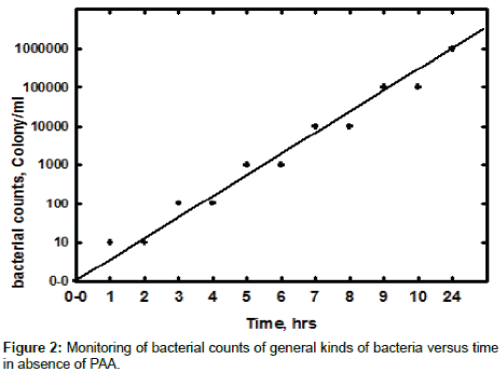
Figure 2 shows the relation between bacterial counts with elapsed time in circulating water system. The data proved that the bacterial growth reached more than one million colonies per ml in just 24 hour, which indicate that conditions from temperature, nutrients and pH are suitable for ideal growth of general kinds of bacteria. The most kind of bacteria in this situation is aerobic bacteria and as we noticed in the cooling water system there is no sessile bacteria formed in the first 24 hours. The biofilm formed on the mild steel coupon is noticed after 72 hours, which indicates that the sessile bacteria take more time to be noticed. This experiment was carried out in absence of PAA to establish the required time for injection of the biocide and it is found that the proper time for monitoring the planketonic bacteria is started from 24 hours and 72 for sessile bacteria.
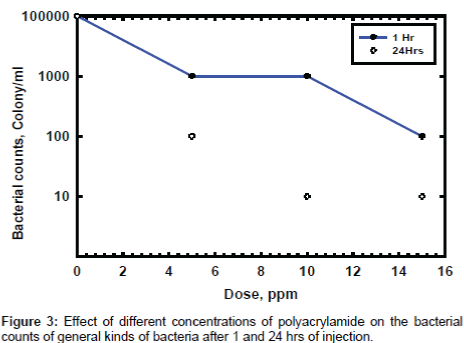
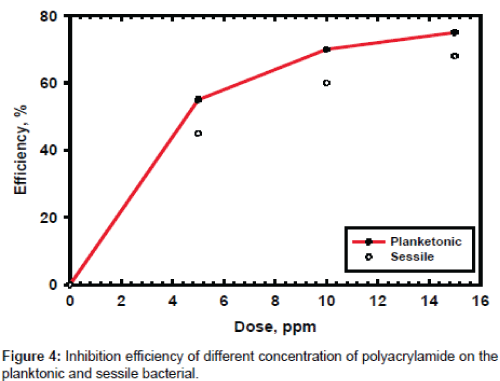
The effect gradual concentration of PAA on the microbial growth at 1 and 24 hours are investigated and the data are listed in Figure 3. The data show that the time is very important in the inhibition of microbial growth, which is meaning the PAA need more time to give its highest effect. These results proved that the PAA has low penetration effect, which is also clearly noticed in the gained data in Figure 4.
The planketonic bacteria are quickly affected by the presence of PAA because the inhibition of bacterial growth in these kinds of bacteria is directly attacked by the biocide and there is no need for penetration effect. In case of sessile bacteria the biocide must have additional properties to get the highest effect as penetration properties and these properties is existed in surfactants. The PAA shows moderate effect in sessile bacteria and need an extensional time to get the required effect. The time is very important if we need to use this biocide in the field of application so it is recommended to use it in case of inhibition of growth of planketonic bacteria but if we need to use it in sessile bacteria it is recommended to add suitable surfactant with a suitable dose to get the highest performance of biocidal activity of PAA.
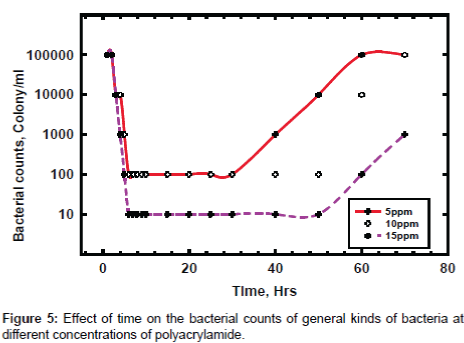
The biocidal activity has been evaluated in the three doses for PAA with time and the aim of this experiment is determining the performance time per each dose to identify the time of cyclic injection. The water is circulated till the bacterial counts reaches one hundred thousand colony per ml and then the PAA is injected with 5, 10 and 15 ppm and then samples with time are taken till the bacterial counts return again to the start number of colony per ml, see Figure 5. The results show that bacterial counts is rapidly affected in all doses but there are difference in the effective duration time, since the time of cyclic injection at bacterial counts of 1000 colony per ml are 40, 55 and 70 hours at 5, 10 and 15 ppm respectively. To protect the cooling water from the infection of general kinds of bacteria, it is preferable to use PAA at 15 ppm and it is recommended to make the cyclic injection after 70 hours from first addition. To achieve the same results in presence of sessile bacteria it is recommended to add suitable surfactant in addition of PAA to improve its quality in mitigation of microbial activity.
Electrochemical measurements
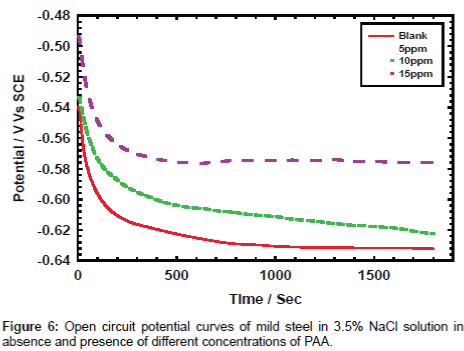
Open circuit potential: Figure 6 shows the open circuit potential of mild steel electrode at different concentration of PAA. The results show that the values of the registered open circuit potentials are shifted towards more positive potential. The values of open circuit potential are -630.0, -621.0, -609.0 and -574.0 and as we see the values shifted gradually to more positive values, which represent the concentration of PAA starts from zero, 5, 10 and 25 ppm of PAA. Shifting the values of open circuit potential to more positive prove that the behavior go to control the corrosion of mild steel in chloride media.
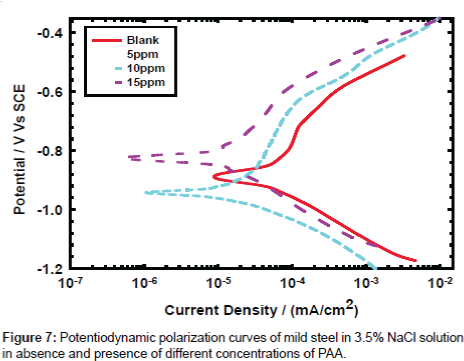
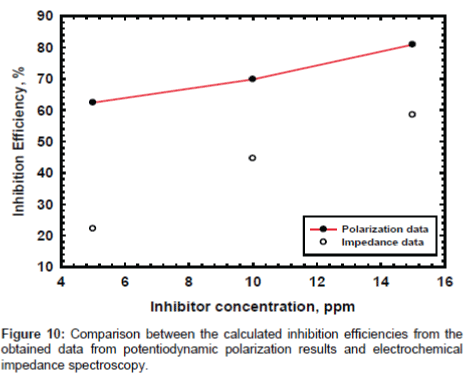
Potentiodynamic polarization: The effect of PAA concentration on the corrosion behavior of mild steel in sodium chloride solution was also tested by using the potentiodynamic polarization method and results are presented in Figure 7. The results show that the corrosion rate is decreased with increasing the concentration of PAA and the inhibition efficiency is increased gradually from 62.4, 69.8 and 80.9 for the concentrations of 5, 10 and 25 respectively. On the other hand the current densities were estimated in absence and in the presence of PAA by using Gamry soft ware and the obtained data are 31.50, 11.90, 8.90 and 6.03 μA cm-2 in absence and in the presence of 5, 10 and 25 ppm respectively. For the corrosion behavior results at the previous concentrations it is acceptable in case of the main aim is using the PAA as biocide but if we want to use it as corrosion inhibitor we recommend that increasing the dose to improve the performance of inhibition efficiency.
The potentiodynamic polarization data is presented in Table 1 and the results show that the OCP shifted to more positive potential and the data are -630.0, -621.0, -609.0 and -574.0 for zero inhibitor concentration, 5, 10 and 25 ppm respectively. The current density decreases gradually with the increasing of inhibitor and in the same time the corrosion rate decrease with the increment of concentration. By the way the inhibition efficiency is increase with concentration PAA concentration.
| Inhibitor concentration ppm | OCP (mV)Vs. SCE | Ecorr (mV)Vs. SCE | ba(mV dec-1) | bc(mV dec-1) | Icorr (µA cm-2) | C.R, mpy |
|---|---|---|---|---|---|---|
| IE % | ||||||
| Blank | -630.0 | -888.0 | 284.4 | 169.1 | 31.50 | 18.38 |
| 5 | -621.0 | -842.0 | 172.7 | 154.4 | 11.90 | 6.91/62.4% |
| 10 | -609.0 | -834.0 | 162.3 | 146.3 | 8.90 | 5.55/69.8% |
| 15 | -574.0 | -823.0 | 160.8 | 139.7 | 6.030 | 3.51/80.9% |
Table 1: Electrochemical parameters and inhibition efficiency for mild steel in 3.5% NaCl containing different concentrations of PAA.
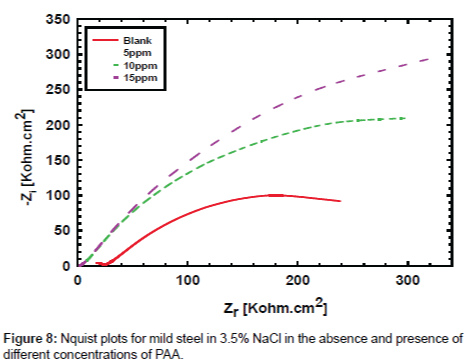
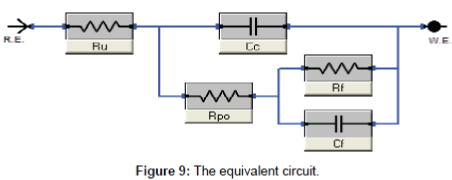
Electrochemical impedance measurements: The Nyquist plots curves of electrochemical impedance spectroscopy curves, which show the relation between the impedance and the concentration of the inhibitor, are presented in Figure 8. The data shows that the trend is clearly seen i.e., the impedance increases with increase the PAA concentration. The impedance parameters obtained from the equivalent circuit are listed in Table 2. Rf represents the solution resistance between working electrodes and the reference electrode, Ru and Cc are the resistance and capacitance of oxide layer, RPo and Cf are the resistance and capacitance of protective film. The percentage inhibition efficiency (IE%) was calculated from the Rp resistance values. From the data cited in Table 2 the inhibition efficiencies are 22.2, 44.6 and 58.5 for 5, 10 and 25 respectively. It is well known that the impedance is inversely proportional with the corrosion rate and this behavior is seen from the Rp values of untreated and treated solution, since the Rp values are 249.5, 320.0, 450.0 and 600.0 for untreated and treated with 5, 10 and 25 ppm of PAA respectively. It is clearly noticed that the impedance values were increased with increasing of the inhibitor concentration. Although the calculated inhibition efficiency from the impedance curves are lower than which calculated from polarization curves the same trend is obtained as mentioned in Figures 9 and 10. These results proved that the PAA has moderate effect against corrosion, so it is recommended to use PAA as biocide for controlling the bacterial growth in planketonic and sessile bacteria and in the same time it is safe for the metal surface and we can predict that the PAA could be have a synergistic effect if it added with a good corrosion inhibitor.
| Inhibitor concentration ppm | Rf (ohm cm2) |
Cc (uFcm-2) |
Ru (ohm cm2) |
Cf ( uFcm-2) |
Rpo (ohm cm2) |
I.E, (%) |
|---|---|---|---|---|---|---|
| Blank | 4.561 | 0.01319 | 25.28 | 1.29 | 249.5 | ------- |
| 5 | 5.00 | 0.01291 | 2.58 | 3.25 | 320.0 | 22.2 |
| 10 | 4.00 | 0.01333 | 2.30 | 4.20 | 450.0 | 44.6 |
| 15 | 4.00 | 0.01442 | 2.28 | 5.09 | 600.0 | 58.5 |
Table 2: Electrochemical Impedance parameters and inhibition efficiency for mild steel in 3.5% NaCl containing different concentrations of PAA.
Conclusions
Polyacrylamide with concentration range of 5, 10 and 25 ppm is showed acceptable results in both planketonic and sessile bacteria in circulating water which is considered the main task of our paper. The polyacrylamide is also subjected to corrosion control of mild steel insalt water to magnify its benefits to be used as biocide and corrosion inhibitor in cooling water. The cyclic injection of polyacrylamide is also tested to suggest the proper time for the re addition process for continuous control of bacterial growth and it is found that if we use the dose of 25 ppm we can recommended that the cyclic injection could be done after 70 hours from the first addition of biocide. The electrochemical measurements prove that the trend or behavior of polyacrylamide is stable in all electrochemical techniques with narrow difference in inhibition efficiencies.
References
- Videla HA (1996) Manual of Biocorrosion. Lewis Publishers, Boca Raton, Florida, USA.
- El-Shamy AM, Soror TY, El-Dahan HA, Ghazy EA, Eweas AF (2009) Microbial Corrosion Inhibition of Mild Steel in Salty Water Environment. Materials chemistry and physics 114: 156-159.
- Borenstein SW (1994) Microbiologically Influenced Corrosion Handbook. Woodhead, Cambridge, UK.
- Neelam G, Abhinav A (2014) Microbes in Process. Control of Corrosion Caused by Sulfate-Reducing Bacteria. Chapter 14: 337- 362.
- El-Shamy AM, Zakaria Kh, Abbas MA, Zein El AS (2015) Anti-bacterial and anti-corrosion effects of the ionic liquid 1-butyl-1-methylpyrrolidinium trifluoromethylsulfonate. Journal of Molecular Liquids 211: 363-369.
- Little BJ, Ray R, Wagner P, Lewandowski Z, Lee WC, et al. (1991) Biofouling 3: 43.
- Hazziza LJ, Helary G, Sauvet G (1995) J Appl Polym Sci 58: 77-84.
- Tashiro T (2001) Mater Eng 286: 63-87.
- Seter M, Thomson MJ, Stoimenovski J, MacFarlane DR, Forsyth M (2012) Dual active ionic liquids and organic salts for inhibition of microbially influenced corrosion. Chem Commun (Camb) 48: 5983-5985.
- Sauvet G, Fortuniak W, Kazmierski K, Chojnowski J (2003) J Polym Sci Part A: Polym Chem 41: 2939-2948.
- Scott S (2010) The Most Probable Number Method and Its Uses in Enumeration, Qualification, and Validation. Journal of validation technology,pp: 35-38.
- Neoh KG, Kang ET (2011) Combating bacterial colonization on metals via polymer coatings: relevance to marine and medical applications. ACS Appl Mater Interfaces 3: 2808-2819.
- Nurdin N, Helary G, Sauvet G (1993) J Appl Polym Sci 50: 663-670.
- Lewis MA (1991) Water Res 25: 101-113.
- Garcia MT, Ribosa I, Guindulain T, Sanchez-Leal J, Vives-Rego J (2001) Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment. Environ Pollut 111: 169-175.
- Milne AJ, Hails GW (1977) 4021392. Int Paint Company Ltd., London, United States of America.
- Kiil S, Weinell CE, Pedersen MS, Dam-Johansen K (2001) Ind Eng Chem Res 40: 3906-3920.
- Kiil S, Weinell CE, Pedersen MS, Dam-Johansen K (2002) Chem Eng Res Des 80: 45-52.
- Evans SM, Leksono T, McKinnell PD (1995) Mar Pollut Bull 30: 14-21.
- Weaver KD, Kim HJ, Sun J, MacFarlane DR (2010) Elliott Green Chem 12: 507-513.
- Stoimenovski J, MacFarlane DR, Bica K, Rogers RD (2010) Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm Res 27: 521-526.
- Busetti A, Crawford DE,Earle MJ,Gilea MA, Gilmore BF, et al. (2010) Green Chem 12: 420-425.
- Cybulski J, Wisniewska A, Kulig-Adamiak A, Dabrowski Z, Praczyk T, et al. (2011) Tetrahedron Lett 52: 1325-1328.
- Cybulski J, Wisniewska A, Kulig-Adamiak A, Lewicka L, Cieniecka-Rosionkiewicz A, et al. (2008) Long-alkyl-chain quaternary ammonium lactate based ionic liquids. Chemistry 14: 9305-9311.
- Ismail AIM, El-Shamy AM (2008) Engineering behaviour of soil materials on the corrosion of mild steel. Applied clay science 42: 356-362.
- Smith EA, Prues SL, Oehme FW (1997) Environmental degradation of polyacrylamides. II. Effects of environmental (outdoor) exposure. Ecotoxicol Environ Saf 37: 76-91.
- Kay-Shoemake JL, Watwood ME, Lentz RD, Sojka RE (1998) Polyacrylamide as an organic nitrogen source for soil microorganisms with potential effects on inorganic soil nitrogen in agricultural soil. Soil Biology and Biochemistry 30: 1045-1052.
- Gao JP, Lin T, Wang W, Yu JG, Yuan SJ, et al. (1999) Accelerated chemical degradation of polyacrylamide. Macromolecular Symposia 144: 179-185.
- Ver Vers LM (1999) Determination of acrylamide monomer in polyacrylamide degradation studies by high-performance liquid chromatography. J Chromatogr Sci 37: 486-494.
- Postgate J (1984) Mechanical properties and phase transition of biomedical titanium alloy strips with initial quasi-single phase state under high-energy electropulses. Journal of the Mechanical Behavior of Biomedical Materials 42: 100-115.
- Standard Methods for Examination of Water and Waste Water (1975) American Public Health Association, New York, USA.
- Fontana MG, Green ND (1978) Corrosion Engineering. McGraw-Hill Company.
- Ghazy EA, El-Shamy AM, Mahmoud MN, Abdel Samie ME (2007) Effect of CTAB as inhibitor for microbial corrosion of mild steel. Egypt J biotechnol.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12647
- [From(publication date):
June-2016 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 11654
- PDF downloads : 993