Research Article Open Access
Contributions of Procalcitonin in the Treatment of Neonatal Late Onset Sepsis: A Prospective Observational Study
Aude du Mesniladelee1, Valérie Champion1, François Kieffer1, Mohamed Ali Lachtar1, Inès de Montgolfier1, Laurence Foix l’Hélias1,2 and Delphine Mitanchez1,2*
1Division of Neonatology, Department of Perinatology, Armand Trousseau Hospital, Paris
2Sorbonne University, UPMC Univ Paris, Paris, France
- *Corresponding Author:
- Delphine Mitanchez
Sorbonne University, UPMC Univ Paris, Paris, France
Tel: 331 44736191
Fax: 33144736892
E-mail: delphine.mitanchez@aphp.fr
Received date: May 26, 2016; Accepted date: May 31, 2016; Published date: June 05, 2016
Citation: du Mesniladelee A, Champion V, Kieffer F, Lachtar MA, de Montgolfier I, et al. (2016) Contributions of Procalcitonin in the Treatment of Neonatal Late Onset Sepsis: A Prospective Observational Study. J Preg Child Health 3:256. doi:10.4172/2376-127X.1000256
Copyright: © 2016 du Mesniladelee A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Duration of antibiotic treatment in neonatal late onset sepsis is empirical. Objectives: a) To study the evolution of procalcitonin during treatment of secondary sepsis in new-borns, b) To evaluate the reduction of antibiotic exposure using its serial measurements. Methods: This single-center prospective observational study was conducted in a level II neonatology unit in the Armand Trousseau Hospital in Paris, France. All neonates hospitalized in the unit between December 2011 and January 2013 with suspected infection after 5 days of life and serum procalcitonin concentration >0.6 μg/L were included. Serial procalcitonine, C-reactive protein and blood culture survey was performed during antimicrobial therapy. Antimicrobial therapy was administered for 10 days after the last positive blood culture. Results: 54 infective episodes were observed in 46 neonates, born at a mean term of 32 weeks (range: 26-40) and infected at mean age of 19 days (7-40). Staphylococci was found in 31 infective episodes (57.4%), other microorganisms in 12 (22.2%), and none bacteria in 11 episodes (20.4%). The main cause was central line infection (85.2%). On day 5, 80% of procalcitonin measurements were <0.6 μg/L compared to 60% of C-reactive protein <5 mg/L measurements. If antimicrobial therapy had been discontinued when serum procalcitonine level was <0.6 μg/L, or the decrease from the maximal procalcitonin level was at least 80%, the duration would have been 5 days shorter. Conclusion: The use of procalcitonine in neonatal late onset sepsis as a guide to duration of treatment may limit the prescription of antibiotics. This should be further examined in a controlled study
Keywords
New-born; Procalcitonin; C-reactive protein; Late onset sepsis
Introduction
Late onset sepsis in neonates (principally nosocomial) is a major concern and challenge in neonatal units. These infections are a source of prominent mortality and morbidity, as well as prolongation of duration of hospitalisation [1,2]. In the United States, the incidence varies between 5 and 32% [3], while in France, the latest reported incidence ranged between 7.5 and 14.2% in neonatal units [4]. This high incidence is explained, in part, by the increasing use of diagnostic and therapeutic invasive procedures, and the higher survival rate of low-birth weight premature babies.
The greatest challenge consists to diagnose the infection the earliest as possible to allow the prompt initiation of antimicrobial therapy. While the clinical manifestations are the most common diagnostic indicators, they lack sensitivity and specificity.
Procalcitonin (PCT) has been studied as an early marker of early and late onset neonatal sepsis [5-7]. It is also currently under investigation as a guide for decision-making in the duration of antibiotic therapy in early sepsis [8].
There are many data in both adults and children on PCT used as a guide to the duration of treatment. In the PRORATA study, PCT was used in hospitalized adults to determine the duration of treatment of severe septicaemias [9]. Consequently, the duration of antimicrobial therapy was shortened by >2 days on the basis of the evolution of serum PCT concentrations, without increases in morbidity and mortality. Similarly, in children, the evolution of PCT enabled a five day shortening of antimicrobial therapy in the treatment of community-acquired lung diseases [10]. The efficacy of PCT-guided decision making on the duration of antibiotic treatment has never been studied in secondary sepsis of the neonate. Since the duration of antimicrobial therapy has not been the object of precise recommendations, PCT is likely to significantly shorten the duration of treatment of these infections and, consequently, to lower the costs as well as the risk of promoting the selection of multi-resistant bacteria.
The aim of our study was to examine the evolution of PCT, routinely used in our service, compared with that of C-reactive protein (CRP), during secondary neonatal sepsis. A secondary objective consisted in evaluating the reliability of PCT as a mean of determining the duration of treatment and the reduction in antibiotic exposure.
Study Sample and Methods
This study was performed between December 2011 and January 2013 in the neonatal intensive care unit of Trousseau hospital, in Paris, France, as part of a protocol of standard care. The study was approved by the « Comité d'Ethique Appliqué à la Recherche » (Société Française de Néonatologie).
During the study period, all consecutive new-borns hospitalised in the unit who, presented with secondary sepsis were considered for inclusion in this study. Secondary sepsis was defined as an infectious disease developing after 5 days of life and during which the PCT concentration was ≥ 0.6 μg/L within 24 h after the clinical suspicion of infection. All clinical and laboratory data were collected prospectively.
Standard protocol of care
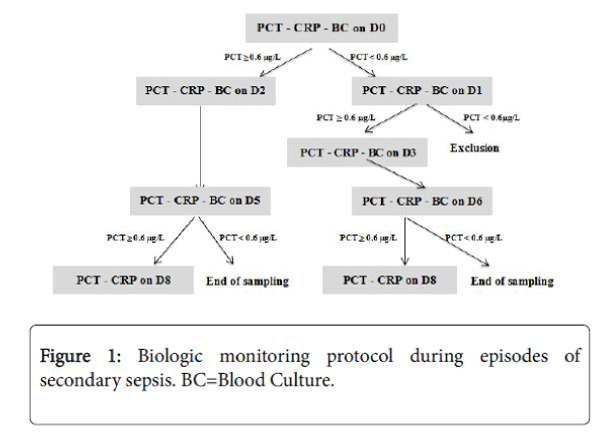
As soon as a neonate presented with manifestations consistent with infection, such as apnoea, fever or bradycardia, blood was collected for cultures and measurements of serum PCT and CRP concentrations. Figure 1 shows our standard monitoring protocol. Day zero (D0) was the day of initial manifestations of infection. If the blood PCT concentration was positive (≥ 0.6 μg/L) on D0, follow up blood samples were collected on D2 and D5.
If the concentration remained ≥ 0.6 μg/L on D5, another sample was obtained on D8. If, on the other hand, the PCT value on D0 was negative (<0.6 μg/L), another measurement was made on D1. If the PCT value on D1 remained negative, the neonate was considered noninfected and was excluded from the study. If, PCT was positive on D1, further measurements were made on D3, D6 and, if needed, on D8. At least 3 peripheral blood cultures were sampled, and additional cultures were obtained until PCT became negative. Supplemental microbiologic examinations, such as lumbar puncture in case of major inflammatory syndrome or pertinent clinical manifestations, urinary analyses and cultures in absence of clear infective focus, or stool cultures when a digestive disorder was suspected, were performed on a case-by-case basis.
Antimicrobial therapy was started as soon as clinical manifestations suggesting an infection had appeared, regardless of the PCT results. If PCT value was ≥ 0.6 μg/L, antimicrobial therapy was administered for 10 days after the last positive blood culture. The choice of antimicrobial, left to the primary physician’s discretion, consisted usually of an initial empiric regimen of cefotaxime, vancomycin and gentamicin, subsequently adapted to the culture and sensitivity of the organism identified. It could be immediately adapted to the patient’s environment, especially in cases of multiresistant organisms. The anthropometric and clinical characteristics of new-born were recorded at birth and on the day of infection. The delivery date was calculated on the basis of the last menstruation. The patients’ ages are expressed in days from the day of birth.
PCT was measured by sandwich chemiluminescence immunoassay in heparinised plasma or serum samples, using a Liaison® analyser (Diasorin, Saluggia, Italy), and the Liaison® BRAHMS® PCT reagent, reference 318.101 (ThermoFisher Scientific Inc., Waltham, MA), capable of returning the results within 1 h. CRP was measured by immunoturbidimetry of heparinised serum or plasma samples, using a Modular P analyser (Roche diagnostics, Indianapolis, IN) and the Tina-Quant C-Reactive Protein Gen 3 reagent, reference 04956912 190 (Roche diagnostics), which returns its results within 30 min. For this study, the upper normal limit of CRP serum concentration was 5.0 mg/L.
Statistics
All statistical computations have been done using the R software. All survival analysis was performed using the “survival” R package and R version 3.0.2.
Four different protocols for the management of antibiotic treatment were defined and tested to determine the length of treatment. The reference duration protocol treatment (denoted ref) was defined as the max number of days of vancomycine, cefotaxime, gentamycine and other antibiotics. This was the antibiotic treatment actually received by the patients. Three other hypothetical protocols were then defined. The first, denoted “CRP”, corresponded to the protocol where the treatment was stopped as soon as the CRP biomarker was below the threshold of 5 mg/L. The second protocol, denoted “pct1”, corresponded to the stop of treatment as soon as the PCT biomarker was below the threshold of 0.6 μg/L. Finally, for the third protocol, denoted “pct2”, the treatment was stopped as soon as PCT biomarker was below the threshold of 0.6 μg/L or an 80% decrease from the max PCT level was observed.
Cox model was used to take into account the selected individual and sepsis covariates on the duration of antibiotic treatment for the four protocols. The covariates included in the model were: gestational age (29-32 SA, ≤ 28 SA)/birth weight/low birth weight (<10th percentile)/ central catheter/severity (2 or more positive blood cultures).
Non parametric survival estimation was performed for each protocol type using the classical Kaplan-Meier-estimate. Since the sample size was small, survival analysis using log rang difference tests was used to assess whether the observed differences between the Kaplan-Meier curves were significant or not.
Results
We identified 50 neonates with suspected infection eligible for inclusion in our study. After the exclusion of four patients for whom PCT was negative on D1, sepsis was confirmed in 46 neonates between the ages of 7 and 40 days. Among them, eight developed two infectious episodes during the study, representing a total of 54 episodes. The term of neonates at birth was 32 GA (26-40) and birth weight was 1757 g (590-4030). They were aged on average of 19 days on the day of infection (7-40).
Infection characteristics
Among the 54 cases of infection, 47 (87.0%) were associated with a central catheter, one (1.8%) was attributed to a peripheral catheter, four (7.4%) developed in patients suffering from necrotising enterocolitis and two (3.7%) in patients suffering from meningitis. In 31 episodes (57.4%), the infection was caused by a coagulase negative Staphylococcus, while five were due to Klebsiella pneumoniae , three to Enterobacter cloacae , and one each to Enterobacter aerogenes , Escherichia coli , Flavimonas and Pseudomonas, respectively; no microorganism was identified in 11 episodes. The infectious organisms were identified by blood cultures obtained at the time of diagnosis in 41 episodes. No organism was identified with alternate microbiologic examinations. The two cases of meningitis were diagnosed by positive blood culture and increased leucocyte count in the spinal fluid.
The antimicrobials used and the duration of treatment are shown in Table 1.
| Number of prescriptions | Duration of prescription Days (mean) |
|
|---|---|---|
| Vancomycin | 48 | 9 |
| Cefotaxime | 50 | 6 |
| Gentamicin | 52 | 2 |
| Imipenem | 5 | 11 |
| Metronidazole | 5 | 9 |
| Ceftazidime | 2 | 8 |
| Amoxicillin | 2 | 8 |
| Ciprofloxacin | 1 | 5 |
Table 1: Antimicrobials prescribed in all patients and duration of administration.
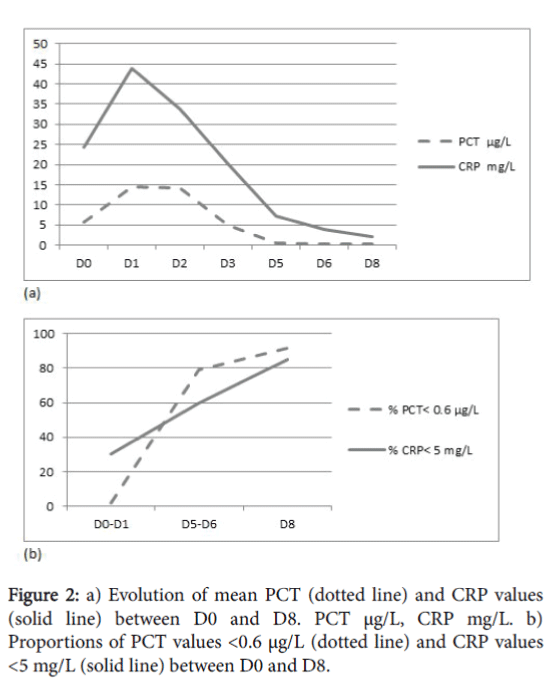
The evolution of the mean values of PCT and CRP between D0 and D8, and the proportions of PCT<0.6 μg/L and CRP<5 mg/L at each dosage are shown in Figure 2.
Between D5 and D8, 80 to 95% of the PCT values were <0.6 μg/L. On the other hand, 60 to 85% of the CRP values were <5 mg/L between D5 and D8. On D5, 11 PCT samples were positive, including eight coagulase negative Staphylococcus and one Klebsiella pneumoniae infections of central catheters, one Enterobacter cloacae infection of a peripheral venous line and one Enterobacter aerogenes meningitis .
Using the Cox model, we analysed the influence of clinical variables on the duration of antibiotic treatment (gestational age, birth weight, central line and severity of sepsis). We found that only birth weight and the presence of central line were related to longer treatment duration (data not shown).
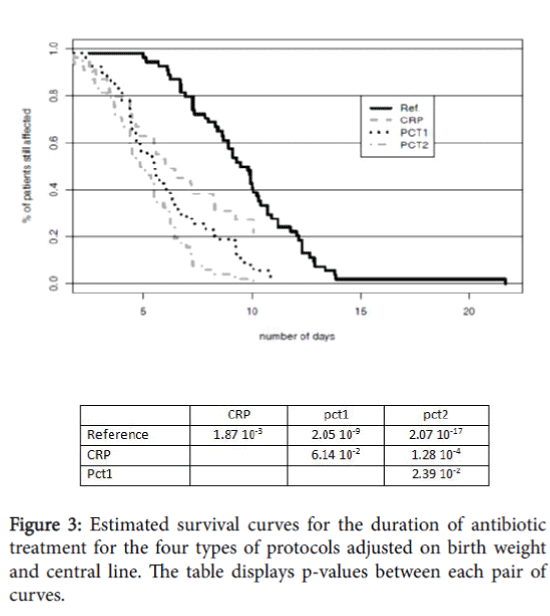
The estimated survival curves for the duration of antibiotic treatment for the four protocols tested (ref, CRP, pct1, pct2) are shown in Figure 3.
The durations were adjusted on birth weight and central line. All three hypothetical protocols CRP, pct1, pct2 clearly showed significant differences with the reference protocol.
In order to assess the gain in terms of shortened antibiotic treatment, the median durations for the four protocols are presented in Table 2.
| Protocol type | Median (days) | 95%CI |
|---|---|---|
| Reference | 9.8 | [8.68-10.42] |
| CRP | 6.25 | [5.48-8.29] |
| pct1 | 5.48 | [4.66-6.43] |
| pct2 | 4.86 | [4.36-5.94] |
Table 2: Median treatment duration for the four protocol types.
The three hypothetical protocols could allow a significant decrease of the treatment duration: 3.5 days for CRP, 4.5 days for pct1 and almost 5.0 days for pct2.
Discussion
This study shows that the decrease in PCT concentrations is more rapid than those of CRP during secondary sepsis in the new-born. The use of the evolution of PCT values to set the duration of antibiotic treatment would significantly reduce the length of treatment. If antibiotic therapy would be discontinued when PCT value is <0.6 μg/L or has decreased in 80% of its maximal value, the limitation of unnecessary antibiotic use would be on average 5 days. To the best of our knowledge this is the first study of this kind conducted in neonates.
PCT remained positive beyond D5 in a few patients, one of whom presented with Enterobacter aerogenes meningitis . The rise in PCT and the persistence of positive values is known to be related to the severity of the infection [11]. In this study, 50% of the new-borns suffered from indwelling catheter-related coagulase negative Staphylococcus infections that were not particularly severe.
The various microorganisms isolated during this study were similar to the principal bacteria described in earlier reports. The study by Vergnano et al. [12] described the main microorganisms causing secondary septicaemia in new-born as a function of the underlying disorders. Coagulase negative Staphylococcus was the most common germ, followed by group B Streptococcus and Staphylococcus aureus . In another study, the authors also found coagulase negative staphylococcus in 54% of cases of septicaemia [13]. These observations are closely concordant with our own results.
Currently, treatment strategies of secondary sepsis of the new-born are based on good practice recommendations. There is no study that allows to precisely identifying the antimicrobial regimens most appropriate for the treatment and the duration [14]. The first-line antimicrobials that are recommended are vancomycin, gentamicin and cefotaxime, while meropenem can be prescribed as second-line treatment. These recommendations are concordant with our own practice. Several studies have underscored the negative consequences of prescribing empiric antimicrobials, such as the development of resistance. For example, enterobacteria are becoming increasingly resistant to cephalosporins and the bacteria secreting extendedspectrum beta-lactamase is tremendously growing [12,15].
Once sepsis has been proven and antimicrobial has been chosen, a decision must be made regarding the duration of treatment. In this study, vancomycin was prescribed on average for 9 days and cefotaxime for 6 days. This is in agreement with Russel et al. [14] who recommend a minimum of 5 days of treatment in case of negative microbiologic examinations, 14 days in case Staphylococcus aureus infection, and 21 days in case of meningeal involvement. Measurements of PCT should enable a choice of more individual, objective and adapted duration of treatment. The study by Bouadma et al. [9] found that the prescription of antimicrobials might be shortened by over 2 days, using the evolution of PCT during the course of major septicaemia in adults hospitalised in intensive care units. Similarly, antimicrobial therapy could be shortened by 5 days in the treatment of community-acquired lung infection in children [10]. Our study also showed that the duration of antimicrobial therapy for secondary sepsis in neonates could be shortened by 5 days by using the serum concentrations of PCT. Stocker et al. [5] found similar results in neonatal early sepsis: a PCT-guided-decision making resulted in a shortening of 22 h of antibiotic therapy. These are highly encouraging results given the bacteriologic resistance and the noxious consequences of administering unnecessary antimicrobials to an immature organism. Indeed, besides the selection of resistant bacteria, the indiscriminate prescription of antimicrobials in the neonatal period is likely to have long-term adverse effects. The use of broad-spectrum antimicrobials promotes the implantation of unusual organisms among the normal flora. While these can be eliminated from more mature subjects, several studies have observed their persistence after implantation in a new-born. These elements of the digestive flora cause anomalies of the immune system and modify the metabolic programming [16]. A secondary increase in the prevalence of diabetes and obesity, and changes in the immune system with, in particular, the development of lung disease and allergies in adults, have all been reported [17-21].
Our results are limited by the small sample size. However, they were obtained, using a protocol routinely implemented in our service, which was developed from the use of antimicrobials reported by others [14]. In our series, we did not report severe sepsis that may modify the evolution of PCT value and the evaluation of duration of treatment. Furthermore, the use of PCT-guided treatment in ventilated patients may be different as a variety of neonatal disorders like perinatal asphyxia or respiratory distress syndrome impact the concentration of PCT [22]. A randomized controlled international intervention trial is currently going on to test the efficacy and the safety of PCT-guided treatment in early neonatal sepsis in term and near-term neonates [8]. A similar protocol should be designed in late-onset sepsis in neonates.
Such study should stratify the participants according to the term of birth, and according to the presenting infections and culprit microorganisms. It might contribute valuable additional information with respect to the reliability of PCT and, above all, its ability to safely shorten the duration of antimicrobial therapy in secondary sepsis.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the national research committee (Comité d'Ethique Appliqué à la Recherche (Société Française de Neonatologie) and with the 1964 Helsinski declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- Stoll BJ, Hansen NI, Chapman AI, Fanaroff AA, Hintz SR, et al. (2004) Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama 292: 2357-2365.
- Payne NR, Carpenter JH, Badger GJ, Horror JD, Rogowski J (2004) Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Paediatrics 114: 348-355.
- Sohn AH, Garrett DO, Cochran RL, Grohskopf LA, Levine GL, et al. (2001) Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr 139: 821-827.
- Lachassinne E, Richard EL, Gaudelus J (2004) Epidemiology of nosocomial infections in neonates. Arch Pediatr 11: 229-233.
- Stocker M, Fontana M, El Helou S, Wegscheider K, Berger TM (2010) Use of procalcitonin-guided decision-making to shorten antibiotic therapy in suspected neonatal early-onset sepsis: Prospective randomized intervention trial. Neonatology 97: 165-74.
- Sastre JB, Solis DP, Serradilla VR, Colomer BF, Cotallo GD (2007) Evaluation of procalcitonin for diagnosis of neonatal sepsis of vertical transmission. BMC Pediatr 7: 9.
- Auriti C, Fiscarelli E, Ronchetti MP, Argentieri M, Marrocco G, et al. (2012) Procalcitonin in detecting neonatal nosocomial sepsis. Arch Dis Child Fetal Neonatal Ed 97: 368-370.
- Stocker M, Hop WC, Van-Rossum AM (2011) Neonatal procalcitonin intervention study (NeoPInS): Effect of procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis: A multi-centre randomized superiority and non-inferiority Intervention Study. BMC Pediatr 10: 89.
- Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, et al. (2010) Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 375: 463-474.
- Esposito S, Tagliabue C, Picciolli I, Semino M, Sabatini C, et al. (2011) Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med 105: 1939-1945.
- Pacifico L, Osborn JF, Natale F, Ferraro F, De Curtis M, et al. (2013) Procalcitonin in pediatrics. AdvClinChem 59: 203-263.
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, et al. (2011) Neonatal infections in England: The NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed 96: 9-14.
- Vergnano S, Sharland M, Kazembe P, Mwansambo C (2005) Neonatal sepsis: An international perspective. Arch Dis Child Fetal Neonatal Ed 90: 220-224.
- Russell AB, Sharland M, Heath PT (2012) Improving antibiotic prescribing in neonatal units: time to act. Arch Dis Child Fetal Neonatal Ed 97: 141-146.
- Samanta S, Farrer K, Breathnach A, Heath PT (2011) Risk factors for late onset gram-negative infections: A case-control study. Arch Dis Child Fetal Neonatal Ed 96: 15-18.
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, et al. (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118: 511-521.
- Russell AR, Murch SH (2006) Could peripartum antibiotics have delayed health consequences for the infant? Bjog 113: 758-765.
- Risnes KR, Belanger K, Murk W, Bracken MB (2011) Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1,401 US children. Am J Epidemiol 173: 310-318.
- Strachan DP (2000) Family size, infection and atopy: The first decade of the "hygiene hypothesis". Thorax Suppl 1: 2-10.
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, et al. (2004) The gut microbiota as an environmental factor that regulates fat storage. ProcNatlAcadSci USA 101: 15718-15723.
- Blaser M (2011) Antibiotic overuse: Stop the killing of beneficial bacteria. Nature 476: 393-394.
- Bhandari V (2014) Effective biomarkers for diagnosis of neonatal sepsis. J Pediatric Infect Dis Soc 3: 234-245.
--
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11084
- [From(publication date):
June-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10252
- PDF downloads : 832