Congenital Cystic Adenomatoid Malformation (CCAM): A Case Report and Imaging Findings
Received: 02-Feb-2024 / Manuscript No. roa-24-128080 / Editor assigned: 05-Feb-2024 / PreQC No. roa-24-128080 / Reviewed: 19-Feb-2024 / QC No. roa-24-128080 / Revised: 23-Feb-2024 / Manuscript No. roa-24-128080 / Published Date: 29-Feb-2024
Abstract
Congenital cystic adenomatoid malformation is an atypical manifestation of cystic lung disease, alternatively referred to as congenital pulmonary adenomatoid malformation. We report a case of a 45-day-old infant presenting with respiratory distress. The thoracic CT scan revealing a Type I congenital cystic adenomatoid malformation.
Keywords
Cystic; Malformation; Pulmonary; Imaging
Introduction
Congenital cystic adenomatoid malformation (CCAM) is an atypical manifestation of cystic lung disease, alternatively referred to as congenital pulmonary adenomatoid malformation (CPAM). It represents a non-inherited, hamartomatous anomaly affecting the terminal respiratory structures, with an unknown etiology [1, 2].
Case Report
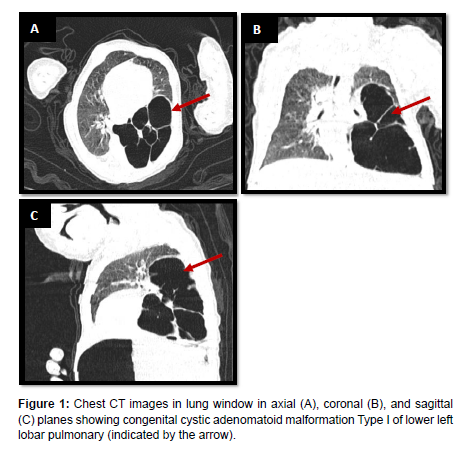
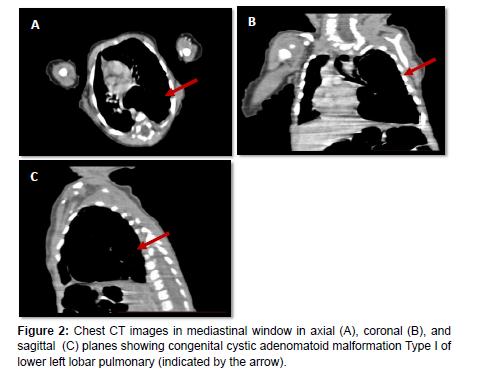
Our case involves a 45-day-old infant presenting with persistent respiratory distress since birth. This prompted a thoracic CT scan, revealing a conglomerate of lower left lobar pulmonary cysts separated by thin septa, pushing the upper lobe upwards and the mediastinum laterally, bordered by lung parenchyma, communicating with the left lower lobar bronchus, without a feeding artery. The largest cyst measures 39x40mm, suggestive of a Type I congenital cystic adenomatoid malformation (Figures 1 and 2).
Discussion
The origin of CCAM remains uncertain, and the fundamental anomaly during development leading to CCAM is described as either bronchial atresia or the arrest of maturation in bronchopulmonary segments occurring before the 17th week of gestation [2].
Constituting 25% of congenital pulmonary malformations, CCAM is predominantly diagnosed in neonates and infants [2]. The incidence of prenatally diagnosed CCAM is reported to be 1 in 25,000 to 35,000 pregnancies, with potential manifestations in older children and adults as an incidental discovery due to recurrent infections. The mortality rate for prenatally diagnosed cases varies between 9% and 49% [2].
CCAM commonly presents with respiratory distress (RD) in neonates and infants, with spontaneous pneumothorax being an uncommon occurrence [1]. Documented correlations include associations with extralobar intra-abdominal sequestration,bronchopulmonary sequestration, and bronchial atresia in live births and stillbirths. Additionally, a few cases report a close association between CCAM and polyhydramnios [1].
The manifestation of CCAM is typically unilateral and predominantly confined to the lower lobe, with rare instances occurring in the lower left lobe [1].
Five distinct types of CCAM have been identified based on the embryological level of origin and histological characteristics [3]. Type 0 represents the rarest form, originating from the trachea or bronchus, presenting with severe and often fatal outcomes characterized by small cysts. Type 1 is the most prevalent form, accounting for 50% to 70% of cases, originating from the distal bronchus or proximal bronchiole, and is associated with a substantial mass effect that may lead to hydrops. Type 2 accounts for 15% to 30% of cases, originating from terminal bronchioles, featuring small cysts and solid areas. Type 3 constitutes 5% to 10% of cases, presenting with cysts in macrocystic lesions and a less favorable prognosis. Type 4 accounts for 5% to 15% of cases, featuring large cysts linked to malignancy, particularly pleuropulmonary blastoma, originating from the alveoli [3].
Diagnosis of CCAM is typically achieved through imaging studies, with confirmation obtained through histopathology [2].
Prenatal diagnosis of CCAM can typically be established through ultrasonography [2].
Congenital diaphragmatic hernia (CDH) and Bronchopulmonary sequestration (BPS) are the two primary and crucial differential diagnoses for CCAM [4].
CCAM, excluding type III, can be distinguished from bronchopulmonary sequestration (BPS) by showcasing cysts within the lung mass. Additionally, color Doppler or power Doppler can illustrate the systemic blood supply of BPS and the pulmonary blood flow in CCAM, serving as a means to differentiate type III CCAM from BPS [4].
CCAM can also be mistakenly diagnosed by radiology as a pulmonary cyst, emphysematous bubbles, or pneumothorax. Chest X-rays may reveal a mass containing air-filled cysts [2].
CT scan is recognized as the gold standard for radiographic characterization of congenital lung malformations, providing high spatial resolution and rapid acquisition times [4]. The typical presentation involves multiocular cystic lesions characterized by thin walls surrounded by normal lung parenchyma. However, the presence of superimposed infection can complicate the overall appearance [2].
The use of magnetic resonance imaging (MRI) in perinatal imaging offers advantages such as high contrast resolution without ionizing radiation exposure, facilitating the measurement of congenital lesion size and uninvolved lung volume, impacting counseling, fetal prognosis, and management. However, its prolonged scanning time often requires general anesthesia for most children over 6 months old, making the “feed and wrap” technique impractical, with potential spatial resolution limitations [4].
One significant risk associated with CCAM is the potential development of bronchioloalveolar carcinoma or other types of malignant transformations, such as sarcoma or blastoma [2].
The outcome of CCAM surgery is generally positive, with a low incidence of postoperative complications and mortality, shorter hospitalization periods, and a significant reduction in the risk of recurrence. However, surgery can potentially result in varying degrees of respiratory failure [2].
Conclusion
Increased awareness of this rare condition among radiologists and pediatricians can facilitate early diagnosis and appropriate treatment, ultimately enhancing outcomes and the quality of life for affected patients [1,2].
References
- Monica Salerno , Francesco Sessa , Giuseppe Cocimano , Salvatore Roccuzzo, Massimiliano Esposito et al. (2022) Congenital Cystic Adenomatoid Malformation (CCAM) Type II: A Rare Case of Sudden Infant Death. Children 9: 1830.
- Maysaa Badour, Bara’a Hussain, Ali Hammed, Sawssan ali, Saeed falyon (2021) A rare case of congenital cystic adenomatoid malformation: Mimics pneumonia manifestations. Ann med surg 66: 102433.
- Kamal Alshamiri M, Hatem Abbod B (2017) Congenital cystic adenomatoid malformation. Int J Pediatr Adolesc Med 4:159-160.
- Moti Chowdhury M, Subhasis Chakraborty (2015) Imaging of congenital lung malformations. Semin Pediatr Surg 24: 168–175.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sara Z, Majda A, Sanae J, Lina B, Nazik A, et al. (2024) Congenital CysticAdenomatoid Malformation (CCAM): A Case Report and Imaging Findings. OMICSJ Radiol 13: 542.
Copyright: © 2024 Sara Z, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 326
- [From(publication date): 0-2024 - Mar 09, 2025]
- Breakdown by view type
- HTML page views: 274
- PDF downloads: 52