Confocal Laser Endomicroscopy: The Potential Role of In Vivo Characterization of Neoplasia in the Gastrointestinal Tract
Received: 31-Mar-2014 / Accepted Date: 22-Jul-2014 / Published Date: 24-Jul-2014 DOI: 10.4172/2161-0681.1000181
Abstract
Confocal laser endomicroscopy (CLE) is a novel microscope which enables real-time imaging with detailed mucosal and sub mucosal architectures throughout the gastrointestinal tract. New optical technology using the principle of light reflection, scattering and refocusing dramatically increases image resolution thus has the potential of real time optical biopsy. Two types of CLE system are commercially available; endoscope-based type (eCLE) which integrated CLE in the tip of scope and probe-based type (pCLE) which uses a probe through the accessory channel of a traditional endoscope. Clinical data applying CLE for the detection of neoplastic lesions in the gastrointestinal tract have been increasingly reported including esophagus, stomach and colon. The probe based system has a probe that can be used in the bile ducts to evaluate biliary strictures. More recently, a needle based CLE which passes through a fine-needle aspiration needle has been introduced into pancreatic cystic lesions. CLE has demonstrated promising in vivo data in various clinical arenas. Further validation regarding reproducible image classification, inter and intra observer variability, learning curve, and cost effectiveness remain to be demonstrated.
Keywords: Confocal laser endomicroscopy; Chromoendoscopy; Barrett’s esophagus; Esophageal cancer; Colon cancer; Endoscopic ultrasonography; Biliary stricture; Pancreatic cystic neoplasia
314885Introduction
Recent innovation of endoscopic imaging technology has enabled gastroenterologists to visualize detailed mucosal architecture and even subepithelial vascular structures of the gastrointestinal lumen. The technology is developed to potentially allow real-time, in-vivo histological assessment called “optical biopsy” on the interest region under endoscopic observation. Confocal laser endomicroscopy (CLE) is one of the emerging endoscopic modalities, and it has been introduced for the detection of pre-cancerous lesions in the gastrointestinal tract. Early detection of neoplastic changes in the gastrointestinal tract is devoted to the treatment in early stage cancer and theoretically leads to better prognosis. Promising data have been published especially in the esophageal and colon cancer surveillance. CLE has recently been applied to pancreatobiliary diseases as well. This article will review the current status of CLE in the diagnosis of neoplasia in the field of gastroenterology.
Technical aspect
CLE provides unique imaging features that complement the use of conventional endoscopy. CLE visualizes below the tissue surface with subcellular resolution over a smaller region of tissue and can be considered as point detection modality. The principle of CLE is based on tissue illumination with a low-power laser with subsequent detection of the fluorescent light reflected from the tissue. The laser is focused at a specific depth and only light reflected back from that tissue layer is refocused through a pinhole effect. Rejection of scattered lights from the layer out of focus provides increasing imaging resolution. The area of focus is scanned in the horizontal and vertical planes and an image is reconstructed [1,2].
Since confocal imaging relies on tissue fluorescence, local and/or intravenous contrast agents are required in gastrointestinal tract imaging. Fluorescein sodium (Akorn Pharmaceuticals, Lake Forest, Illinois, USA) is the most commonly used. Fluorescein is administered intravenously and may highlight the vasculature, lamina propria, and intracellular spaces of this tissue. A cross sectional survey of 16 international academic medical centers performing 2272 gastrointestinal CLE procedures with intravenous 2.5 - 5 ml of 10% sodium ?uorescein showed no serious adverse events were reported. Mild adverse events occurred in 1.4% of individuals, including nausea, vomiting, transient hypotension without shock, injection site erythema, diffuse rash and mild epigastric pain [3]. The fluorescent contrasts can be also be applied topically (acriflavin [Sigma Pharmaceuticals, Clayton, Victoria, Australia], tetracycline, cresyl violet [AnaSpec, Inc, San Jose, Calif] through a spraying catheter [2]. With intravenous administration of fluorescein, the agent initially starts as a blood pool agent and perfuses the capillaries, then passes into the extracellular matrix and the lamina propria. After some time the fluorescein may pass into the epithelium. Since fluorescein only occasionally crosses the lipid membrane and does not stain cell nuclei. The recommended dose to start with is 2.5 ml of 10% fluorescein sodium, to be repeated if needed. An experimental data investigating contrast dynamics and image quality over time after injection of the substance showed that in gastrointestinal tract the contrast and image quality decreased significantly after 8 minutes of fluorescein injection [4]. In clinical setting, imaging may begin within 30 seconds and optimal imaging occurs 8 - 10 minutes. Topical fluorescence (0.2% acriflavine), in contrast, crosses cell membranes but does not penetrate into the deeper layers of the gastrointestinal mucosa. Moreover, acriflavin is a known carcinogen thus it may not be suitable for routine clinical use [5,6].
Two types of CLE system were initially developed: one is endoscope-based type (Pentax, Tokyo, Japan / Optiscan, Victoria, Australia) which integrated CLE in the tip of scope, and another is probe-based type (Mauna Kea Technologies, Paris, France) which uses a probe through the accessory channel of a traditional endoscope. Endoscope-based CLE (eCLE) collects images at a scan rate of 0.8-1.6 frames per second with an adjustable depth of scanning ranging from surface to 250 mm, a field of view of 475×475 mm, a lateral resolution of 0.7 um. At the time of this review, the eCLE is no longer commercially available. CellvizioR is the first and only probe-based CLE (pCLE) compatible with all flexible video-endoscopes and enables a seamless procedure session from diagnosis and therapeutic such as endoscopic mucosal resection. The latest model of Cellvizio pCLE designed for gastrointestinal tract applications include Ultrahigh definition (UHD) GastroFlexTM for Esophagogastroduodenoscopy (EGD), Ultra high definition (UHD) ColoFlexTM for colonoscopy, CholangioFlexTM for cholangioscopy in endoscopic retrograde cholangiopancreatography, and AQ-FlexTM 19 for EUS-FNA procedures. All probes generate dynamic images (12 frames per second). The depth of imaging for GastroFlex/ColoFlex is 55-65 um with the UHD probes having a resolution down to 1 micron and CholangioFlex/AQ-Flex is 40 to 70 mm with a resolution down to 3 microns (Table 1). The newly developed needle-based CLE (nCLE) AQ flex 19 miniprobe which can be passed through a 19-gauge (G) endoscopic ultrasonography (EUS)-guided fine-needle aspiration (EUS-FNA) needle enables needle-based CLE under EUS guidance. Becker et al. reported a feasibility study of nCLE for in vivo histology of various organs in a porcine model by using either EUS-guide or natural-orifice transluminal endoscopic surgery (NOTES) procedure [7]. The confocal miniprobe was inserted through the 22-gauge needle, and puncture of various intra-abdominal structures and organs was performed (lymph nodes, diaphragm, ovaries, liver, spleen, and pancreas). Real-time in-vivo histologic image sequences were recorded with acceptable image quality, but the 22 gauge needle was not robust enough for clinical use. The miniprobe compatible with a 19 gauge needle was developed and available for clinical use and was tested in an initial technical feasibility study in the pancreas in humans [8].
| GastroFlex/ColoFlex | CholangioFlex | AQ-Flex 19 | |
|---|---|---|---|
| Procedure | EDG/Colonoscopy | ERCP | EUS-FNA |
| Accessory Channel | 2.8 mm | 1.2 mm | 19 G FNA needle |
| Depth (um) | 55-65 | 40-70 | 40-70 |
| Field of View (um) | 240 | 320 | 325 |
| Lateral Resolution (um) | 1 | 3.5 | 3.5 |
Table 1: Characteristics of pCLE probes
Development of Criteria
A standardization of terminology, categorization of images is also essential for new advanced imaging tools. In February 2009, a group of expertise in pCLE was assembled based on their early experience in a variety of gastrointestinal conditions. Since pCLE is a new technology and there was a limited number of clinical trials prior to the meeting, the standards were largely based on expert opinion, and consensus development. The consensus conference involved presentation of standard images and specific features of each gastrointestinal condition, together with group discussion and consensus development (Miami criteria) (Table 2) [9]. The Miami classification is now considered to be the standard reference criteria for pCLE. Additional studies have also suggested other classification systems or refinements of the criteria for pCLE in gastrointestinal neoplasm. This process of validation and refinement of criteria is at different stages in each organ system or disease state.
| Barrett’s Esophagus | |
| Normal squamous epithelium | flat cells without crypts or villi bright vessels within papillae (intra papillary capillary loops |
| Non dysplastic Barrett‘s esophagus | uniform villiform architecture columnar cells dark ‘goblet’ cells |
| High grade dysplasia | villiform structures dark, irregularly thickened epithelial borders dilated irregular vessels |
| Adenocarcinoma | disorganized/loss of villiform structure and crypts dark columnar cells dilated irregular vessels |
| Stomach | |
| Normal gastric mucosa | round regular crypts “cobblestone“ appearence of normal glands |
| Gastric dysplasia | irregular crypt lumen dark, irregular, thickened epithelium |
| Gastric adenocarcinoma | completely disorganized epithelium fluorescein leakage dark irregular epithelium |
| Colon | |
| Normal colonic mucosa | round crypt structures dark goblet cells regular, narrow vessels surrounding crypts |
| Hyperplastic polyp | crypts with slit or stellate openings (pits) bright non-thickened, uniform epithelium dark ‘goblet’ cells small vessels |
| Adenoma | irregular or villiform structures dark, irregularly thickened epithelium decreased goblet cells |
| Adenocarcinoma | disorganized villiform or lack of structure dark, irregularly thickened epithelium dilated vessels |
| Biliary | |
| Normal bile duct | fine, reticular gray pattern |
| Biliary malignancy | irregular structures interspersed with bright areas of tortuous dilated blood vessel |
Table 2: pCLE-based Miami classification system
Esophagus
Clinical context: Barrett’s esophagus is well established precancerous condition of the esophagus and the incidence of esophageal cancer continues to rise in the USA. The emphasis on early detection of esophageal cancer has been devoted to treatment of early stage disease and theoretically leads to better prognosis. Thus, new imaging modalities have been applied for the purposes of detection and characterization of neoplastic tissue in the setting of Barrett’s esophagus and showing promising data.
Imaging characteristics of Barrett’s Esophagus: CLE demonstrates the lining of BE as columnar cell and dark ‘goblet’ cells. Irregular capillaries and epithelial structures suggest the presence of dysplasia.
Miami classification system for Barrett’s esophagus as follows (Table 2): Normal squamous epithelium is characterized by flat cells without crypts or villi, bright vessels within papillae (intra papillary capillary loops); Non dysplastic Barrett‘s esophagus by uniform villiform architecture, columnar cells, and dark ‘goblet’ cells; High grade dysplasia by villiform structures, dark, irregularly thickened epithelial borders, and dilated irregular vessels; and Adenocarcinoma by disorganized/loss of villiform structure and crypts, dark columnar cells, dilated irregular vessels. Subsequently, six additional pCLE criteria (Kansas city criteria) were proposed to diagnose dysplasia in BE and are as follows: 1. Epithelial surface appears saw-toothed, 2. Goblet cells not easily identified, 3. Gland are not equidistant, 4. Glands are unequal in size and shape, 5. Cells are enlarged, 6. Cells are irregular and not equidistant from one another [10]. None of these Kansas City criteria had adequate accuracy or agreement individually and the presence of two or more criteria provided the best accuracy for differentiation between dysplasia (71%, area under the curve=0.71, P=0.05) and non-dysplasia (71%, area under the curve = 0.74, P=0 .03).
Diagnostic performance for Barrett’s associated neoplasia: Since the first in vivo study reported that CLE detected Barrett’s esophagus (BE) associated neoplasia with a sensitivity and specificity of 92.9% and 98.4%, respectively, with excellent inter- and intra-observer agreement [11], there have been emerging data on the role of CLE for the management of BE. In a prospective randomized crossover study comparing targeted biopsies by CLE (eCLE) from endoscopically inapparent neoplasia with standard quadrant protocol, CLE targeted biopsy almost doubled the diagnostic yield for neoplasia and two-thirds of patients did not need any mucosal biopsies on the basis of normal CLE findings [12]. An initial pCLE study based on the Miami criteria demonstrated a high negative predictive value in predicting high grade dysplasia (HGD) or early esophageal adenocarcinoma (EAC) in BE, however sensitivity, specificity, positive predictive value, negative predictive value were 75%, 88%, 44.4% and 98.8%, respectively [13]. Preliminary studies of pCLE for surveillance of BE from Europe and U.S. showed comparable results to that of conventional approach by the excellent specificity and negative predictive value but showed room to improve sensitivity [14,15]. The recent largest multicenter randomized study has demonstrated that adding pCLE to high definition white light endoscopy (HD-WLE) increased sensitivity (34.2% by HD-WLE, 68.3% by HD-WLE or pCLE, p=0.002) and improved sensitivity of narrow band imaging (NBI) (45.0% by HD-WLE or NBI, 75.8% HD-WLE, NBI or pCLE, p=0.01). Overall, the combination of pCLE with HD-WLE led to the recognition of 41 additional locations with HGD/EC compared with HD-WLE alone [16]. Of note, the negative predictive value (NPV) of pCLE used in combination with HD-WLE was 91% and was 95.6% when combined with both HD-WLE and NBI. Table 3 summarized results of key trials on the diagnostic performance of CLE.
| Study | Organ / Targeted tissue | Detection | CLE | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Sharmaet al. [15] | Esophagus Barrett’s | HGD/EAC | pCLE | 68.3 | 87.8 |
| Pech et al. [20]) | Esophagus | SCC | eCLE | 100 | 87.0 |
| Li [23] | Stomach | Gastric neoplasia | eCLE | 88.1 | 98.6 |
| Kiesslich et al.[26] | Colon Polyps and random sites |
Neoplasia | eCLE | 97.4 | 99.4 |
| Hurlstone et al. [28] | Colon Polyps |
Neoplasia | eCLE | 97.4 | 99.3 |
| Meining et al.[34] | Colon Polyps |
Neoplasia | pCLE | 93 | 92 |
| Buchner et al.[34] | Colon Polyps |
Neoplasia | pCLE | 91 | 76 |
| Meining et al.[49] | Bile ducts Indeterminate stricture |
Malignancy | pCLE | 98 | 67 |
| Konda et al.[55] | Pancreas Cysts |
PCN | nCLE | 59 | 100 |
Table 3: Prospective In Vivo Study of CLE in Gastrointestinal Cancer
The improved sensitivity with the detection of additional neoplastic areas could lead to the decision of the course of treatment with more widespread endoscopic mucosal resection (EMR) and/or ablative therapies. Therefore, a case can be made for real-time decision making in those patients evaluated by using pCLE. A high NPV offers a higher degree of confidence in confirming the absence of high grade dysplastic lesions for a better informed decision. One case series illustrated the potential role of pCLE during therapeutic procedures that include localization of pathology, targeting of resections, guiding which therapy to use, and determining adequacy of treatment [17]. A prospective, multicenter, randomized, clinical trial was conducted to assess whether addition of pCLE to high-definition white light to aid in determination of residual BE for those who had undergone ablation therapy. The study was closed after the interim analysis due to low conditional power resulting from lack of difference between groups and there was no evidence that the addition of pCLE to high-definition white light imaging for detection of residual BE or neoplasia can provide improved treatment [18]. The revised pCLE criteria was developed among the Kansas city group and to better predict the presence of HGD and ECA in BE patients [10]. By testing multiple sets of images by the expertise in pCLE and gastrointestinal pathologists, only criteria with either a mean sensitivity or specificity ≥ 70% was considered to have high yield in prediction of dysplasia. The overall accuracy of the Kansas city criteria in diagnosing dysplasia was 82% (95% C.I.: 77.5 – 85.0). The sensitivity and specificity in diagnosing dysplasia using the criteria were 76 and 85%, respectively. To predict dysplasia, PPV and NPV were 76.05 (68.84, 81.88) and 84.97 (68.91 – 91.62), respectively. The overall agreement between the reviewers was substantial with a κ of 0.61 (0.53 – 0.69). No difference was found in agreement between experienced (κ=0.66 (0.53 – 0.79)) and non-experienced (κ=0.57 (0.48 – 0.68), P = 0.22) observers. The learning curve was also assessed by comparing the accuracy of the first 30 videos to the last 45, and there was no significant difference from the last 45 (83 (76 – 89) vs. 81% (75 – 85), P=0.51) [10].
Squamous cell cancer of the esophagus: A few studies have been reported to date for the use of eCLE in the management of squamous cell cancer (SCC) of the esophagus [19,20]. One study reported the diagnostic efficacy of eCLE for patients who were referred for suspected early SCC [20]. eCLE detected all cancerous lesions correctly with two false positive lesions. The overall accuracy was 95%, and the sensitivity and specificity were 100% and 87%, respectively, with acceptable intra- and interobserver agreement [20]. Larger scale control study is to be performed to determine the clinical usefulness of CLE for the management of SCC of the esophagus.
Stomach
Clinical context: EMR and endoscopic submucosal dissection (ESD) have widely recognized to achieve curative resection for early superficial gastric cancer. Given the geographic difference of prevalence of gastric cancer, the majority of trials have been reported from Asia. Current standard practice to detect early gastric cancer where endoscopic resection is indicated is using chromoendoscopy with detailed inspections of the disease extent, histology assessment by biopsy, and prediction of vertical invasion of cancer. Undifferentiated gastric cancer is not amenable to endoscopic therapy. CLE has been explored as a tool for detection and characterization of gastric neoplasia. Imaging characteristics
CLE demonstrates the lining of gastric mucosa as glandular appearance and goblet cells are recognizable by CLE. An observational preliminary report showed that villus-like appearance with goblet cell in intestinal metaplasia and distorted pit appearance with atypical gland in differentiated adenocarcinoma [21,22].
Miami classification system for the stomach as follows: Normal gastric mucosa is characterized by round regular crypts, “cobblestone“ appearance of normal glands; Gastric dysplasia by irregular crypt lumen, dark, irregular and thickened epithelium; Gastric adenocarcinoma by completely disorganized epithelium, fluorescein leakage and dark irregular epithelium.
Diagnostic performance: An initial feasibility in-vivo study reported high diagnostic yield and accuracy (91% sensitivity, 97% specificity, and 95% accuracy) but 40% of the images were excluded for interpretation because of suboptimal quality [23]. Following prospective study evaluated diagnostic value of eCLE for the diagnosis of early gastric cancer [24]. The study proposed diagnostic classification of non-cancerous lesions and cancer/high-grade intraepithelial neoplasia and then applied this to referral patients with known or suspicious gastric cancer comparing with HD-WLE by using histopathology as a gold standard. eCLE diagnosis had a higher sensitivity (88.9%), specificity (99.3%) and diagnostic accuracy (98.8%) for gastric superficial cancer/high-grade intraepithelial neoplasia lesions than HD-WLE diagnosis (sensitivity, 72.2%; specificity, 95.1%; and accuracy, 94.1%) (p<0.05). CLE has been also applied to effectively diagnose early gastric cancer suitable for ESD. Repeated biopsies to obtain precise histology results are sometimes needed but it could cause submucosal fibrosis which may increase the incidence of procedural complications. In the cohort of patients who are scheduled for ESD, one prospective comparative study reported that the overall accuracy of eCLE diagnosis of gastric adenomas and adenocarcinomas was significantly higher at 94.2% versus 85.7% for conventional endoscopic biopsy (P=0.03) and the overall accuracy of CLE diagnosis of differentiated and undifferentiated adenocarcinomas also was higher (95.4% compared to 84.2%) but it did not differ significantly (P=0.146) [25]. Same group has recently published the comparative study of pCLE in the pre-ESD management [26]. The study showed that the overall accuracy for the diagnosis of adenocarcinoma was 91.7% for pCLE compared to 85.2% for conventional endoscopic biopsies (P=0.065) and the combined accuracy of conventional endoscopic biopsies and pCLE was 98.1%.
Colon
Clinical context: Screening colonoscopy aims at detecting precancerous lesions and thus preventing death from colorectal cancer. Polyp or tissue characterization of in vivo may be of benefit during endoscopic procedures, particularly with flat or subtle lesions or areas of previous resection. Advances in endoscopic technology have been applies and colon is one of the most widely investigated gastrointestinal organs by CLE.
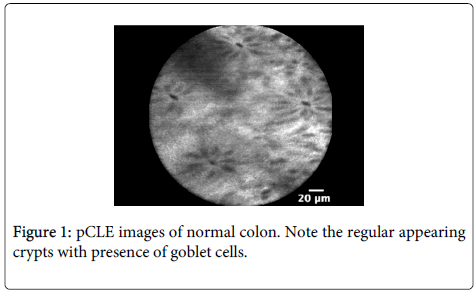
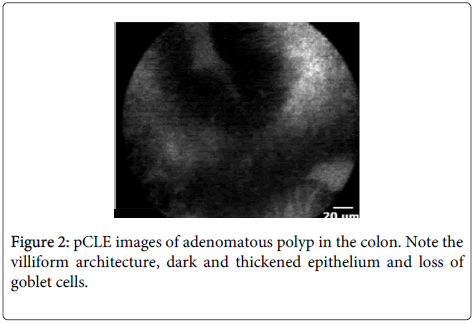
Imaging characteristics: CLE readily identifies mucin-containing goblet cells and columnar epithelial cells within the surface of the colonic mucosa (Figures 1 and 2). Miami classification system for colonic mucosa as follows: Normal colonic mucosa is characterized by round crypt structures, dark goblet cells, regular and narrow vessels surrounding crypts; Adenoma by irregular or villiform structures, dark and irregularly thickened epithelium and decreased goblet cells; Adenocarcinoma by disorganized villiform or lack of structure, dark and irregularly thickened epithelium and dilated vessels.
The Mainz classification [27] was originally reported and used mainly in eCLE. This classification consists of three categories (normal, regenerative or neoplastic) and several studies have demonstrated a high degree of accuracy and interobserver agreement [28-31]. A new classification system based on the Mainz classification has been proposed for pCLE consisting of three vessel categories and seven crypt categories [32] (Table 4). Based on the Mainz pCLE criteria, the interobserver agreements on vessel and crypt architecture were ‘fair’ (?= 0.29 and 0.27, respectively). When the classification was reduced to neoplasia vs. non-neoplasia, overall agreement was ‘moderate’ (kappa 0.56). Overall sensitivity and specificity for predicting neoplasia was 66% and 83%, respectively [33].
| Crypt architecture | |
|---|---|
| Type 1 | Regular luminal openings, size, and distribution of the crypts, covered by a homogeneous layer of epithelial cells, including goblet cells |
| Type 2a | Aggregation/branching of otherwise normal crypts; normal amount of goblet cells |
| Type 2b | Star-shaped luminal crypt openings (narrow lumen) with regular or reduced amount of goblet cells |
| Type 2c | Star-shaped luminal crypt openings (wide lumen) with regular or reduced amount of goblet cells |
| Type 2d | Both aggregation/fusion of regular-shaped crypts and star-shaped luminal crypt openings (combination of type 2a, 2b or 2c) |
| Type 2e | Decreased number of crypts, irregular size of crypts, and irregular distribution of crypts, with regular or reduced amount of goblet cells |
| Type 3 | Variable width of epithelial lining with tubular-shaped crypts and loss of goblet cells (striped dark epithelium); irregular and decreased volume of lamina propria |
| Vessel architecture | |
| Type 1 | Hexagonal, honeycomb appearance that presents a network of capillaries outlining the luminal openings of the crypts |
| Type 2 | Hexagonal, honeycomb appearance with mild (or no) increase in the number of capillaries or increased amounts of normal vessels without leakage |
| Type 3 | Dilated and distorted vessels with elevated leakage; irregular architecture with little or no orientation to adjunct tissue |
Table 4: pCLE-based Mainz classification system for colonic tissue
Diagnostic performance: The first prospective in vivo trial of 69 patients during screening colorectal cancer, eCLE detected the presence of neoplastic changes with high diagnostic yield (97.4% sensitivity, 99.4% specificity, 99.2% accuracy) (26). Similar results of high accuracy in diagnosing intraepithelial colonic neoplasia using the eCLE were followed from other institutions [27,32]. The preliminary in vivo observational study of 13 patients reported the applicability and higher sensitivities and specificities (93% and 92%, respectively) of pCLE for screening colorectal neoplasia [34]. Following prospective comparative study to virtual chromoendoscopy when considering histopathology as gold standard demonstrated that pCLE had higher sensitivity compared to virtual chromoendoscopy (91% and 77%, respectively ; P=0.010) with similar specificity (76% and 71%, respectively ; P=0.77) between pCLE and virtual chromoendoscopy [35]. Taking the consideration of the summarized results of various studies reporting NBI with a sensitivity of 90%-95% and a specificity of 80%-85% for differentiation between neoplastic and non-neoplastic lesions among endoscopists with extensive experience [36-39], this initial prospective study confirmed overall effectiveness of pCLE in classifying colorectal lesions. On the other hand, even though various trials have investigated the role of advanced digital chromoendoscopy and they have been shown to improve the sensitivity in vivo diagnosis of colonic neoplasia, especially with the use of pit pattern characterization, those studies did not eliminate the need for histopathology as gold standard because these techniques were shown to lack specificity. Ideally, small, low-grade neoplastic polyps could be diagnosed definitively by advanced endoscopy without the need for histological confirmation (“in vivo biopsy”), which could reduce substantial cost and delays. One of the disadvantages of pCLE system is difficulty maintaining stabilization of the probe on the surface of targeted lesions during observation along with the very small field of view. One study reported that nearly a quarter of pCLE videos (12% of all lesions) did not demonstrate any crypts or vessels and therefore could not be interpreted, and most of the excluded pCLE images were reported to be made of polypoid lesions [40]. The difference of the reported rate of technical success may be attributed to that of operators’ expertise [35,41]. To overcome this technical issue, using a cap on the tip of the endoscope has been suggested. Systems development to solve technical problems is underway. Other essential element for this new advanced imaging technology is standardized image classification and interobserver agreement. A study from an international collaborate group simplified previously described Miami classification system to differentiate non-neoplasia versus neoplasia [42]. Interobserver agreement was moderate too good for the classification of neoplasia (?=0.55) and in distinguishing between neoplastic and non-neoplastic lesions, sensitivity, specificity, and accuracy were 76%, 72% and 75%, respectively. Of note, when only used better quality images, interobserver agreement for classification of neoplasia was higher (?=0.83), as were sensitivity of 88%, specificity of 89 % and accuracy of 88 %. It is difficult to make direct comparisons of the sensitivities and specificities between eCLE and pCLE. In clinical practice, real-time interpretation during procedure is necessary for decision-making. One study comparing real-time and blinded offline diagnosis tried to address this question and demonstrated that the overall accuracy of real-time pCLE diagnosis (accuracy 79%, sensitivity 81%, specificity 76%) were comparable to that of offline pCLE diagnosis (83%, 88%, and 77%, respectively) [43].
As previous studies indicated a combination of CLE and virtual chromoendoscopy would likely increase overall in vivo diagnostic yield of colon neoplastic lesions, trials on CLE and NBI have been recently reported [44]. The study investigated diagnostic yield for the combined CLE and NBI for 65 polyps with high quality videos assuming histopathology as gold standard, and showed that accuracy, sensitivity, specificity were all high (95%, 94%, 97%, respectively). The majority of studies have been conducted in the tertiary referral center for specialized population and it is operator dependent, time consuming procedure, and needs the learning curve [45]. The room to improve inherent instrumental disadvantages such as difficult maintaining mechanical stabilization of the probe on the target surface, the very small field of view still exists. Currently, a small cap is often used in those studies to improve stabilization but this is not currently used during routinely during colonoscopy. If such techniques are further validated, they have the potential to reduce the risk associated with removal of non-neoplastic small polyps and can make screening or surveillance colonoscopy less costly without compromising on the quality, and could broaden the applicability to community practice settings.
Bile ducts
Clinical context: CLE has been introduced in the management of suspected biliary malignancy, indeterminate biliary stricture and pancreatic cyst. Indeterminate biliary strictures are observed on diagnostic imaging modality with negative cytopathologic evaluation such as brushing cytology and/or endoscopic biopsy and frequentry encountered given the suboptimal accuracy rates of sampling techniques.
Imaging characteristics: In an initial single-center prospective study of patients with indeterminate biliary stricture, CLE characterized benign bile duct by reticular arrangement of dark-grey bands on a light-grey background, in contrast, malignancy by a black/dark-grey-background with irregular large white streaks [46]. CLE visualizes epithelial and subepithelial components and incorporates dynamic information such as blood flow, contrast uptake, and leakage.
Miami classification system for bile duct mucosa as follows: Normal bile duct is characterized by fine, reticular gray pattern; Biliary malignancy by irregular structures interspersed with bright areas of tortuous dilated blood vessel.
The presence of benign inflammatory structuring condition induced false-positive cases resulting in a lower specificity in a following multicenter study based on Miami criteria. One study has recently proposed specific criteria (Paris classification) for the benign inflammatory condition: multiple thin white bands (vascular congestion), dark granular patterns with scales, increased spaces between scales (>20 µm), thickened reticular structure [47]. The Paris classification has not yet validated and further prospective studies are warranted.
Diagnostic performance: An initial single center study reported the diagnostic yield of pCLE for cholangiocarcinoma with an accuracy rate of 86%, sensitivity of 83%, and specificity of 88%, respectively [46], which was confirmed by other single center studies [48]. In a large prospective multicenter study of pCLE for indeterminate pancreatobiliary strictures compared with endoscopic retrograde cholangiopancreatography (ERCP) [49], pCLE demonstrated the sensitivity, specificity, positive-predictive value, and negative-predictive value for detecting cancerous strictures were 98%, 67%, 71%, and 97%, respectively, compared with 45%, 100%, 100%, and 69% for index pathology where ERCP examiners were unblended for the preprocedural patient history and the results of tissue sampling were also obtained in an unblinded manner. Diagnostic accuracy for combination of ERCP and pCLE was significantly higher compared with ERCP with tissue acquisition (90% versus 73%; P=0.001). The same study consortium later reported the standardized terminology adopted for pCLE findings using the Miami Classification criteria [50] for pancreaticobiliary. They demonstrated that any single criteria was not high enough to be sensitive for predicting malignancy however, combining two or more criteria significantly increased the sensitivity and predictive values. Thick white bands (>20 μm), thick dark bands (>40 μm), dark clumps or epithelial structures are most suggestive of malignancy with sensitivity, specificity, positive predictive value, and negative predictive value of 97%, 33%, 80%, and 80%, respectively, and inter-observer variability was moderate for most criteria [51]. Further studies should evaluate the accuracy of pCLE in a blinded manner and assessment of interobserver variation in using the proposed classification.
Pancreas
Clinical context for pancreatic cysts: Another interest of CLE for pancreatobiliary disease is pancreatic cystic lesion. Widespread use of cross sectional imaging has recognized increasing number of pancreatic cysts. The management algorithm relies on cyst fluid analysis including fluid appearance, cytology, tumor marker, chemistry in combination with endosonographic cystic wall characteristics however the yield of accurate diagnosis is suboptimal [52,53].
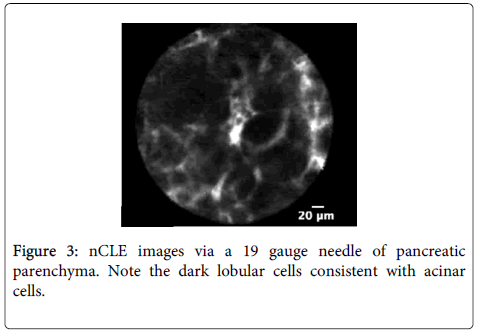
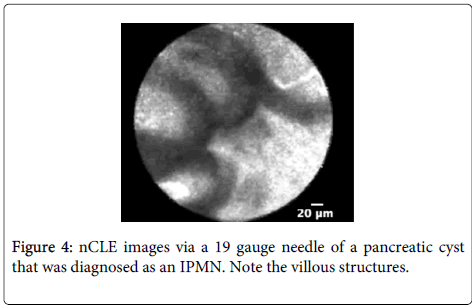
Imaging characteristics: A multicenter trial was conducted to develop criteria for pancreatic cystic neoplasm including intraductal papillary mucinous neoplasm (IPMN) or mucinous cystadenoma (MCA) [54]. CLE described structures in pancreatic parenchyma blood vessels as white bands thin or thick, acinar cells as dark lobular structures, adipose cells as grey ovals ? 60 microns, pancreatic ductal, sub-epithelium as thin grey bands, and fibrous strands ultrathin straight bright bands. Imaging characteristic of IPMN on CLE was reported villous epithelial structures and no other feature was strongly associated with pancreatic cystic neoplasm.
Diagnostic performance: A multicenter pilot in vivo trial to assess the nCLE device in pancreatic cysts to investigate the diagnostic potential of pancreatic cysts has been reported [55]. The presence of epithelial villous structures (Figures 3 and 4) based on nCLE was identified in pancreatic cystic neoplasia (PCN) both in the initial feasibility trial and in the multicenter trial, and provided a sensitivity of 59%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 50% for the diagnosis of PCN.
Adding irregular and dark aggregates of cells which was proposed to be the feature associated with neoplasia as a parameter did not change the sensitivity. The study showed the potential of nCLE to be as an adjunct tool in the current algorithm of PCNs given the high positive predictive value of epithelial structures. Patients identified with villous structures on nCLE may be diagnosed with PCN despite non-diagnostic cytology results of equivocal fluid analysis. Further studies should focus on a wide range of pathologies, image classifications, and validation of the high specificity to apply this modality for clinical decision making in clinical practice.
Conclusion
CLE is a novel optical modality which enables real-time detailed observation of architectural distortions in the gastrointestinal tract. It has been introduced with the intent of increasing diagnostic yield of early gastrointestinal cancers. Though the usage is still limited primarily in research institutions and tertiary referral centers, growing evidence of clinical applicability and technical progress have been reported. CLE alone is still suboptimal to replace standard biopsy protocol for the surveillance of neoplasia in the gastrointestinal tract. American Society for Gastrointestinal Endoscopy (ASGE) developed PIVI (Preservation and Incorporation of Valuable endoscopic Innovation) initiative primarily in order to direct endoscopic technology development and minimize the possibility that potentially valuable innovations are prematurely abandoned. Once endoscopic technologies meet an established PIVI threshold, those technologies are appropriate to incorporate into clinical practice. This is a societal initiative encouraging and supporting new technologies applicable to clinical use [55,56]. These thresholds have been established for Barrett’s esophagus and small colon polyps and can help guide further studies and even guideline development. CLE may be useful for detailed characterization and targeting tissue acquisition particularly when used with a red-flag technique (e.g. digital chromoendoscopy) in upper and lower luminal tract. Furthermore, CLE may provide a surrogate marker for histology where tissue is difficult to obtain in the setting of EUS/ERCP for biliary stricture and pancreatic cystic neoplasia. The controversy of time commitment and cost effectiveness need to be addressed. The current role of CLE for the management of early cancers in the gastrointestinal tract is to compensate for the inherent limitations of endoscopic biopsy and increase efficiency of endoscopy rather than to replace conventional biopsies. Further studies focusing on the reproducible image classification, inter- and intraobserver variability, and learning curve also remain to be warranted.
References
- Wang TD (2005) Confocal microscopy from the bench to the bedside. GastrointestEndosc 62: 696-697.
- ASGE Technology Committee, Kantsevoy SV, Adler DG, Conway JD, Diehl DL, et al. (2009) Confocal laser endomicroscopy. GastrointestEndosc 70: 197-200.
- Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, et al. (2010) The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment PharmacolTher 31: 548-552.
- Becker V, Von Delius S, Bajbouj M, Karagianni A, Schmid RM, et al. (2008) Intravenous application of fluorescein for confocal laser scanning microscopy: evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. GastrointestEndosc 68: 319-323.
- (1997) Microbes in action. Seeley HW, Van Demark PJ, Lee JJ, editors. (4thedn), Macmillan, New York.
- Caulfield MJ, Burleson GR, Pollard M (1979) Ozonation of mutagenic and carcinogenic polyaromatic amines and polyaromatic hydrocarbons in water. Cancer Res 39: 2149-2154.
- Becker V, Wallace MB, Fockens P, Von Delius S, Woodward TA, et al. (2010) Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model. GastrointestEndosc 71: 1260-1266.
- Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, et al. (2011) First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas. GastrointestEndosc 74: 1049-1060.
- Wallace M, Lauwers GY, Chen Y, Dekker E, Fockens P, et al. (2011) Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 43: 882-991.
- Gaddam S, Mathur SC, Singh M, Arora J, Wani SB, et al. (1961) Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrett's esophagus. Am J Gastroenterol 106: 1961-1969.
- Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, et al. (2006) In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. ClinGastroenterolHepatol 4: 979-987.
- Dunbar KB, Okolo P, Montgomery E, Canto MI (2009) Confocal laser endomicroscopy in Barrett's esophagus and endoscopicallyinapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. GastrointestEndosc 70: 645-654.
- Pohl H, Rösch T, Vieth M, Koch M, Becker V, et al. (2008) Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett's oesophagus. Gut 57: 1648-1653.
- Bajbouj M, Vieth M, Rösch T, Miehlke S, Becker V, et al. (2010) Probe-based confocal laser endomicroscopy compared with standard four-quadrant biopsy for evaluation of neoplasia in Barrett’s esophagus. Endoscopy 42: 435–440.
- Wallace MB, Sharma P, Lightdale C, Wolfsen H, Coron E, et al. (2010) Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. GastrointestEndosc 72: 19-24.
- Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, et al. (2011) Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. GastrointestEndosc 74: 465-472.
- Konda VJ, Chennat JS, Hart J, Waxman I (2010) Confocal laser endomicroscopy: potential in the management of Barrett's esophagus. Dis Esophagus 23: 21-31.
- Wallace MB, Crook JE, Saunders M, Lovat L, Coron E, et al. (2012) Multicenter, randomized, controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett's esophagus. GastrointestEndosc 76: 539-547.
- Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, et al. (2009) Confocal laser endomicroscopy for superficial esophageal squamous cell carcinoma. Endoscopy 41: 99-106.
- Pech O, Rabenstein T, Manner H, Petrone MC, Pohl J, et al. (2008) Confocal laser endomicroscopy for in vivo diagnosis of early squamous cell carcinoma in the esophagus. ClinGastroenterolHepatol 6: 89-94.
- Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, et al. (2008) Classification of gastric pit patterns by confocal endomicroscopy. GastrointestEndosc 67: 843-853.
- Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, et al. (2006) Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy 38: 886-990.
- Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, et al. (2006) Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy 38: 1110-1114.
- Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, et al. (2011) Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut 60: 299-306.
- Jeon SR, Cho WY, Jin SY, Cheon YK, Choi SR, et al. (2011) Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. GastrointestEndosc 74: 772-780.
- Bok GH, Jeon SR, Cho JY, Cho JH, Lee WC, et al. (2013) The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia. GastrointestEndosc 77: 899-908.
- Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, et al. (2004) Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127: 706-713.
- Hurlstone DP, Baraza W, Brown S, Thomson M, Tiffin N, et al. (2008) In vivo real-time confocal laser scanning endomicroscopic colonoscopy for the detection and characterization of colorectal neoplasia. Br J Surg. 95: 636-645.
- Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, et al. (2007) Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. ClinGastroenterolHepatol 5: 1235-1241.
- Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, et al. (2007) Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 132: 874–882.
- Sanduleanu S, Driessen A, Gomez-Garcia E, Hameeteman W, de Bruïne A, et al. (2010) In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. ClinGastroenterolHepatol 8: 371–378.
- Kuiper T, van den Broek FJ, van Eeden S, Wallace MB, Buchner AM, et al. (2011) New classification for probe-based confocal laser endomicroscopy in the colon. Endoscopy 43: 1076-1081.
- Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, et al. (2005) A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. GastrointestEndosc 62: 686-695.
- Meining A, Saur D, Bajbouj M, Becker V, Peltier E, et al. (2007) In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. ClinGastroenterolHepatol 5: 1261-1267.
- Buchner AM, Shahid MW, Heckman MG, Krishna M, Ghabril M, et al. (2010) Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology 138: 834-842.
- Rex DK (2009) Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology 136: 1174–1181.
- Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, et al. (2007) A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut 56: 373-379.
- East JE, Suzuki N, Saunders BP (2007) Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study GastrointestEndosc 66: 310-316.
- Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, et al. (2006) Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol 101: 2711-2716.
- Kuiper T, van den Broek FJ, van Eeden S, Fockens P, Dekker E (2012) Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol 107: 543-550.
- Buchner AM, Gomez V, Heckman MG, Shahid MW, Achem S, et al. (2011) The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. GastrointestEndosc 73: 556-560.
- Gómez V, Buchner AM, Dekker E, van den Broek FJ, Meining A, et al. (2010) Interobserver agreement and accuracy among international experts with probe-based confocal laser endomicroscopy in predicting colorectal neoplasia. Endoscopy 42: 286-291.
- Shahid MW, Buchner AM, Raimondo M, Woodward TA, Krishna M, et al. (2012) Accuracy of real-time vs. blinded offline diagnosis of neoplastic colorectal polyps using probe-based confocal laser endomicroscopy: a pilot study. Endoscopy 44:343-348.
- Shahid MW, Buchner AM, Heckman MG, Krishna M, Raimondo M, et al. (2012) Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: a feasibility study Am J Gastroenterol 107: 231-239.
- Kuiper T, Kiesslich R, Ponsioen C, Fockens P, Dekker E (2012) The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. GastrointestEndosc 75: 1211-1217.
- Meining A, Frimberger E, Becker V, Von Delius S, Von Weyhern CH, et al. (2008) Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. ClinGastroenterolHepatol 6: 1057-1060.
- Caillol F, Filoche B, Gaidhane M, Kahaleh M (2013) Refined Probe-Based Confocal Laser Endomicroscopy Classification for Biliary Strictures: The Paris Classification. Dig Dis Sci 58: 1784–1789.
- Giovannini M, Bories E, Monges G, Pesenti C, Caillol F, et al. (2011) Results of a phase I-II study on intraductal confocal microscopy (IDCM) in patients with common bile duct stenosis. SurgEndosc 25: 2247-2253.
- Meining A, Chen YK, Pleskow D, Stevens P, Shah RJ, et al. (2011) Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. GastrointestEndosc 74: 961-968.
- Wallace M, Lauwers GY, Chen Y, Dekker E, Fockens P, et al. (2011) Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 43: 882-891.
- Meining A, Shah RJ, Slivka A, Pleskow D, Chuttani R, et al. (2012) Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy 44: 251-257.
- Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, et al. (2006) International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6: 17-32.
- Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, et al. (2012) International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12: 183-197.
- Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, et al. (2013) A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 45: 1006-1013.
- Sharma P, Savides TJ, Canto MI, Corley DA, Falk GW, et al. (2012) ASGE Technology and Standards of Practice Committee. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett's Esophagus. GastrointestEndosc 76: 252-254.
- Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, et al. (2011) The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. GastrointestEndosc 73: 419-422.
Citation: Tomizawa Y, Konda VJA (2014) Confocal Laser Endomicroscopy – The Potential Role of In Vivo Characterization of Neoplasia in the Gastrointestinal Tract. J Clin Exp Pathol 4:181. DOI: 10.4172/2161-0681.1000181
Copyright: © 2014 Tomizawa Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14820
- [From(publication date): 9-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 10211
- PDF downloads: 4609