Comprehensive Utilization of High Alumina Coal Fly Ash Under the Recyclable Economy Model
Received: 02-May-2018 / Accepted Date: 27-Jun-2018 / Published Date: 02-Jul-2018 DOI: 10.4172/2469-9764.1000128
Abstract
The latest research progress on the utilization of fly ash with high concentration alumina is summarized in this paper. High-alumina fly ash has been accidentally found in central and western regions of Inner Mongolia in recent years. The high content of Al2O3 (up to 50%) and the enrichments of some useful elements, such as gallium, titanium and light rare earth make it a valuable renewable resource. A comprehensive technique route utilizing fly ash was researched and developed. After the summarize of properties of fly ash with high concentration alumina, the main methods of utilization are introduced: sintering, acid, alkali, acid and alkali combination method. The processes, productions of the four methods and the research status are described. Advantages and disadvantages of various methods are pointed out and corresponding solutions are proposed.
Keywords: High-alumina fly ash; Comprehensive utilization; Alumina; Recyclable economy
Introduction
The distribution of the world's bauxite resources is uneven, Highgrade bauxite resources are mainly distributed in Australia, and other countries. From the geological origin analyzes, bauxite resources presence is associated with the coal resources, mainly for aluminum resources enrichment in coal gangue and kaolin which, on the other hand, due to the large power consumption of aluminum process, the production of aluminum is matched with energy.
Fly ash, generated during the combustion of coal for energy production, is an industrial by-product. The annual production of fly ash worldwide is about 500 million tones at 75-80% of the total ash produced in 1997 year [1]. The present-day utilization of ash on worldwide basis varied widely from a minimum of 3% to a maximum of 57%, yet the world average only amounts to 16% of the total ash [1]. The increasing disposal of the large amount of fly ash has taken up many valuable agricultural land, cause human and plant health problem [2]. Because of the environmental problems caused by the fly ash, considerable research has been undertaken on the subject worldwide. Fly ash typically contains silica (30 ~ 45%), alumina (25 ~ 52%), magnetite, Fe2O3 (6 ~ 15%). Currently, the utilization of fly ash mainly focused on construction materials as a low-cost adsorbent for the removal of organic compounds and zeolite synthesis [3-5].
Coal is China's major consumer of energy, accounting for more than the proportion of China's primary energy consumption by 75%. Although China was developing wind, nuclear, biomass and other emerging energy industry in recent years, but China's energy consumption structure will not occur essentially unchanged in a fairly long period. China's coal is mainly used for coal-fired power plants, accounting for about 70% of coal consumption, and this date is expected will account for 50% in 2030 [6-8].
With the advancement of technology and the increasing scarcity of bauxite, alumina and other aluminum products extract from other sources rather than bauxite become a new trend [9]. The Soviet Union because of political reasons were purchased bauxite embargo in seventies of last century, under this condition development use fly ash and coal waste as raw material to limestone sintering produce alumina (aluminum hydroxide) and cement technology, and in Poland were tested plant. Chinese coal fly ash is rich in Al2O3, which content was more than three times than bauxite resources. China is launch a new round craze of extracting alumina from fly ash, which mainly Datang International Power Generation Co., Ltd., the company developed a fly ash pre desilication soda lime sintered produce alumina technology and achieved industrial production, there are some other Chinese enterprises and research institutions, the ongoing develop utilization fly ash extraction aluminum with a variety of process route.
This paper focus on fly ash which exists in Inner Mongolia, Shanxi and other areas of China, based on analysis it`s composition and nature, the research status of silica and alumina extracted from this kind of fly ash were analyzed, and pointed out that the author views about existing problems and proposed development direction.
The Nature and Application of High Alumina Coal Fly Ash
Formation and properties of high-alumina fly ash
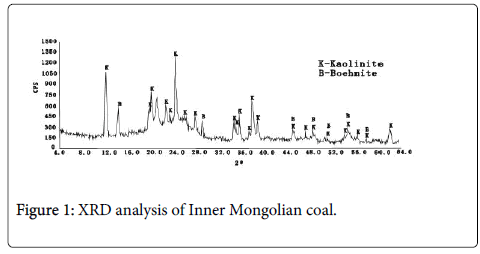
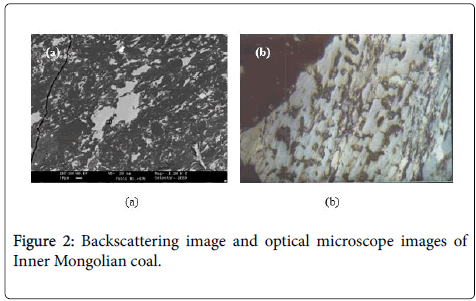
Paragntic properties of coal, aluminum and gallium in central and western regions of Inner Mongolia coal field. Minerals in coal are precursor of fly ash, and their composition and distribution characteristics highly influence the physical and chemical properties of fly ash (Figures 1 and 2).
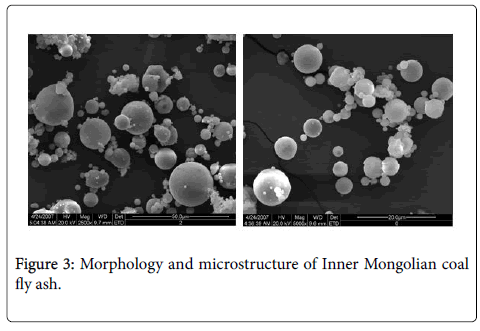
The XRD results of Inner Mongolian coal (Figure 1) showed that the main minerals were Kaolinite and Boehmite. The contents of Kaolinite and Boehmite were 27.2% and 8.7%, respectively. From the backscattering image (Figure 2a), lumpy aluminum minerals can be observed apparently. these aluminum minerals were also dispersed in cell cavities from the optical microscope image (Figure 2b). Some rare elements, such as gallium in the form of Al-Ga symbiotic mineral, also rich in central and western regions of Inner Mongolia coal field according to the recent researches from China University of Mining. Unlike the normal fly ash, most of these particles are merely partial fused and higher viscosity (Figure 3). Because of the effect of melt surface tension, many glass microballons were formed with the shape of nearly spherical and other irregular particles.
Nature of high alumina coal fly ash
Coal fly ash is a product of coal combustion, the nature of coal fly ash depends on the coal species, fineness of pulverized coal, combustion method, and the collection and transport of fly ash and another factors manner. Coal fly ash properties including physical properties, chemical properties and the nature of the phase structure.
The physical properties of the coal fly ash mainly comprise particle size distribution, density, surface area, etc. The particle size distribution of fly ash related coal fly ash sources and power plant equipment. There is no essential difference between high alumina fly ash and the general fly ash in the physical properties such as the particle size, etc., generally the range within 10-100 μm particles density, surface area and other properties can refer to the nature of general coal fly ash [10].
The chemical composition of coal fly ash include silica, alumina, iron oxide, calcium oxide, and magnesium oxide, etc. Usually silicon oxide content is between 20-60%, alumina content is 5-35% [11]. One of the main characteristics of high alumina fly ash is high alumina content of about 40%, up more than 48-50% [12]. Elements contained of Oxides in the high alumina fly ash is single, silica, alumina content accounted for more than 80% of total, the remaining is small amount of iron oxide, titanium oxide, calcium (magnesium), unburned carbon and some trace elements [13]. The minerals in coal include silicates, oxides, carbonates, etc., the formation is influence due to the chemical composition of pulverized coal, the combustion conditions, the cooling process, etc., the difference fly ash is presence big different on phases [14].
Coal fly ash is composite structure mixture consisting crystalline, vitreous and a small amount of unburned carbon. the vitreous is accounted the major part of the fly ash, but the crystalline substances sometimes are higher, accounting for 11-48%. The crystalline material mainly includes mullite and quartz, in addition to magnetite, anhydrite, tricalcium aluminate [15]. The oxides vitreous form is SiO2, Al2O3, Fe2O3(Fe3O4), CaO, MgO, Na2O [16].
Through the analysis of several fly ash from China, it displays whose main phase are mullite and vitreous, and containing a few other phase compositions [17].
Utilization of high aluminum fly ash resources
Currently, classification and application of fly ash is usually use the U.S. ASTM C618 standards, the different physical and chemical properties fly ash has its different application areas, mainly for building materials, such as fly ash used in the manufacture of cement, mixed with concrete, manufacturing brick, ceramic, etc., It can also be used in agriculture, forestry, sewage treatment and other industries [18].
In some of the developed regions of China, such as Jiangsu province, Shanghai and other places, the utilization of fly ash up to 100% [19]. However, the coal-producing areas in China, such as Shanxi, Inner Mongolia and other places, which concentrated large-scale power plants, emissions of fly ash cannot be achieved using completely by existing technology, it most still stockpiling by damming the way, lead to local enormous economic and environment pressure [20]. A considerable part of the coal fly ash from these areas has the unique characteristics, its high alumina and silica content, composition is relatively single, it offers the possibility that utilization as an aluminous and siliceous resource. China's various research institutes and enterprises focus on this kind of fly ash in recent years, separation and extraction of alumina and silica become a research hotspot, and gradually expand the industrial experiment, get the different chemical products, the main methods used by the Pre-desilication soda lime sintering process, sintering method of limestone, digestion acid, acid and alkali combination method etc.
The Main Utilization Process of High Alumina Fly Ash
Pre-desilication soda lime sintering method
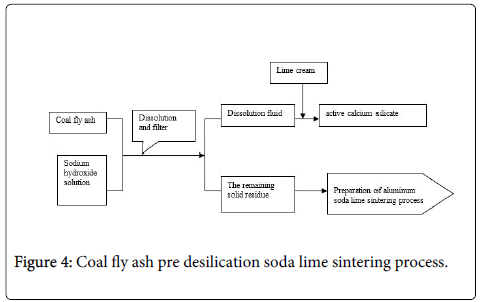
Pre-desilication soda lime sintering process using sodium hydroxide solution mixed with coal fly ash, dissolve silica under certain conditions to obtain sodium silicate solution, after lime causticization reaction get the active calcium silicate byproduct (Figure 4). The remaining solid residue can be used as a base raw material to extraction alumina by lime sintered method. The main process route is as follows:
The basic chemical reaction is as follows:
SiO2+NaOH → Na2SiO3+H2O
Na2SiO3+Ca(OH)2 → CaSiO3+NaOH
Sun Junmin and other extraction silica successfully using the process shown in Figure 1 and preparation active calcium silicate byproduct. Get the higher alumina/silica remaining solid residue, it can be extracted alumina by using soda lime sintering.
The basic process is: Mass concentration of 40% NaOH solution with a quantity of fly ash at 80-150°C reaction conditions, in order to obtain the sodium silicate solution and the corresponding alkali dissolution solid ash. The preparation of active sodium silicate can be achieved by the process of sodium silicate solution lime causticization. The resulting solid ash A/S is improved to 2 or more, it can use soda lime sintering process to extraction alumina.
Guodong [21] studied conventional pressure, pressure and microwave three kinds of fly ash alkaline elution methods and investigated the factors of silicon and aluminum dissolution rate during the reaction of fly ash and alkali. Include heat treatment temperature, dissolution time, dissolution temperature, alkali concentration, liquid-solid ratio. Then studied the microstructure of fly ash before and after treatment. Zhan-jun [22] used geology research methods to study the high aluminum fly ash formation and properties, also studied substances evolution regular pattern and the inherent mechanism which its follow-up process to extract alumina material. Lay the foundation for the development of the fly ash utilization technologies route. It also uses alkali soluble ash sintering method to achieve the extraction of silica and alumina. The presence of sodium silicate and sodium aluminate solution were simultaneously in said process, and therefore, can achieve synthesis in the process of molecular sieve products which achieve diversification [23]. While using pre-desilication soda lime fly ash sintering method to achieve the extraction of silica and alumina. The presence of sodium silicate and sodium aluminate solution were simultaneously in said process, and therefore, it can synthesis molecular sieve products which achieve diversification [24].
The main objective of alkali-soluble is to achieve the separation silicon from fly ash, and get higher A/S solid ash, conducive to the subsequent treatment process. Using alkali-soluble methods cannot be achieved silica separation completely, generally the separation is up to about 50%, the remaining solid residue A/S can reach up to 2.39 [25,26]. Most studies suggest that the impact of the extraction rate increased due to the mullite form part exist in the fly ash not dissolution.
It can take advantage the Na2O in the remaining solid residue filtered after the high alumina fly ash by pre-silicon desilication treatment. In order to save alkali consumption in subsequent extraction of alumina. while the subsequent soda lime sintering process and related equipment are available for mature reference industrialized achieve a smaller degree of difficulty. However, A/S is an important factor to affect the running costs, how to further improve the removal rate of silicon, obtain a high aluminum-silicon ratio remaining solid residue will be an important research direction. At the same time, it should also be pay attention to the problem that may bring secondary slagging pollution.
Limestone sintering method
The alumina of high alumina coal fly ash is mainly in the mullite phase. The basic principle of limestone sintering method is: By hightemperature sintering reaction, mullite and quartz were converted into seven twelve calcium aluminates and dicalcium silicate, then using sodium carbonate dissolved the sodium aluminate solution to achieve the separation of silicon and alumina. Sintering and dissolution process includes the following chemical reactions:
CaCO3 → CaO+CO2
SiO2+2CaO → 2CaO·SiO2
7(3Al2O3·2SiO2)+64CaO →3(12CaO·7Al2O3)+14(2CaO·SiO2)
12CaO·7Al2O3+12Na2CO3+5H2O →14NaAlO2+12CaCO3+10NaOH
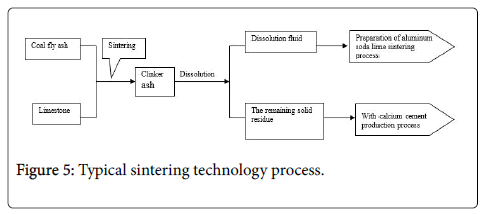
Erin Liu et al. [27] use limestone sintering process combined production alumina and cement, and the industrialization experiment was prepared sandy alumina products. The basic process shown in Figure 2. Fly ash and limestone mixing and then make high temperature sintering conditions at 1390-1440°C, raw material saturation ratio at 0.6-1.2. The resulting clinker A/S is 0.65-1.1, clinker saturation ratio of 0.65-1.1. The resulting clinker elution with sodium carbonate solution at 60-80°C keeping 15-50 min, and then solidliquid separation, after separating off the crude liquid go through desilication, carbonization, calcination and other processes to get the alumina product. Solid calcium silicon slag produced by washing, ingredients, grinding, calcination processes to get cement product.
Limestone sintering, alumina dissolution rate of more than 82%, Self-powdering up to 100% [28]. Limestone sintering process using in the formulation of dry ingredients, directly sintered using a rotary kiln, and after sintering, clinker can powder itself, no additional additional crushing, grinding and other processes. However, handling the calcium silicate slag was a problem, although it can produce cement with calcium, but there are a limited radius of cement sales, marketing and other issues cannot be fully digested it (Figure 5).
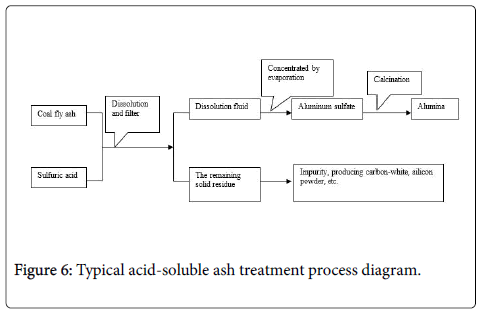
Acid dissolution method
Acid dissolution method mainly uses sulfuric acid or hydrochloric acid, the dissolution of the fly ash under certain conditions, get the corresponding aluminum salt solution, achieve the separation of silicon and alumina (Figure 6). For example, sulfuric acid, its main process is as follows:
The process includes the following chemical reactions:
Al2O3+3H2SO4 → Al2(SO4)3+3H2O
Al2(SO4)3 → Al2O3+3SO3
Jinguo [29] use concentrated sulfuric acid and fly ash reaction under certain conditions, obtain an aluminum sulfate solution and further calcined alumina. The basic process is: the fly ash was ground to 200-400 mesh, calcined at 300 ~ 760°C and activation 1 ~ 1.5 hours; Then fly ash and 60% ~ 98% H2SO4 solution in a weight ratio of 1:1 ~ 6 were mixed and heated to 160 ~ 330°C for 1 ~ 1.5 hours, the filter separated the residual acid and the filtration residue reactant; Residue was added 2.5 ~ 5 times amount of water in the 65 ~ 90°C boiling solution of 30 ~ 45 minutes, the reaction was eluted, the residue was removed by filtration, the filtrate was concentrated by evaporation after cooling, precipitated crystals of aluminum sulphate.
Aluminum sulfate crystallization elevated temperature dehydration obtain anhydrous aluminum sulfate; continue heating up the anhydrous aluminum sulfate decomposition γ-Al2O3 and recovered SO3 fumes.
Shen [30] improvements on the basis of the original process, reducing the amount of H2SO4, significantly the proportion of 1:1 ~ 2:1, and then calcined at 200-400°C conditions, then crushed and dissolution. This method reduces the sulfuric acid consumption, water consumption and energy consumption, reducing the cost of production significantly.
Ash is mostly spherical beads, during the reaction with sulfuric acid, the surface of fly ash takes shape large amounts of radioactivity needle lamellae, the after-elution measurement results show, aluminum, iron and other elements conversion into a soluble sulfate salt form, in the washing process to achieve separation of the aluminum-silicon, aluminum extraction rate can reach 80-85%.
The remaining solid residue mainly component is silicon dioxide, also contain small amounts of alumina, iron oxide and so on. After a simple cleaning and calcined can obtained carbon-white, silicon powder and other products.
By acid dissolution method can effectively achieve the separation of Al-Si, the extraction rate of alumina is high, remaining high silicon content of residual ash can also be recovery and utilization effectively, almost no residual slag exists. However, acid treatment of fly ash also has some industrialized bottlenecks. First, the problem of acid corrosion the equipment, the course of the acid-soluble reaction using the high concentration sulfuric acid, it has corrosion problems when the storage and transportation process. Second, the acid roasting generated SO2 make pollution problem during the reaction and subsequent process of aluminum sulfate calcinations, it will produce a lot of acid vapor and SO2 gas, the problem of recycling these gasses also need to be concerned. Furthermore, the presence of aluminum sulfate solution impurity is also a problem. During the Acid dissolution process, iron, titanium and other impurities are also converted into the solution as form of sulfate, affect the quality of the final alumina product.
Acid alkaline combined method
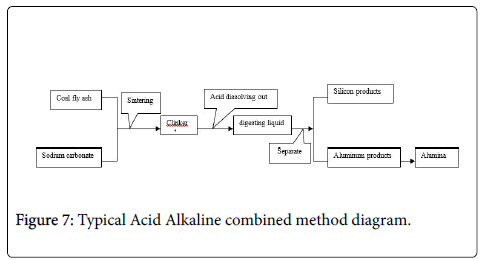
Acid alkaline combined method using coal fly ash and sodium carbonates evenly mixed, calcined a certain time under the medium temperature, and then use hot water or acid soluble out the alumina silicon mixture dissolution (Figure 7).
Then use acid precipitated the silicon and aluminum step by step to achieve separation. The main process line shown in Figure 4.
Pan et al. [31] using the following process to achieve separation of alumina and silicon, obtained alumina and silicone products: (1) After grinding the fly ash mixed with Na2CO3 by weight percentage that fly ash: sodium carbonate ratio=1:0.5 ~ 5, calcined at 600°C ~ 1000°C of 0.5 ~ 2 hours. (2) A amount of water added to the calcined product, the percentage by weight that sintered material: water=1:50 ~ 200, extraction at 60°C ~ 95°C temperature for 0.5 hour ~ 2 hours to obtain a filtrate after filtration. (3) Filtrate carbon two hours after filter to obtain H2SiO4 and Al (OH)3 mixture. (4) Added a certain concentration industrial hydrochloric acid in the mixture, leach at a temperature of 50°C ~ 90°C for 20 ~ 60 minutes, then filtered under conditions that pH value less than 3.0, obtained silicone solids and filtrate. (5) The filtrate was further processed to obtain NaAlO2, then carbonization, filtered to obtain Al (OH)3, calcinations at a temperature conditions of 800 ~ 1200°C keeping 1 ~ 3 hours to obtain ultrafine alumina powder, the resulting silicone solid residue purified and separated to get pure silicone residue.
There are several other researchers have been studied acid alkaline combined method extraction silica and alumina [32-34], the basic idea is dissolution after calcination make the silicon oxide and aluminum oxide transferred into the liquid phase, then adjusting the pH distribution precipitation achieve separation of silicon and aluminum and impurities. removing.
Acid Alkaline combined method almost consumed residue completely, achieved the maximum degree of reduction of fly ash and resource utilization. However, this method is generally a long process, require a higher degree of precision control, the use of acid- alkaline appears certain futile cycles may cause high costs of industrialization and other issues.
Conclusion and Outlook
By Pre-desilication soda lime sintering method, Limestone sintering method, Acid dissolution method, Acid Alkaline combined method extract silica and alumina is the research focus of Chinese high alumina fly ash utilization currently. Different methods have their advantages and disadvantages. To sum up the main as follows: (1) Predesilication soda lime sintering method can be obtained a higher A/S ash on the basis of calcium silicate products, it can borrow, bauxite soda lime sintered treatment, this technology is mature and reliable, the main application bottleneck problem of the method is the A/S further improvement and the alkaline pollution in secondary slagging. (2) Limestone sintering method achieved a co-production of extracted aluminum and cement, the process is simple, and the equipment is sophisticated, but there are problems with excessive calcium and large cement production. (3) Acid dissolution method can effectively achieve the silicon and aluminum separation and utilization, and the small amount of waste, the effective realization of reduction. However, this method proposed test for equipment selection and runs, while should responding to acid gases, corrosion and other problems to pay close attention. (4) Acid Alkaline combined method can make fly ash achieved minimization and recycling effectively, but the process is more longer, and the control requirements is high, should further strengthen study on the process, control parameters, operating costs and other aspects. In addition, there are still basic research is weak at the process research of refinement utilization, application technology and equipment research inadequate, estimated shortage on the environmental benefits evaluation and secondary negative pollution impacts.
In summary, the research of high alumina fly ash utilization conducts active in recent years, especially using a variety of process route to achieve the alumina and silicon separation and extraction, obtain different chemical products. Although there are still some problems in terms of technology, equipment, etc. But the corresponding industrialization work has been started. The fly ash as silicon and aluminum resources will gradually break through technical barriers and solution, the resulting social, environmental and economic benefits will become increasingly apparent.
References
- Joshi RC, Lothia RP (1997) Fly ash in concrete: production, properties and uses. In: Advances in concrete technology, Gordon and Breach Science Publishers, Amsterdam, Netherlands 2: 269.
- Ahmaruzzaman M (2010) A review on the utilization of fly ash. Progress in Energy and Combustion Science 36: 327-363.
- Ge XL, Zhai JW, Feng YL (2012) The Comprehensive Utilization of Coal Fly Ash[J]. Advanced Materials Research 347-353: 1362-1365.
- Iyer RS, Scott JA (2001) Power station fly ash - a review of value-added utilization outside of the construction industry. Resour Conserv Recycl 31: 217-228.
- Matheswaran M, Karunanithi T (2007) Adsorption of chrysoidine R by using fly ash in batch process. J Hazard Mater 145: 154-161.
- Gupta VK, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45: 1446-1453.
- Sun JM, Chen P (2013) Resourcing Utilization of High Alumina Fly Ash. Advanced Materials Research 652-654: 2570-2575.
- Dizge N, Aydiner C, Demirbas E, Kobya M, Kara S (2008) Adsorption of reactive dyes from aqueous solutions by fly ash: kinetic and equilibrium studies. J Hazard Mater 150: 737-746.
- Thepparat K, Pravasant P, Navadol L (2010) Synthesis of Zeolite from Coal Fly Ash: Its Application as Water Sorbent. Engineering Journal 14: 37-43.
- Querol X, Moreno N, Umana JC, Alastuey A, Hernandez E, et al. (2002) Synthesis of zeolites from coal fly ash: An overview. Int J Coal Geol 50: 413-423.
- Yang S, Lin L, Li SP, Li Q, Wang XT, et al. (2017) Assessment and comparison of three high-aluminum fly ash utilization scenarios in Inner Mongolia, China using an eco-efficiency indicator. Waste Management & Research the Journal of the International Solid Wastes & Public Cleansing Association Iswa 35: 515-524.
- Koukouzas NK, Zeng R, Perdikatsis V, Xu W (2006) Mineralogy and geochemistry of Greek and Chinese coal fly ash. Fuel 85: 2301-2309.
- Sibanda V, Ndlovu S, Dombo G, Shemi A, Rampou M, et al. (2016) Towards the Utilization of Fly Ash as a Feedstock for Smelter Grade Alumina Production: A Review of the Developments. Journal of Sustainable Metallurgy 2: 167-184.
- Yu C, Li H, Jia X (2015) Heavy metal flows in multi-resource utilization of high-alumina coal fly ash: a substance flow analysis. Clean Technologies & Environmental Policy 17: 757-766.
- Ahmaruzzaman M (2010) A review on the utilization of fly ash. Progress in Energy and Combustion Science 36: 327-363.
- Zhang Z (2007) Extract of alumina and other useful resources from high alumina fly ash. Xi'an: Northwestern University.
- Shao L, Chen J, Shi Y, Lu J (2007) Minerals in feed coal and their contribution to high-alumina fly ash in the Jungar Power Plant. Coal Society 32: 411-415.
- Dash B, Das BR, Tripathy BC, Das SC (2008) Acid dissolution of alumina from waste aluminium dross. Hydrometallurgy 92: 48-53.
- Han H, Jiang T (2001) Fly ash utilization technology. Beijing: Chemical Industry Press.
- Qian J, Wu C, Wang Z (2001) Fly ash mineral composition (a) fly ash utilization, pp:Â 26-31.
- Wu G, Peng K (2005) Application of Microwave-Heating by Alkaline Solution to Leach Silicon and Aluminum from Fly Ash. Modern Scientific Instruments.
- Zhang Z, Sun J, Yao Q (2016) Research on the extraction of amorphous SiO2 from high-aluminium fly ash. Acta Mineralogica Sinica 27: 137-142.
- Cao DZ, Selic E, Herbell JD (2008) Utilization of fly ash from coal-fired power plants in China. Journal of Zhejiang University-Science A 9: 681-687.
- Bai G, Shen B, Qin J (2008) Study on the surface micropography and chemical composition change in extracting aluminum from high-aluminum fly ash. Coal Conversion 31: 71-74.
- Bai G, Xu P, Qin J (2009) One kind of 4A zeolite production methods: China CN200810080297.1.
- Pan A, Yang S, Ma R (2008) The method of high purity alumina and silica gel extraction from fly ash: China CN200810017869.1.
- Liu A, Zhao J, Wu S (2005) The method using fly ash and limestone joint production of alumina and cement: China, CN200410090949.1.
- Sun P, Li G, Tong J (2007) Study on sintering process of raw materials in ectracting alumina from fly ash of coal industry power plate. Journal of China Coal Society 32: 744-747.
- Qin J, Zhai Y (2011) Extraction of Alumina from Coal Fly Ash with Sulfuric Acid Leaching Method. The Chinese Journal of Process Engineering 11: 254-258.
- Shen B (2008) Study on sulfate extraction of aluminum from fly ash [D]: Xi'an University of Architecture and Technology.
- Pan A, Yang SB, Ma R (2008) Method for extracting high purity alumina from fly ash and silica gel: China, CN200810017869.1.
- Ma H, Yang J, Wang Y (2007) The use of high alumina fly ash production of alumina and silica clean production process: China, CN 101041450A.
- Ji H, Ma Y, Wu P (2007) Study of activating process of extracting high-purity nano-alumina from fly ash[J]. Environmental Chemistry 26: 448-451.
- Zhao P, Li H, Lin W (2007) A method for extraction alumina and waste slag to produce cement from high alumina fly ash: China, CN200710017304.9.
Citation: Peng X, Tonglin Z (2018) Comprehensive Utilization of High Alumina Coal Fly Ash Under the Recyclable Economy Model. Ind Chem 4: 128. DOI: 10.4172/2469-9764.1000128
Copyright: © 2018 Peng X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5072
- [From(publication date): 0-2018 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 4195
- PDF downloads: 877