Compliance of Maize Flour Brands in Nairobi County to Food Fortification Standards and Minerals Bioaccessibility in Ugali Made from Fortified Maize Flour
Received: 05-Sep-2022 / Manuscript No. SNT-22-73779 / Editor assigned: 07-Sep-2022 / PreQC No. SNT-22-73779 (PQ) / Reviewed: 21-Sep-2022 / QC No. SNT-22-73779 / Revised: 21-Mar-2023 / Manuscript No. SNT-22-73779 (R) / Published Date: 28-Mar-2023
Abstract
Micronutrient deficiencies remain a public health challenge in Kenya with high deficiencies in iron and zinc. Food fortification is one of the interventions used to alleviate micronutrient deficiencies. However, the bio accessibility of the micronutrients in fortified foods is key in determining the success of the intervention. The objective of this work was to determine compliance of commercially milled maize flour brands in Nairobi County to national fortification standards and bio accessibility of minerals from fortified maize flour. Ninety six maize flour samples were randomly collected from Nairobi County and sorted to remove duplicate samples yielding 52 analytical samples. The samples were analyzed for zinc and iron content using atomic absorption spectroscopy, while vitamins were determined using high performance liquid chromatography. Samples are considered compliant if they meet the national standards in any two of the three indicator micronutrients (Iron, Zinc and Vitamin A). An in vitro digestion protocol was used to determine mineral bio accessibility. From the analysis, 26.9%, 57.7% and 26.9% of the samples complied with fortification standards for zinc, iron and vitamin A, respectively. The compliance levels for the B vitamins ranged from 34.5% to 100%. The bio accessibility for iron ranged from 45.1% to 52.2% while that for zinc ranged from 12.8% to 22.3%. There was significant difference in bio accessibility based on the mineral type (p<0.0001). Ascorbic acid levels didn’t have any effect on the bio accessibility of either mineral (p=0.5794) and neither was there any interaction between the type of mineral nor the ascorbic acid levels (p=0.8333). Compliance to the national food fortification standards is low despite efforts by both government and private sector players. The bio accessibility of iron was higher than of zinc 2 folds-3 folds. The addition of ascorbic acid had no significant effect on the minerals bio accessibility.
Keywords: Micronutrient deficiencies; Fortification; Compliance; Bio accessibility; In vitro digestion; Ascorbic acid
Introduction
Worldwide micronutrient deficiencies are estimated to affect over a third of the population resulting in delayed growth, increased susceptibility to ill health and a lowering of the cognitive capacity of the affected population [1]. In Kenya, micronutrient malnutrition is of great public health concern with iron and zinc deficiency levels being high in children and women. Over twenty percent (21.8%) of preschool children and 36.1% of pregnant women are iron deficient, respectively while 83.3% of pre-school children and 82.3% of nonpregnant women are zinc deficient . Ugali a form of thick porridge made from maize flour is a common delicacy consumed across the County. Compared to other foods, Ugali is a highly regarded and consumed as the main source of calories and other nutrients for the Kenyan population as well as for economic reasons compared.
Insufficient intake of micronutrients leading to deficiencies is a public health concern worldwide. Deficiencies in vitamin A, iron, iodine and zinc have been reported on a global scale while other micronutrient deficiencies have been reported at regional levels. Zinc and iron deficiencies are majorly attributed to monotonous diets lacking in these minerals and inadequate intake of the same minerals from foods taken by the general population [2]. A more likely cause for the deficiencies is poor bioavailability of zinc and iron from dietary sources, especially plant based foods.
Food fortification is one of the most effective means of fighting micronutrient deficiencies and has been widely adopted all over the world [3]. In order for this to be more effective in terms of improving human health as a result of minimizing the effects of micronutrient malnutrition, fortification of staple foods is practiced as it has a wider population reach.
In Kenya, food fortification is mandatory (Food, Drugs and Chemical Substances Act, 2015) with four food vehicles of salt, maize flour, wheat flour and cooking oils/fats being used as channels for improving uptake of various micronutrients of public health concern [4-6]. Fortification of maize flour with various micronutrients can result in an improved nutrition profile for the maize flour which has an effect on the micronutrient intake of populations reliant on maize as a staple food. The nutritional improvement of maize flour during fortification is critical during the assessment of nutrient intake of populations consuming a large proportion of their daily meals as products made from maize flour. Other than food fortification, the Kenyan government implements other strategies such as supplementation, dietary diversification and other public health measures such as malaria control, deworming and hygiene promotion [7].
Quality food fortification data collection with regards to compliance to the set national standards is a universal challenge for large scale fortification implementing countries with a key consideration of the fortification program being the bioavailability of the added fortificants [8]. Sodium Iron Ethylenediaminetetraacetate (NaFeEDTA) is the preferred form of iron used in the premix for maize flour fortification in Kenya (Food, Drugs and Chemical Substances Act, 2015). Bioavailability of NaFeEDTA has been shown to be higher compared to that of ferrous sulphate (reference point for iron bioavailability in foods) in cereal diets with high phytate content and similar in low extraction flours. Micronutrients affect nutritional status only to the extent at which the body is able to absorb them from a food matrix. The absorption of the essential minerals such as iron and zinc from the fortified foods is dependent on the form of micronutrient in the fortificant and is also affected by other food components such as phytates [9-11]. Compliance with food fortification standards is determined through analysis of foods for the micronutrients of interest while regulatory monitoring which can include internal monitoring at food industry, external checks by government food control agencies through factory audits, import checks by government agencies and, market level surveillance by government food control agencies are used to track progress.
Data on compliance levels to food fortification standards in Kenya is scanty given that not many studies or surveys have been done in the recent past to ascertain compliance levels. In order to frequently check whether the food industry is providing to the consumers well-fortified maize flour, it is important for market and industry surveillance exercise to be conducted. Studies regarding bio accessibility of minerals in fortified staple foods in Kenya are also limited. Review of available evidence on the effect of fortifying maize flour with iron on reduction of the risk of anemia concluded that it was uncertain that the fortified maize flour resulted in positive outcomes in children above 2 years of age and adults [12]. Nutrient availability of fortified foods has important implications on the overall health of the people consuming the foods. The absorption of micronutrients from fortified foods is determined by the amount of bioavailable micronutrients from the food matrix which will determine the success of a fortification program. The objective of this work was therefore to determine the level of compliance of commercially milled maize flours in Nairobi County with the set standards for maize flour fortification in Kenya and to establish the bio accessibility of iron and zinc from Ugali made from fortified maize flour.
Materials and Methods
Maize flour samples were collected from retail outlets, wholesale shops and supermarkets in all the 17 sub counties of Nairobi. The following information was recorded for each sample: (i) Date of sampling, (ii) Sample source (manufacturer), (iii) Sampling point e.g. supermarket, wholesaler, retail shop or kiosk, (iv) Brand name, (v) Date of manufacture (vi) Expiry date (vii) Batch number and (viii) Labelling as fortified, among others [13].
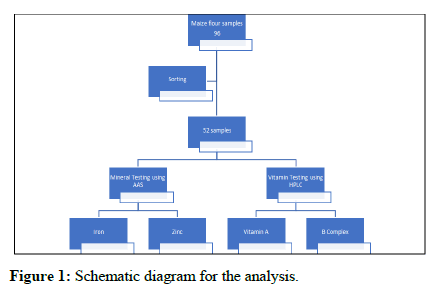
A total number of 96 samples of maize flour were collected and brought to the food fortification reference laboratory in JKUAT (Figure 1). Samples with similar batch numbers for any flour type were sorted to avoid duplication resulting in 52 samples (Table 1). Samples with the same batch numbers were composited and an analytical sample of approximately 200 g drawn. All individual samples selected for analysis were thoroughly mixed using blenders to ensure homogeneity before drawing laboratory samples of about 5 g (Table 2).
| Sub County | No. of samples analysed | Sub County | No. of samples analysed |
|---|---|---|---|
| Dagoreti North | 8 | Kibera | 3 |
| Dagoreti South | 1 | Langata | 2 |
| Embakasi Central | 1 | Makadara | 2 |
| Embakasi East | 2 | Mathare east | 9 |
| Embakasi North | 1 | Roysambu | 4 |
| Embakasi South | 2 | Ruaraka | 4 |
| Embakasi West | 2 | Starehe | 2 |
| Kamkunji | 3 | Westlands | 3 |
| Kasarani | 3 | County | 52 |
Table 1: Samples collected per Sub County.
| Brand | No. of samples | Brand | No. of samples | Brand | No. of samples |
|---|---|---|---|---|---|
| A | 3 | K1 | 1 | S2 | 1 |
| A1 | 1 | K2 | 1 | S3 | 1 |
| A2 | 1 | L | 1 | S4 | 2 |
| B | 1 | L1 | 1 | S5 | 1 |
| C | 1 | M | 3 | S6 | 1 |
| C1 | 1 | M1 | 1 | T | 1 |
| D | 1 | M2 | 1 | T1 | 1 |
| D1 | 2 | M3 | 1 | T2 | 1 |
| H | 1 | P | 4 | T3 | 2 |
| H1 | 1 | P1 | 1 | U | 2 |
| J | 1 | P2 | 1 | W | 1 |
| J2 | 3 | S | 1 | W1 | 2 |
| K | 1 | S1 | 1 | ||
| Note: *The brand names are coded to seal identity of individual brands obtained from the market e.g. A is a brand from 1 company and 3 of them were collected from the field. | |||||
Table 2: Maize flour brands in Nairobi County analyzed for fortification compliance.
Ashing and mineral determination
The reagents used for this process were all of analytical grade. The iron and zinc content of the samples were determined by the method described by Shongwe. In brief, samples of 2 g-4 g were weighed exactly in triplicate crucibles. They were then charred over a hotplate until smoking ceased. The charred samples were placed in a furnace and incinerated at 550°C for 5 h-6 h. The crucibles were then removed from the furnace and cooled. Approximately 5 mL 1 N HNO3 was then added to the ash and transferred into a 100 mL volumetric flask. The crucibles were then washed several times with 1 N HNO3 to ensure complete removal of the ash and filtered using Whatman® filter paper No. 541. The filtrate was then diluted to 100 ml mark with 1 N HNO3. The samples were then analyzed using an AAS (Shimadzu AA-700, Japan) at 248.3 nm for iron and 213.9 nm for zinc. Calculation of concentration was done using standard curves made for each element [14]. Certified reference samples of known concentration were used for quality control in each case. Determination of iron and Zinc content was done in triplicates and the average taken as the final value.
Vitamin A extraction and determination
The retinol content was determined by the method described by Zahar & Smith. To a 50 ml glass stoppered centrifuge tube, 2 g-5 g of ground sample were added followed by 10 ml of absolute ethanol containing 0.1% (w/v) ascorbic acid followed by 2 ml 50% (w/v) Potassium Hydroxide (KOH). The tubes were stoppered, agitated carefully and placed in a water bath at 80°C for 20 min. During this period, the tubes were agitated periodically to ensure complete digestion of fat. After saponification the tubes were cooled with running water and then placed in an ice water bath. Fifteen (15) milliliters of hexane containing 0.01% (w/v) Butylated Hydroxytoluene (BHT) was added. The tubes were again stoppered and mixed vigorously with a vortex (XH-C, Hinotek, China) for 1 min, allowed to stand for 2 min and again vortexed for 1 min. Five (5) milliliters of cold water (1°C) were added to each tube and the tubes inverted 10 times. Centrifugation (Hermle ZK 496, LFT Labortechnik, Germany) was done at 1000 x g for 10 min. The upper organic layer was accurately removed by pipette into a rotary flask (tube) and the solvent was evaporated under vacuum at 40°C using a rotary evaporator (Hahnvapor HS-2005V, Hahnshin S and T Co. LTD, Korea). The residue was immediately re-dissolved in 1 ml of methanol. The samples were then injected into SHIMADZU HPLC (NEXERA UFLC, Japan. Column C-18 ODS size 250 mm × 4.6 mm × 0.5 um. Model 2DA series), the conditions were: Mobile phase, methanol: Water (95:5); flow rate=1 ml/min; photodiode array detector at wavelength 325 nm filter, volume injected=20 ul.
B vitamins extraction and determination in maize flour
The B vitamins were determined using the method by Ekinci and Kadakal. Briefly, approximately five grams (5 g) of the samples were weighed in a 50 ml falcon tube. The samples were then mixed with 20 ml of acidified deionized water and extraction enhanced by using a mechanical shaker (KS 250 basic, IKA LARBORTECHNIK, Germany) for one hour. The samples were then centrifuged for 10 minutes at a speed of 2500 rpm and acceleration of 2 g. The supernatant was then filtered using syringe filters 25 mm 0.45 μm and 1.5 ml collected in the HPLC vial ready for analysis.
Chromatographic experiments were conducted using HPLC (SHIMADZU HPLC NEXERA UFLC Liquid Chromatograph (LC), Japan) equipped with an SIL-20HT auto sampler, SPD-M20A diode array detector and a quaternary pump LC-20AD. A binary gradient method was used. The mobile phase channel A was 100 mM KH2PO4 (pH= 7.0), mobile phase channel B was methanol and the flow rate was 1.0 mL/min. The ration of two mobile phases ranged from 95:5 to 68:32 at the end of the elution process. The column compartment was at 4°C (Oven Model CTO-10ASvp) and detection done at 250 nm (SPD-M20A diode array detector) after injecting 20 μL. Column used in the study was: 5 μm SUPELCO C-18 stationary phase in 4.6 mm × 250 mm. Vitamin standards were obtained from sigma Aldrich (UK) [15-17].
Standard used as reference
In this analysis, fortification compliance was based on the East African standards KS EAS 768 for fortified maize flour as shown in the Table 3. A sample was considered compliant if the level of the elements tested was at least at the minimum levels or within the ranges provided in the standards.
| Element | Standard (mg/kg) |
|---|---|
| Iron | 21 |
| Zinc | 33-65 |
| Vitamin A | 0.5-1.4 |
| Vitamin B1 | 3 |
| Vitamin B2 | 2 |
| Vitamin B3 | 14.9 |
| Vitamin B6 | 2 |
| Folic acid | 0.6-1.7 |
| Note: Specification for maize flour KS EAS 768. | |
Table 3: Maize flour fortification standards KS EAS 768.
Flour samples for preparation of Ugali
Maize flour samples were obtained from a local manufacturer. The samples were delivered to the food fortification laboratory of Jomo Kenyatta University of Agriculture & Technology (JKUAT) and stored in a cool dry place at room temperature until analyzed.
Ashing and mineral determination
The iron and zinc content of the samples were determined by the method described by Shongwe as indicated above.
Preparation of Ugali
Ugali was prepared from the maize flour as described in the Kenyan food recipes book (FAO/GoK, 2018). Briefly, 200 ml of water was brought to boil. Maize flour (100 g) was gradually added to the boiling water while stirring continuously with a wooden spoon until the desired consistency was achieved. It was turned periodically until cooked taking about 10 minutes [18].
The Ugali was analyzed for bio accessibility of the minerals at the different recommended dietary allowances for children under 3 years old.
In vitro digestion
The in vitro digestion protocol was performed as described by Penugonda, Fiorentino, Alavi, and Lindshield with slight modifications. Approximately 1 g of Ugali was used for in vitro digestion. Three independent replications of the in vitro digestion procedure were carried out for all of the Ugali samples. Mineral (iron and zinc) bio accessibility was assessed in both the presence and absence of ascorbic acid. These were added to the Ugali at the start of the oral digestion phase at quantities of 15 mg, 40 mg and 50 mg.
Oral phase: All the chemicals used were of analytical grade. Salivary digestion was first simulated by adding 1.5 mL of α-amylase (1.055 g of alpha amylase was dissolved into a solution of 137 mM NaCl, 2.7 mM KCl, 10 mM NaH2PO4 and 1.8 mM KH2PO4 in NaCl 0.9%) at pH 6.9 followed by incubation at 37°C for 10 minutes while shaking in a water bath. For the gastric step, the pH of the mixture was adjusted to 2 using 1 M HCl and 2.5 ml pepsin (0.2 g pepsin into 5 ml 0.1 N HCl) added. The mixture was then incubated at 37°C for 2 hours while shaking in a water bath (Maxsturdy 30, Daihan Scientific, South Korea).
Intestinal phase: For this phase, the pH was then increased to 7 using 1 M NaHCO3. The mixture was then shaken and 12.5 ml bile extract and pancreatin (0.05 g pancreatin and 0.3 g bile extract in 25 mL 0.1 M NaHCO3) were then added and the mixture incubated for 2 hours at 37°C. The mixture’s pH was then adjusted to 7.2 using NaOH and placed in dialysis tubes with saline solution for 24 hours at room temperature.
Evaluation of bio-accessible nutrients
The bio accessibility of nutrients (iron and zinc) in the samples was determined by simulated in vitro absorption by use of dialysis tubes. This process involved the passive transmembrane diffusion of nutrients from one medium to another. A 10 mL portion of each sample (previously digested) was loaded to the inside of dialysis tubing (Britchemicals Ltd.) by pipettes. The outside compartment of the dialysis tubing of each sample was loaded with 25 mL solution of 0.9% NaCl with albumin. The samples were then be dialyzed at room temperature for 24 hours. The volumes of the solution outside and inside the dialysis tubing were measured and emptied in separate test tubes for nutrients analysis [19]. The final digestion extract was centrifuged at 4600 rpm for 10 min at 25°C (Hermle ZK 496, LFT Labortechnik, Germany) and the supernatant filtered. Analysis was done by atomic absorption spectrophotometer (Shimadzu AA-700, Japan) for iron and zinc. The nutrients that passed to the outside of the dialysis tubing were reported to have corresponded to the bio-accessible nutrients.
The bio accessibility (%) of Fe and Zn in the Ugali was calculated using the equation shown below.

Statistical analysis
The compliance data was analyzed using means, frequencies and percentages based on the fortification standards in the country. Data on bio accessibility were analyzed in triplicates and the data reported as the average ± standard deviation. Analysis of Variance (ANOVA) was used to compare the means at a confidence level of 95%. Mean separation was conducted using the Tukey Kramer multiple comparison procedure (Microsoft Excel 2007, Microsoft Corp., Redmond, WA, USA).
Results and Discussion
Compliance of minerals and vitamins
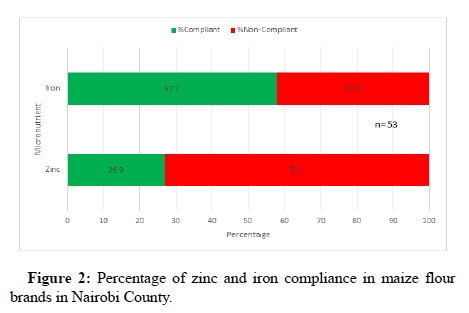
The results for analyzed samples from Nairobi County showed low levels of compliance. Out of the 52 samples analyzed, 26.9% of them complied with the set national fortification standard of 33 mg/kg-65 mg/kg for zinc while 57.7% of the samples complied with the set national standard of 21 mg/kg for iron as shown in Figure 2. The levels of zinc in the analyzed samples ranged from 4.32 mg/kg to 66.5 mg/kg with an average of 26.4 mg/kg while those for iron ranged from 11.3 mg/kg to 33.3 mg/kg with an average of 20.7 mg/kg. A survey conducted in Nigeria for compliance of fortified maize flour to iron fortification standards, revealed varied compliance levels ranging from 1%-21%. The current results show a big decline in compliance for zinc in maize flour from 77.7% while that for iron declined slightly from 59.3%. Hence, the current results demonstrate a decline in compliance with regards to the food fortification standards [20]. This could limit the expected impact of the food fortification program in the country. This low compliance could be as a result of various factors such as non-enforcement of the food fortification regulation leading to complacency amongst the maize millers.
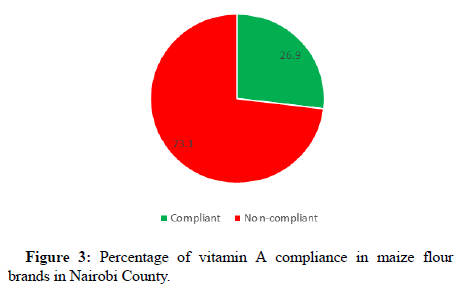
Almost one-third (26.9%) of the samples complied with the set standard for vitamin A which has a range of 0.5 mg/kg to 1.4 mg/kg (Figure 3). The ranges for vitamin A in the samples ranged from not being detected to 4.03 mg/kg (Table 4). These results show a drop in compliance levels of the vitamin A in maize flour as a previous study reported compliance levels of 33.3%. This calls for regular monitoring of the fortification process to ascertain whether there is progress in supplying the community with the required micronutrients. In a study conducted in Nigeria, compliance levels for vitamin A ranged from 12.2% to 33.3%. Similarly, low compliance to the vitamin A standards were reported in the Kano region, Nigeria, with the levels of vitamin A in maize flour of between 16% and 32% of the set standard (Chapter, 2012). Therefore, results from this study corroborates well with the findings from elsewhere in Africa, where the levels of compliance are generally low. The compliance picture is necessary for a country to establish whether or not fortified foods contain the required levels of the micronutrients. With this scenario, programs risk implementation ineffectively without achieving the desired nutritional impact.
The low compliance levels for vitamin A could be due to several factors such as the sensitivity of the vitamin to light and oxygen. Processing and storage periods have resulted in up to 40% loss of vitamin A. Ulemu, Ishmael and Lawrence reported losses of up to 61% of vitamin A along the supply chain in maize flour samples analyzed in Malawi. This calls for appropriate storage conditions for the fortified foods and education of consumers on the need to maintain the integrity of the packaging materials during use at the household level to ensure vitamin A availability.
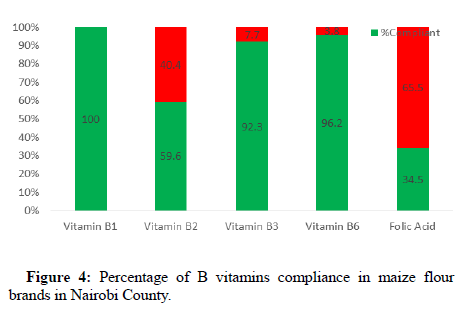
The compliance levels for the B vitamins were generally high compared to vitamin A with levels of 100%, 59.6%, 92.3%, 96.2% and 34.5% being achieved for B1, B2, B3, B6 and folic acid respectively as shown in Figure 4. The ranges for the B vitamins in the samples analyzed are as shown in Table 4.
| Nutrient | Average (mg/kg) | Ranges | Standard (mg/kg) |
|---|---|---|---|
| Iron | 19.7 | 11.3-33.3 | 21 |
| Zinc | 20.3 | 4.32-66.5 | 33-65 |
| Vitamin A | 0.9 | 0-4.03 | 0.5-1.4 |
| Vitamin B1 | 22.5 | 7.5-60.8 | 3 |
| Vitamin B2 | 1.69 | 0.3-25.2 | 2 |
| Vitamin B3 | 114.9 | 11.5-1962.8 | 14.9 |
| Vitamin B6 | 9.3 | 1.4-40.4 | 2 |
| Folic acid | 1.1 | 0.4-30.3 | 0.6-1.7 |
Table 4: Summary of averages and ranges.
A study conducted in Tanzania to analyze maize flour samples for conformity to fortification standards yielded mixed results for compliance of iron (80%), folic acid (20%) and zinc (40%). The results of the study show higher compliance levels for the B vitamins in the current study compared to that of the minerals and vitamin A. These mixed results could probably be a pointer to the varied quality of the premix used for the fortification exercise as well as malpractices during handling and storage of the premix resulting to degradation.
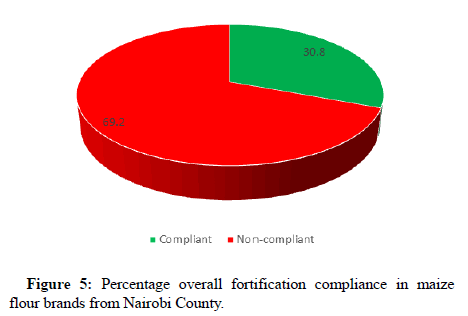
Figure 5 shows that the overall compliance to the national fortification standard based was at 30.8%. This low compliance level is an indicator that the primary objective of providing the micronutrients of interest in Kenya to the general population is not being met. This shows stagnation in compliance to the food fortification standards (GAIN/MOH, 2017).
Bio accessibility of minerals
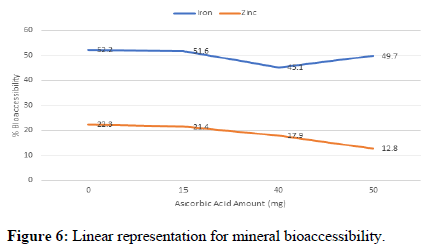
The bio accessible iron and zinc content for the Ugali ranged from 6.8 ppm to 7.9 ppm and 3.2 ppm to 5.6 ppm respectively after the digestion process (Table 5). This represented 45.1% to 52.2% bio accessible iron and 12.8% to 22.3% bio accessible zinc (Table 6).
| Ascorbic acid amounts/fortification level in maize flour | 0 mg Mean ± SD | 15 mg (1-3 y) Mean ± SD | 40 mg (0 m-6 m) Mean ± SD | 50 mg (6 m-12 m) Mean ± SD |
|---|---|---|---|---|
| Iron-15.1 ppm | 7.9 ± 1.2a | 7.8 ± 1.2a | 6.8 ± 0.9a | 7.5 ± 2.6a |
| Zinc-24.6 ppm | 5.6 ± 0.4a | 5.4 ± 0.8a | 4.5 ± 0.4b | 3.2 ± 0.2c |
| Note: *Values are mean of three replicates ± SD, numbers in the same row followed by the same letter are not significantly different at n=3; p=0.05. | ||||
Table 5: Absolute bio accessible iron and zinc from Ugali*.
| Ascorbic acid amounts/Fortification level in maize flour | 0 mg Mean ± SD | 15 mg (1-3 y) Mean ± SD | 40 mg (0 m-6 m) Mean ± SD | 50 mg (6-12 m) Mean ± SD |
|---|---|---|---|---|
| Iron-15.1 ppm | 52.2 ± 7.6 | 51.6 ± 7.9 | 45.1 ± 6.2 | 49.7 ± 17.4 |
| Zinc-24.6 ppm | 22.3 ± 1.7 | 21.4 ± 3.4 | 17.9 ± 1.5 | 12.8 ± 0.8 |
Table 6: % Bio accessible iron and zinc.
There was a significant difference (p<0.0001) in bioaccessibility depending on the type of mineral involved with iron showing a higher bioaccessibility proportion compared to zinc. The bioaccessibility levels for both minerals showed a downward trend as the amount of ascorbic acid increased from 0 mg to 50 mg. Heat treatment of grains (rice, barley, buckwheat, wheat and oat) and pulses (chickpea, green gram, black gram, red gram, cowpea and faba bean) produced contrasting effects on iron and zinc bioavailability. These results showed that whereas iron bioavailability was enhanced by between 7% and 12% that for zinc was decreased by between 11.4% and 63%. This contrasting effect of heat on the bioaccessibility of the two minerals has also been demonstrated by Hemalatha and colleagues.
The higher percentage bioaccessibility of iron compared to that of zinc could be attributed to the difference in valence states of the two minerals with ferric iron with a valence of 3 being more bioaccessible compared to zinc with a valence of 2. Furthermore, the lower bioaccessibility levels of zinc comparative to iron could also be associated to the interaction of zinc with the other food components upon application of heat. Studies have shown an interaction of non-heme iron with the zinc content of foods leading to a lowering effect on the absorption of zinc. At a molar ration of 1:1 for Fe:Zn, zinc absorption was inhibited with the inhibition levels being directly proportional with increase in molar ratio. In the current study, the molar ration of Fe:Zn was 0.9:1 and this conforms to the findings from the above study by Solomon and colleagues (Table 7).
| Minerals | Molar ratio |
|---|---|
| Fe:Zn | 0.9:1 |
| Zn:Fe | 1.1:1 |
Table 7: Minerals molar ratios in Ugali.
For effective iron absorption the molar ration of ascorbic acid: Iron should be 2:1. In the present study, the ascorbic acid: iron molar ratios ranged from 0.25:1 to 0.8:1 way lower than the recommended level for enhancement of iron absorption (Table 8). These low levels are as a result of the amounts of ascorbic chosen for the study since they were based on daily requirements for children under 3 years of age. However, the decline in bioaccessibility with increase in ascorbic acid amounts cannot be conclusively explained from the current work.
| AA level | 15 mg | 40 mg | 50 mg |
|---|---|---|---|
| AA: Fe molar ratio | 0.25:1 | 0.6:1 | 0.8:1 |
| AA: Zn molar ratio | 0.23:1 | 0.6:1 | 0.75:1 |
Table 8: Ascorbic acid to minerals molar ratios.
The trends for bioaccessibility with varying amounts of ascorbic acid are shown in Figure 6 below.
There was no significant difference in the amount of ascorbic acid on bioaccessibility (p=0.5794) neither was there interaction of the mineral type and ascorbic acid (p=0.8334). The results from the study indicate that at higher levels of ascorbic acid, bioaccessibility of iron and zinc from Ugali was lower compared to that at lower ascorbic acid levels an indication that ascorbic acid only enhances bioaccessibility to a certain extent beyond which there is no effect on the bioaccessibility.
In a study to determine the effect of cooking on stability of micronutrients in tortilla, showed that the mineral content in tortilla did not show any significant difference compared to that of the fortified corn flour. The percentage of iron and zinc bioaccessibility in spontaneously fermented dried and blanched vegetables showed no significant differences with cooked vegetables. This is a clear indication of the stability of the micronutrients used in the fortification process. However, this stability can only be useful if what is contained in the final product can be made available for absorption in the human body.
Ascorbic acid has been shown to enhance absorption of iron from food matrix. Addition of ascorbic acid to foods and removal of inhibitors such as phytates are associated with increased absorption of iron from iron fortified foods. Addition of ascorbic acid to test meals enhanced absorption of iron from 2% to 7% in human trails. However, the results from this study show a contrast to the commonly observed effect of ascorbic acid with the bioaccessible levels of both minerals declining with increase in the amount of ascorbic acid. In a review of ascorbic acid effect on iron absorption, Bendich and Cohen observed that the enhancement effect of ascorbic acid on iron absorption decreased at higher doses. Hurrell, et al., reported a low effect of ascorbic acid on iron absorption in human studies. The effect of ascorbic acid on iron absorption is dependent on the amount of ascorbic acid added to a food item, the amount of iron present in a food and the presence and nature of iron inhibitors.
Ascorbic acid had no effect on the absorption of zinc in human studies possibly due to lack of reduction of zinc by ascorbic acid as it happens with iron. The effect of ascorbic acid in enhancing absorption has been demonstrated for iron but not zinc. In a study by Adetola and colleagues, they reported no effect of ascorbic acid on iron bioaccessibility in a food to food fortification process involving baobab and pearl millet porridge. This was attributed to the low ascorbic acid: Iron ratio which was lower that the recommended level for effective iron absorption of 2:1. From the current study, the molar ratio of ascorbic acid: Iron was less than 1:1. This poses programmatic challenges in enhancing mineral absorption as the levels of ascorbic acid used in this study were the RDAs for children under 3 years of age and for effective absorption more ascorbic acid would be required.
The bioaccessibility of micronutrients from the food matrix is a precursor to its bioavailability. Poor bioaccessibility and bioavailability of minerals from plant based foods could also lead to micronutrient deficiencies in humans. This in vitro process provides an indicator of how much minerals could be available for use by the human body upon the digestion process.
Conclusion
Results of this work show that compliance levels are low based on the Kenyan fortification standards. The overall low compliance of the maize flour brands to fortification standards is an indicator that the Kenyan population may not be benefitting from the food fortification program. Further analysis of all the possible barriers to effective fortification process needs to be undertaken and these should include an understanding of the industries processes and an analysis of the quality of premix used in the exercise.
Iron bioaccessibility was generally higher compared to that of zinc despite the application of co-fortification as a means of fighting several micronutrient deficiencies at the same time. Ascorbic acid at higher concentrations seemed to decrease both iron and zinc bioaccessibility. This is a pointer to the need to establish the appropriate levels at which maximum bioaccessibility could be enhanced to avoid use of higher levels of ascorbic acid as an enhancer of iron absorption with no overall benefit to the consumer.
Acknowledgements
The authors wish to acknowledge the financial support received from the European Union for conducting this study.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
References
- Oluyimika YA, Kruger J, White Z, Taylor JR (2019) Comparison between food to food fortification of pearl millet porridge with Moringa leaves and baobab fruit and with adding ascorbic and citric acid on iron, zinc and other mineral bioaccessibility. LWT 106: 92-97.
- Beiseigel JM, Hunt JR, Glahn RP, Welch RM, Menkir A, et al. (2007) Iron bioavailability from maize and beans: A comparison of human measurements with Caco-2 cell and algorithm predictions. Am J Clin Nutr 86: 388-396.
[Crossref] [Google Scholar] [PubMed]
- Bendich A, Cohen M (1990) Ascorbic acid safety: Analysis of factors affecting iron absorption. Toxicol lett 51: 189-201.
[Crossref] [Google Scholar] [PubMed]
- Maigari FU, Sule MS, Maigari A (2012) Levels of vitamin A fortification in flour and vegetable oils sold in Kano metropolis. Chemsearch J 3: 38-42.
- Dunn ML, Jain V, Klein BP (2014) Stability of key micronutrients added to fortified maize flours and corn meal. Ann N Y Acad Sci 1312: 15-25.
[Crossref] [Google Scholar] [PubMed]
- Ekinci R, Kadakal C (2005) Determination of seven water soluble vitamins in tarhana, a traditional Turkish cereal food, by high performance liquid chromatography. ACTA chromatogr 15: 289.
- Ekpa O, Palacios-Rojas N, Kruseman G, Fogliano V, Linnemann AR (2018) Sub-Saharan African maize based foods: Technological perspectives to increase the food and nutrition security impacts of maize breeding programmes. Glob Food Sec 17: 48-56.
- Faria MA, Araujo A, Pinto E, Oliveira C, Oliva-Teles MT, et al. (2018) Bioaccessibility and intestinal uptake of minerals from different types of home cooked and ready to eat beans. J Funct Foods 50: 201-209.
- Garcia-Casal MN, Pena-Rosas JP, De-Regil LM, Gwirtz JA, Pasricha SR (2018) Fortification of maize flour with iron for controlling anaemia and iron deficiency in populations. Cochrane Database Syst Rev 12: CD010187.
[Crossref] [Google Scholar] [PubMed]
- Harika R, Faber M, Samuel F, Mulugeta A, Kimiywe J, et al. (2017) Are low intakes and deficiencies in iron, vitamin A, zinc and iodine of public health concern in Ethiopian, Kenyan, Nigerian and South African children and adolescents?. Food Nutr Bull 38: 405-427.
[Crossref] [Google Scholar] [PubMed]
- Hemalatha S, Platel K, Srinivasan K (2007) Influence of heat processing on the bioaccessibility of zinc and iron from cereals and pulses consumed in India. J Trace Elem Med Biol 21: 1-7.
[Crossref] [Google Scholar] [PubMed]
- Hurrell RF, Reddy MB, Dassenko SA, Cook JD, Shepherd D (1991) Ferrous fumarate fortification of a chocolate drink powder. Br J Nutr 65: 271-283.
[Crossref] [Google Scholar] [PubMed]
- Jing L, Yuwei L, Zhenping H, Qian W (2017) Impact of heat processing on the bioavailability of zinc and iron from cereals and pulses. Int Food Res J 24: 1980-1985.
- Khamila S, Sila DN, Makokha A (2020) Compliance status and stability of vitamins and minerals in fortified maize flour in Kenya. Sci Afr 8: e00384.
[Crossref]
- Kiwango FA, Chacha M, Raymond J (2021) Adequacy of micronutrient fortification in the mandatory fortified food vehicles in Tanzania. Nutr Food Sci 51: 653-663.
- Magee PJ, McCann MT (2019) Micronutrient deficiencies: Current issues. Proc Nutr Soc 78: 147-149.
[Crossref] [Google Scholar] [PubMed]
- Mkambula P, Mbuya MN, Rowe LA, Sablah M, Friesen VM, et al. (2020) The unfinished agenda for food fortification in low and middle income countries: Quantifying progress, gaps and potential opportunities. Nutrients 12: 354.
[Crossref] [Google Scholar] [PubMed]
- Moretti D, Biebinger R, Bruins MJ, Hoeft B, Kraemer K (2014) Bioavailability of iron, zinc, folic acid and vitamin A from fortified maize. Ann N Y Acad Sci 1312: 54-65.
[Crossref] [Google Scholar] [PubMed]
- Muthayya S, Rah JH, Sugimoto JD, Roos FF, Kraemer K, et al. (2013) The global hidden hunger indices and maps: An advocacy tool for action. PLoS One 8: e67860.
[Crossref] [Google Scholar] [PubMed]
- Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, et al. (2018) Effects of water quality, sanitation, handwashing and nutritional interventions on diarrhea and child growth in rural Kenya: A cluster randomized controlled trial. Lancet Glob Health 6: e316-e329.
[Crossref] [Google Scholar] [PubMed]
Citation: Aila OF, Ndaka SD, Nyanchama KB (2023) Compliance of Maize Flour Brands in Nairobi County to Food Fortification Standards and Minerals Bioaccessibility in Ugali Made from Fortified Maize Flour. J Nutr Sci Res 8: 195
Copyright: © 2023 Aila OF, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 2609
- [From(publication date): 0-2023 - Nov 21, 2025]
- Breakdown by view type
- HTML page views: 2253
- PDF downloads: 356