Review Article Open Access
Complex Regional Pain Syndrome: A Systemic Review Of The Literature. The Past, Present And Future Management
| Salibi A1*, Searle AE2 and Lindau TR3 | |
| 1 Research Fellow in Hand Surgery, Royal Derby Hospital, The Pulvertaft Hand Surgery Centre, Uttoxeter New Road, Derby, DE22 3NE, UK | |
| 2 Lead Consultant in Anaesthesia and Pain Management, Royal Derby Hospital, The Pulvertaft Hand Surgery Centre, Uttoxeter New Road, Derby, DE22 3NE, UK | |
| 3 Consultant Hand and Orthopaedic Surgeon, Royal Derby Hospital, The Pulvertaft Hand Surgery Centre, Uttoxeter New Road, Derby, DE22 3NE, UK | |
| Corresponding Author : | Andrej Salibi Royal Derby Hospital The Pulvertaft Hand Surgery Centre Kings Treatment Centre Level 2 Research office, Uttoxeter New Road Derby, DE22 3NE, UK Tel: 00447770800921 E-mail: asalibi@nhs.net |
| Received December 31, 2013; Accepted January 04, 2014; Published January 06, 2014 | |
| Citation: Salibi A, Searle AE, Lindau TR (2014) Complex Regional Pain Syndrome: A Systemic Review of the Literature, the Past, Present and Future Management. J Pain Relief 3:128. doi: 10.4172/2167-0846.1000128 | |
| Copyright: © 2014 Salibi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Complex Regional Pain Syndrome (CRPS) remains one of the most challenging conditions in the field of pain management in general and in the upper extremity in particular. The varied manifestations of phases, symptoms and signs of CRPS have created florid grounds for trials of a number of treatment modalities. While some of these have proven partially effective, many others have not been successful to date. It is very important therefore to establish a multidisciplinary approach based on the best available literature in order to optimise the treatment outcome. We present an evidence based comprehensive review of the management of CRPS stemming from its past, current and most recent practises. It is essential to have guidelines that set an algorithm for the management of patients with CRPS. However, what is more important is to emphasise that CRPS is only the correct diagnosis if all other structural causes have been investigated and ruled out. Over-diagnosis of this condition has led some to question its existence. Therefore, we present an independent opinion on the management of CRPS, with reference to collaborative guidelines within the remits of the current literature.
| Keywords |
| Complex regional pain syndrome/s; Reflex sympathetic dystrophy; Upper extremity/arm; Hand/hand joints; Physiotherapy; Occupational therapy; Treatment |
| Introduction |
| Complex Regional Pain Syndrome (CRPS), previously known as reflex sympathetic dystrophy (RSD), reflex neurovascular dystrophy, Sudeck’s atrophy, causalgia and algo-dystrophy/algo-neurodystrophy, is a broad term used to describe a chronic persistent pain that is disproportionate to any preceding injury and is not related anatomically to a specific peripheral nerve [1]. |
| CRPS is classified into two types: |
| • CRPS-I which gives a classic spectrum of symptoms following trauma but without identifiable peripheral nerve injury. |
| • CRPS-II is associated with an identifiable peripheral nerve injury [2]. |
| Historically, CRPS has been described in three phases throughout the development of the disease as time progresses. However, the disease does not necessarily proceed in these phases as some can be prolonged, shortened or even cease to exist. |
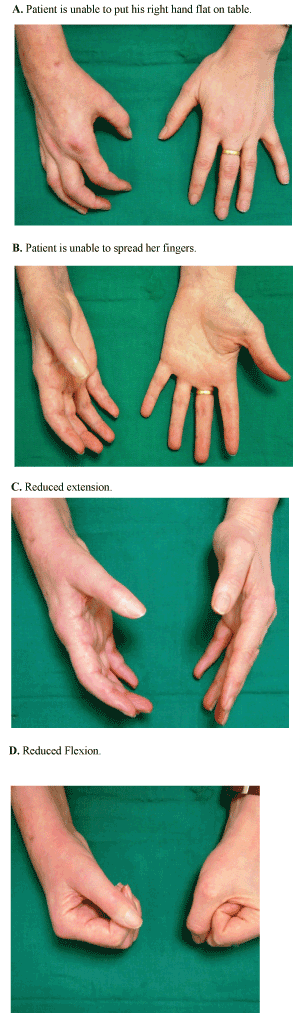
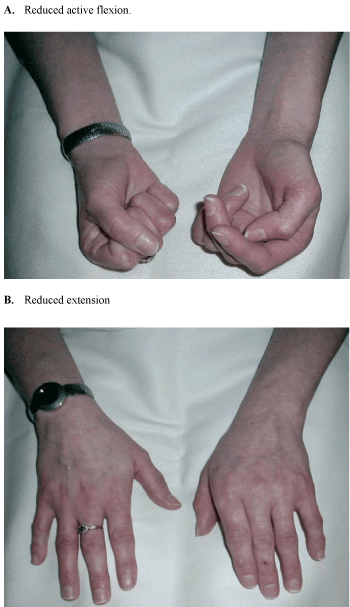
| • The acute phase is characterised by the development of a diffuse severe pain unrelated to a specific dermatome or nerve and local oedema occurring following a traumatic event or even without any clear aetiology (Figure 1). |
| • This is followed by a dystrophic phase, which lasts between three and six months, and where marked oedema, abnormal sweating, skin and soft tissue changes become more prominent (Figure 2). |
| • The final stage is characterised by the presence of atrophic changes. This could include: contractures, waxy and brittle looking skin, and ridged nails, in addition to evidence of demineralisation on bone radiography which may lead to severe deep bone pain [3]. |
| In 1994 the International Association for the Study of Pain (IASP) published the first diagnostic criteria for CRPS which at first seemed to lack specificity. Therefore, this was later revised and modified diagnostic criteria (‘Budapest criteria’) were introduced (Table 1) [4]. |
| The Budapest consensus group in 2004 established that problems do exist in creating a division between the two types of CRPS, and the treatment for both types is the same. Identifying nerve injury in type- II CRPS is relatively dependant on definitive tests of nerve damage, such as Nerve Conduction Velocity (NCV) test or Electro-Myography (EMG), and may be considered unnecessarily painful (even cruel) to CRPS patients. The problem of distinguishing two subtypes is clinically complicated particularly by the fact that there is some evidence to suggest that focal Minimal Distal Nerve Injury (MDNI) affecting nociceptive small-fibers may be associated with post-traumatic CRPS-I. This focal nerve injury which can be demonstrated in the skin of the affected part in most patients studied will remain undetected in most clinical settings [5]. However, the specific definition of nerve injury in CRPS-II does not quantify whether it constitutes “major” or “minor” nerve damage making the distinction between the two types rather unclear. As a result, these indefinite diagnostic distinctions may not impose a significant clinical relevance when considering therapeutic options. The Royal College of Physicians (RCP) in the UK has recently published guidelines for diagnosis, referral and management of CRPS in primary and secondary care. These comprehensive guidelines were developed in conjunction with a wide group of interested disciplines and patient groups and were based on a panel consensus and expert opinion with a reference to the existing literature. The four main pillars of treatment as described in these guidelines include: |
| • Patient information and education |
| • Pain relief (medication and procedures) |
| • Physical rehabilitation |
| • Vocational rehabilitation including psychological intervention [6]. |
| Literature Search |
| The healthcare databases MEDLINE, EMBASE, CINAHL, AMED, PubMed, and TRIP Database were searched and evidence based reviews from the Cochrane library were also included in this review. No data restrictions applied. However, database search only included articles from 1993 until present. MeSH terms used in the search included for example, Complex Regional Pain Syndrome/s, Reflex Sympathetic Dystrophy, Upper extremity/Arm, Hand/Hand Joints, Physiotherapy, etc. Identified articles from both database search and associated references were combined to mainly include: Systematic reviews, meta-analysis of randomized controlled trials, randomized controlled trials (RCTs), double-blinded crossover RCTs, review articles, cohort retrospective comparative reviews/studies, case series and case reports. |
| Treatment options have been ranked in relation to the supporting evidence which were then rated on their quality of evidence (Table 2) and grade of recommendation (Table 3) [7,8]. |
| Treatment Options |
| The historical ambiguity and poor understanding of the pathophysiology of CRPS led to it being one of the most challenging syndromes to treat successfully [9]. As a result of this, and despite a substantial increase in knowledge over the past decade due to ongoing research in this area, there are still many unanswered questions regarding the best treatment modality. Because CRPS exhibits a wide spectrum of presentations stemming from both aetiology and affected body part, its treatment cannot be based on a single treatment modality. Published literature and clinical trials to date have failed to provide strong evidence for a single definitive treatment for CRPS. Instead, it requires a multidisciplinary approach that involves a wide range of services including pain specialists, physical and occupational therapists and psychologists. There is no concrete evidence to support the efficacy of many commonly described interventions. However, we have, through this review, captured the most recent evidence in relation to different aspects of medical treatment, rehabilitation methods and invasive interventions (Table 4). |
| Non-Invasive Modalities |
| Medical treatment |
| A wide variety of oral, topical and parenteral drugs have been used in the treatment of CRPS. Unfortunately, hardly any of these were tested in double-blinded RCTs. This is due to the absence of a genuine diagnostic benchmark in addition to the perceived psychogenic aetiology of CRPS [10]. Although most medications were used on an empiric and theoretical basis, some were favoured more than others because of their combined action, fewer undesirable side effects and evidence provided in literature. |
| The mainstay of treatment choice is dependant to a great extent on whether there is involvement of central sensitisation [11], motor abnormalities [12], and sympathetic efferent features [11]. Therefore, to date there is no individual medication that will treat all aspects [13,14]. |
| In this review we have focused on a number of agents, particularly those included in clinical trials for treatment of CRPS such as α-adrenergic antagonists, corticosteroids, Calcitonin and bisphosphonates, and most recently, intravenous immunoglobulin (IVIG). Tricyclics, gabapentin and pregabalin, carbamazepine, opioids, 5% lidocaine patch, and topical capsaicin were better studied in other related neuralgias, therefore not considered in this review. |
| Antihypertensives and α-adrenergic antagonists |
| The concept of pain in CRPS being sympathetically maintained pain (SMP) or sympathetically independent pain (SIP) is very important from both the diagnostic point of view and in determining the most effective treatment choice. This is particularly important when considering sympatholytic drugs such as α1-antagonists, α2- antagonists, combined α1- and α2 antagonists, and α2 agonists [15-17]. |
| • A systematic review provided evidence that Clonidine (α2- adrenergic agonist), which was used for treatment of SMP CRPS in the past, is rarely considered nowadays [18]. |
| • Phenoxybenzamine (α1 and α2-adrenergic antagonist) seems to work best in the early stages of CRPS particularly in those with less than 3 months duration. |
| • Phentolamine, another similar α-adrenergic antagonist, which is administered by continuous intravenous infusion (IVI), is limited in its use and is mainly used in research [19,20]. |
| • Nifedipine (calcium-channel blocker) is a strong vasodilator and found to have good efficacy with doses of up to 60 mg/day [19,20]. |
| Clinicians should be aware that recent evidence suggested an association between angiotensin converting enzyme (ACE) inhibitors, which are commonly used for the treatment of hypertension, and the onset of CRPS. It is proven that there is an increased risk of developing CRPS in patients on ACE inhibitors particularly when they are used for longer period and in higher doses [21]. |
| Recommendation: Non-selective oral adrenergic medications such as Phenoxybenzamine and Nifedipine may be effective in the acute phase of CRPS where clinical features of altered blood flow and apparent (Level of Evidence: IV, Strength of Recommendation: C). |
| Anti-inflammatory and immunomodulators |
| There is a suggestion that CRPS can be associated with an inflammatory process. This has been established through several small clinical trials following findings of major improvement after administration of corticosteroids particularly in the acute phase [22,23]. Therefore non-steroidal anti-inflammatory drugs (NSAIDs) have been used for the treatment of CRPS. Others, such as cox-2 inhibitors, free radical scavengers (e.g. Vitamin C) and biologics (e.g. Tumour Necrosis Factor TNF-α inhibitors) have also been used, but with a rather low quality evidence to support their efficacy. Few clinical trials presented results supporting the use of NSAIDs for neuropathic pain. A comparative study showed that there is no benefit in treating CRPS-I with NSAIDs [24]. In another study, specific NSAIDS such as Ketoprofen have shown to have substantial anti-bradykinin and antiprostacyclin properties in addition to their normal anti-prostaglandin effect. Cox-2 inhibitors (e.g. Celecoxib) are anecdotally effective but have not been studied within the remits of CRPS treatment [25]. TNF-α inhibitors (e.g. etanercept and infliximab) are known to decrease cytokine levels and subsequently pain in CRPS [26]. The current advances in the development of new biological agents (e.g. Thalidomide) for treating autoimmune and inflammatory conditions may open the horizon to new treatment modalities for CRPS and potentially pave the road to a well-powered RCT, but to date it is still only within the scope of only case reports [27] and clinical use is significantly restricted. Oral corticosteroids have been studied in numerous clinical trials, most of which included their use in the early/acute phase of CRPS. Nevertheless, it is still unknown whether their efficacy remains the same in the chronic cases of CRPS when the proinflammatory cytokines are at their lower levels or in those with a relatively mild inflammatory profile [23]. Two prospective RCTs showed that use of a reducing regime of oral corticosteroids in the early/acute stage of CRPS produced considerable improvements [22,23]. A double-blinded placebo-controlled study of Vitamin C suggested that it decreases the incidence of CRPS following wrist fractures [28]. Di-methylsulfoxide (DMSO) is another free radical scavenging agent, which demonstrated significant pain reduction when applied in a form of (50% cream) for 2 months [29]. |
| Recommendation: There is good evidence to support a short course of steroids in early CRPS. Topical application of DMSO cream is effective in reducing the pain in CRPS. (Level of Evidence: I, Strength of Recommendation: A) |
| Calcitonin and bisphosphonates |
| Calcitonin is a polypeptide hormone produced by the thyroid gland. Its antinociceptic and analgesic properties are well known in the treatment of a wide spectrum of acute and chronic pain conditions [30]. The exact mechanism of action is not clear to date. However, one hypothesis proposes a simple peripheral anti-inflammatory action in addition to a direct effect on specific central nervous system receptors [31]. Another hypothesis suggests that calcitonin alters the descending serotonergic modification on the sensory transmission mediated by C-afferents leading to the analgesic effect [32]. A meta-analysis of several controlled clinical trials confirmed the efficacy of intranasal doses of 100-300 U per day of calcitonin in the treatment of CRPS [33]. Regrettably, Cacitonin has been withdrawn from clinical use due to fears over cancer risk. The associated active bone resorption and vigorous bone remodelling in some patients with CRPS can be painful. Osteoclasts resorb bone by acidifying the extracellular matter in order to dissolve hydroxyapatite activating nociceptive acid-sensing channels. Therefore, inhibiting bone resorption was considered the mainstay of treating pain in CRPS patients with evidence of active bone resorption [34]. Bisphosphonates (e.g. alendronate, ibandronate, risedronate, zoledronate, etidronate, pamidronate) slow bone resorption and therefore were considered for treatment of CRPS in certain circumstances. Systematic review of the literature and multiple clinical trials strongly demonstrated the efficacy of administering both intravenous and oral bisphosphonates with a major improvement in pain among patients with CRPS [2,35-39]. |
| The Royal College of Physician’s guidelines recommend a single I.V infusion (IVI) of 60 mg Pamidronate within the first six months of symptoms. |
| Recommendation: There is evidence to support the use of calcitonin in treatment of CRPS. Patients with active bone resorption respond well to IVI bisphosphonate, with 60 mg Pamidronate being a current recommended treatment. (Level of Evidence: I, Strength of Recommendation: A) |
| Intravenous immunoglobulin (IVIG) |
| It has been suggested that IVIG targets specific autoantibodies deemed to be present in patients with CRPS, in addition to downregulating proinflammatory cytokines [40]. This emerging drug was first considered in treatment of CRPS in a randomised trial, but was never proved better than other treatment options [41]. |
| Recommendation: There is currently no support for IVIG treatment (Level of Evidence: II, Strength of Recommendation: C). |
| Rehabilitation |
| The available literature strongly supports the importance of adjuvant physical (PT) and occupational therapy (OT) in the treatment pathway for patients with CRPS. Over the past decade, it has become clear that in the absence of the ultimate treatment modality for CRPS, the only methodology to overcome the constant gap in medical science is to apply a multidisciplinary approach [42]. |
| From that perspective, the concept of functional restoration came to light and was historically and empirically considered a vital aspect of the rehabilitation process of CRPS patients [43,44]. It has been established that physiotherapy is of “utmost importance” [45]. In a number of RCTs a comparison was made between PT and OT by exploring different elements of their contribution to reduction of pain and impairment in patients with CRPS, in addition to their cost effectiveness. All [46-48] but one trial [49] indicated that PT and OT were effective in reducing pain and improving active mobility in patients with CRPS localised in one extremity and of less than one year duration. However, PT seems to have had the greatest and most rapid effect and was also the most cost-effective overall [46-48]. In the long term, the difference between the two from the functional impairment point of view appeared to be not significant compared to control therapy [49]. |
| The Dutch multidisciplinary evidence-based guideline for treatment of CRPS also considered PT an essential element of functional restoration [50]. From a pragmatic point of view both PT and OT have a vital role in clinical practice and their contribution to treatment cannot be underestimated or isolated. This was further elaborated by the experienced Mayo Anaesthesia group, which emphasised that PT forms “the cornerstone and first line treatment for CRPS” and its activities are designed to complement those of OT [51]. Recent RCP guidelines proposed a number of specialist treatment approaches to be conducted, particularly in tertiary centres, by therapists with CRPS expertise (Table 5) [6]. |
| The current aim of rehabilitation programmes is to shift the focus towards early stages of movement by activation of promoter and primary motor cortices. Therefore, among the list of these recommended approaches, graded motor imagery (GMI) and mirror visual feedback (MVF), have dominated the current ongoing research in the pursuit of the best therapy modality for patients with upper extremity CRPS [52]. We have therefore focused on these two modalities, particularly with their increasing popularity in the recent years among the vast majority of specialists units. |
| Rehabilitation - mirror therapy (mirror visual feedback MVF) |
| MVF was first described and implemented in the treatment of phantom limb pain in 1996 [53]. However, it was not until 2003 that it was used in CRPS patients [54]. Several hypotheses have been proposed in the literature outlining the underlying mechanisms of pain reduction during mirror therapy. It is suggested that limb amputation disrupts the normal relationship between motor intention and the associated sensory feedback. |
| In order to compensate for this dissociation, a mirror is used to superimpose the non-affected extremity in order to create a visual illusion of a pain free movement of the affected one [53,55]. This theory was supported by an analogy with the mismatch between vestibular and visual information associated with nausea. Similarly, in CRPS patients, there is no identifiable nociceptive aetiology, therefore leading to a mismatch between the motor intention, proprioceptive and visual feedback [54,56,57]. Other studies showed that the absence of a normal sensory feedback can change the cortical recognition and produce reorganised body representation that is proportional to severity of pain [58,59]. Therefore, providing an alternative sensory feedback in the form of a visual illusion by using a mirror will eventually decrease pain [60,61]. |
| A controlled pilot study confirmed the effectiveness of mirror therapy, particularly in the early stages of CRPS-I as compared to chronic disease especially when trophic changes supervene [54]. The use of mirror box therapy was assessed in CRPS-I patients and demonstrated an immediate and considerable improvement in range of movement (ROM) of the affected hand, in addition to a significant reduction in pain of more than 50% [62]. A study of two cases demonstrated that the use of mirror therapy in patients with CRPS-II is worthy of further exploration [63]. Many other studies explored the effects of mirror therapy in other conditions such as phantom limb pain, and stroke. However, most of the available literature to date demonstrates relatively low quality evidence. Therefore, firm conclusions cannot be drawn [64]. |
| Recommendation: Mirror therapy is considered an effective first line therapy but no strong evidence is available to confirm its effectiveness in isolation. (Level of Evidence: III, Strength of Recommendation: C) |
| Rehabilitation- Graded Motor Imagery (GMI) |
| • The combination of mirror therapy and motor imagery is called graded motor imagery (GMI). GMI is a comprehensive programme aimed at improving cortical organisation and activation of motor networks [65]. Traditionally, GMI consists of three phases: limb laterality recognition task, an imagined limb movement task (motor imagery) and finally mirror therapy (as described above). |
| • The patient in the first step has to determine whether the pictured hand is left or right [66]. This concept of laterality training depends on an intact body schema, activates premotor cortices and re-establishes left and right orientation within the brain [67]. |
| • In the next step, the patient will be asked to imagine a limb posture imitating what is shown in a picture without moving the affected hand. This will subsequently promote and activate both the premotor and primary motor cortices [66]. |
| • The last step will involve using the aforementioned mirror therapy. |
| Although there is good evidence to support the use of mirror therapy alone for acute CRPS-I [54,68], a RCT predicted that starting with GMI will have a greater impact on reducing limb pain, which as a result may lead to a better recognition of the newly developed relationship with brain cortices [56]. Another RCT provided good evidence that GMI reduced pain and disability in a number of patients with chronic CRPS-I [69]. Given the above encouraging evidence, the use of GMI could be expanded to other similar conditions and potentially used at different stages of CRPS in order to validate the existing literature [67]. A modification was introduced to the GMI programme (mGMI) by integrating the mirror box into the motor imagery step and dividing the mirror therapy step into two separate stages. As a result of this modification, in step 2, imagined hand movements were combined with watching the reflection of the unaffected one in the mirror. Step 3, consisted of mirror therapy with mobilisation of unaffected hand followed by mobilisation of both hands in step 4. Results of this modification demonstrated considerable reduction of pain and improvement of grip strength in patient with non-chronic CRPS-I of the upper extremity [70]. |
| Recommendation: GMI is useful for CRPS patients, particularly those with chronic disease. Further research is required to prove the effectiveness of mGMI as an alternative modality. (Level of Evidence: II, Strength of Recommendation: B) |
| Invasive Modalities |
| Invasive interventions began to expand over the past decade in a relatively progressive manner as the current research has revealed new facets of CRPS aetiology. It has been recommended that these should be applied in appropriately timed manner, and depending on need, they may serve as an adjunctive role for pain relief in order to facilitate reanimation of the affected extremity in addition to complementing the functional goals of the rehabilitation process [6,44]. Numerous therapies have been proposed and some were used in the treatment of patients. These included nerve blocks, drug infusions and implantable pain treatment devices [52]. |
| Sympathetic Nerve Blocks (SNBs) |
| These include blocking the sympathetic nervous system by injecting local anaesthetic agents directly into the sympathetic neural structures such as the stellate ganglion block (SGB) (for upper extremity) or the lumbar sympathetic block (LSB) (for lower extremity) [71]. |
| Historically, the term RSD always implied sympathetic nervous system involvement, which led to the belief that sympathetic blockade should confirm the diagnosis [72]. There is a considerable evidence to suggest coexistent involvement of the sympathetic nerves with other types of afferent fibers in both peripheral and central nervous systems in the SMP type of CRPS [73]. The aim to distinguish SMP from SIP has led to consideration of sympathetic block in most treatment algorithms. However, there is still a considerable difficulty in assessing the sympatholytic effect even when the block clinically appears to be successful [74]. Therefore, the role of these blocks remains empirical due to the lack of strong evidence. This is in addition to the realisation that it is difficult to assess the degree of sympathectomy provided [52]. Several studies were conducted in the aim of establishing block success criteria. In a double-blinded, crossover study, the effect of local anaesthetic (LA) (lidocaine/bupivacaine mixture) vs. saline in SNB was compared. It was found that analgesia was achieved within 30 min in both groups, but with longer duration in the LA group. This showed at least short –term analgesic effect of LA SNB in CRPS patients [75]. In a RCT of SGB vs. guanethidine IV regional block, there was a significant improvement in both groups, but without a difference between the two [76]. A blinded prospective trial compared IV phentolamine infusion with LA SNBs and found significant correlation between the two, concluding that either could distinguish between SMP and SIP [17]. An observational study of 54 SGBs was the first to introduce five strict sympathetic block success criteria. Achieving four out of five criteria was considered a definitely successful blockade. However, only 15 of 54 blocks met the proposed criteria of success, alluding to the fact that clinically there is a relatively high rate of partial or incomplete sympathetic blockade [77]. Interestingly, a systematic review that assessed all available literature regarding LA SNB between 1916 and 1999, found no solid evidence to support the efficacy of SNBs as a treatment for CRPS. Low rate of pain relief in addition to lack of control groups in most of the available case series, led to the overestimation of the efficacy of SNBs in CRPS patients which may explain the inconsistent evidence [78]. |
| Recommendation: Moderate evidence is available to support the efficacy of classic Stellate Ganglia Blocks and Lumbar Sympathetic Blocks, but their main applications remain within the realm of distinguishing SMP from SIP. With the lack of defining criteria of a successful block, their analgesic effects may be enhanced when applied in conjunction with other treatment modalities (Level of Evidence: III, Strength of Recommendation: C). |
| Intravenous Regional Anaesthesia (IVRA) |
| This involves infusion of pharmacological agents whilst the affected limb is under tourniquet ischaemia, allowing interstitial diffusion. Agents commonly used include guanethidine, lidocaine, clonidine and others [79]. A meta-analysis of 11 trials concluded that there is no evidence to support the efficacy of IVRA and in particular for the use of guanethidine in CRPS patients [33]. Other good quality studies have also reported a negative outcome of IVRA. A double-blinded, crossover, RCT found no difference between placebo and guanethidine groups in terms of pain relief and therefore lack of effect of guanethidine over placebo in patients with CRPS-I [80]. Two other RCTs compared various agents with placebo (saline), but all failed to establish a difference in pain relief [81,82]. No significant effect of IVRA with reserpine and guanethidine was found over lidocaine alone as a control group [83]. The only exception to the above is a study which found better response with bretylium compared to lidocaine [84]. One study compared IVRA guanethidine to SGB and demonstrated comparable efficacy [76]. |
| Recommendation: Available literature does not support the use of IVRA guanethidine for the treatment of CRPS. There is low quality evidence to support the use of other agents (Level of Evidence: I, Strength of Recommendation: A). |
| Brachial plexus/spinal block infusions |
| Brachial plexus infusion with local anaesthetic aims to block somatosensory C- and A-afferents in addition to sympathetic efferents, which as a result will block the conduction of action potentials and prevent the release of neuropeptide mediators that lead to peripheral neurogenic reactions [85], and central propagation of pain signals. The brachial plexus is an ideal place for continuous regional techniques because of its neurovascular relation with the upper extremity. Several indications exist for conducting brachial plexus catheter infusions. These include: peri-operative, post-traumatic, and post- operative pain relief, vascular compromise, intractable pain for CRPS and phantom pain [52]. Catheters have been kept in situ for as long as three weeks [86]. In addition to local anaesthetics, adjuvants such as opioid, clonidine and others have been infused [52]. Sympatholysis has reportedly been maintained up to two to three weeks with 0.1-0.2% ropivacaine [87]. |
| Epidural infusions of LA are commonly used and well established for post-operative analgesia. Other medications such as clonidine and/or opioids can be added in order to enhance spinal analgesia and potentiate the degree of pain relief. The most common combination of epidural medications to date includes clonidine with/without bupivacaine. The effectiveness of epidural infusions for the treatment of CRPS has been borne out by several studies. The effectiveness of epidural infusions for the treatment of CRPS has been found using clonidine epidural infusion [88] and with a bupivacaine-opioid mixture [89]. In a similar study, 83% of patients had improvement in pain, swelling, oedema, and dysfunction of the hand. 63% of patients were satisfied with their improvement and 8% reported complete resolution of pain [90]. |
| Intrathecal analgesia has been used in CRPS but with limitation to small studies. Intrathecal baclofen injection in a double-blinded fashion followed by infusion in patients with severe dystonia from CRPS reported good outcomes [91]. |
| Recommendation: The current evidence of the effectiveness of brachial plexus blockade does not extend beyond a few case series. (Level of Evidence: IV, Strength of Recommendation: C). There is moderate evidence to suggest the effectiveness of epidural infusion techniques with a bias towards epidural clonidine. Intrathecal baclofen has proven effective only in severe dystonia component of CRPS (Level of Evidence: III, Strength of Recommendation: C). |
| Botulinum toxin injections (Botox) |
| Botulinum toxin type A (BTA) is a well known acetylcholine (ACh) release blocking agent. This ability to block ACh has led to its rising popularity over the past years. The induction of temporary paralysis of specific muscle groups was the mainstay of its use, particularly in movement disorders. The ability to inhibit the release of various non-cholinergic neurotransmitters and neuropeptides from afferent nerve terminals has provided the platform for its role in neuropathic pain [52]. Regional intradermal injections of BTA showed effective reduction in allodynia when injected at the painful site in patients with post-traumatic neuralgia [92]. However, a pilot study investigating the efficacy and tolerability of intradermal and subcutaneous BTA administration for allodynia in patients with CRPS proved the contrary [93]. In support of the use of BTA, it has demonstrated a profound prolongation of bupivacaine sympathetic blockade and subsequently analgesia when combined with BTA in CRPS patients [94]. |
| Recommendation: There is insufficient evidence to support the use of BTA in CRPS (Level of Evidence: IV, Strength of Recommendation: C). |
| Neurostimulation |
| This involves electrical stimulation of neural tissues by surgical implantation of electrodes into specific areas of the brain or spinal cord [95]. As an invasive treatment option with potential significant complications, it is considered an end-stage treatment. Spinal cord stimulation (SCS) was compared with conservative therapy for patients with CRPS involving the upper extremity demonstrating significant reduction in pain and improvement in quality of life [96]. Both cervical and lumbar SCS were comparable in terms of effectiveness and complication rate [97]. It was also reported that more than 50% reduction in pain in advanced stage CRPS (at least 2 years duration) was achieved following SCS or peripheral nerve stimulation (PNS) [98]. |
| Recommendation: There is some support for the use of neurostimulation with Spinal Cord Stimulation, particularly in the later stages of CRPS (Level of Evidence: II, Strength of Recommendation: C). |
| Ablative techniques |
| Surgical sympathectomy was previously considered a popular option for treatment of a wide array of hyperactive sympathetic syndromes such as hyperhidrosis and Raynaud’s phenomenon. It was also previously regarded as an important treatment modality of sympathetically maintained pain (SMP) in RSD, but as yet, no strong evidence is available to support its widespread use [52,99,100]. |
| Surgical sympathectomy was predominantly performed as an open procedure. However, endoscopic techniques have become the recent trend for upper limb disease [72]. In addition, more recently, radio frequency (RF) ablative techniques have been introduced [101]. |
| The high failure rate (35%) in surgical sympathectomy was caused by poor patient selection, incorrect diagnosis, inadequate resection, re-innervations and contralateral innervation [100]. There is high incidence (44%) of post-sympathectomy neuralgia which may occur six months to two years following ablation [102]. Percutaneous RF ablation to the thoracic T2 sympathetic outflow can give 86% signs of sustained sympathectomy with a very small risk of post-ablation neuralgia syndrome (5%) [101]. SCS can be utilised as an effective treatment of pain in both CRPS and post-ablative neuralgia. Its low morbidity makes it superior to ablative techniques [103]. |
| Recommendation: Surgical sympathectomy gives a high rate of post-sympathectomy neuralgia. Its lack of effectiveness when compared to other sympathetic blocks and neurostimulation, means that it is not recommended (Level of Evidence: III, Strength of Recommendation: C). |
| Conclusions |
| There is currently no strong consensus to determine the optimal treatment modality for CRPS despite the multitude of trials in this area. |
| The aim with the Royal Collage of Physician’s guidelines [6] was to provide a benchmark for both primary and secondary care in order to appropriately diagnose, refer, and manage patients with CRPS. As pain is the most common symptom in CRPS, commencing early treatment with optimised simple analgesia and NSAIDS, and even a short course of steroids may be effective particularly in the early stage. |
| The RCP guidelines recommend introducing medication for neuropathic pain as early as possible if simple analgesia is ineffective. It also favours a one-off treatment with bisphosphonates (Pamidronate 60 mg intravenous single dose) in patients with CRPS of less than six months duration. Spinal cord stimulation is reserved as a last resort in those chronic cases where patients do not respond to other treatment modalities. The most crucial principle of these guidelines is that analgesia should not be considered in isolation, but should be part of an early multidisciplinary integrated approach with the support of Physio (PT) and Occupational (OT) therapy. One of the implied recommendations is for development of referral guidance and pathways to the most relevant clinical team within the treating organisation, and for a co-ordinated response between the disciplines involved. It also identified the need for further high quality research. |
| It is important to emphasise that CRPS is only the correct diagnosis if all other structural causes have been investigated and ruled out. In fact, there are views that CRPS does not exist. The Editorial: “I have a dream ... reflex sympathetic dystrophy RSD or Complex Regional Pain Syndrome- CRPS I does not exist” [104] has been presented to caution the surgeon or doctor not to over-diagnose the condition as CRPS-I and under-diagnose the underlying structural cause of pain, swelling, etc. |
| The fact is that CRPS, as a poorly defined syndrome, shares a wide range of signs and symptoms with many other conditions. Therefore, its definition, diagnostic criteria, aetiology and to a certain extent its treatment, remain subjective to change. It was always assumed that a culprit such as surgery or trauma to the upper extremity is the cause of such a complex and regional array of symptoms. However, as in most cases, the intention, quite often, is to treat the symptoms and not to analyse the underlying issue, which could be hidden within the severity and incongruity of the symptoms of CRPS. It is therefore paramount to rule out instability in fractures and subclinical nerve entrapments, which are relatively common after trauma and surgical procedures and yet amenable to surgical treatment different from CRPS. Hence the clinician needs to constantly reassess and make sure that no alternative cause for pain can explain the symptoms. |
| We therefore put forward an integrated algorithm of our recommendations for suspected cases of CRPS (Table 6). These recommendations, whilst only a guidance, serves as a keystone multidisciplinary approach extrapolated from both our experience in a tertiary hand surgery centre and from the recent RCP guidelines. |
| To conclude, CRPS continues to be a mysterious condition of unknown cause and with various treatments with limited evidence to support it. It is therefore essential to treat the right condition, i.e. CRPS only if there is no hidden underling cause for the dramatic clinical signs and symptoms [105]. |
References
- Bruehl S (2010) An update on the pathophysiology of complex regional pain syndrome. Anesthesiology 113: 713-725.
- Forouzanfar T, Köke AJ, van Kleef M, Weber WE (2002) Treatment of complex regional pain syndrome type I. Eur J Pain 6: 105-122.
- Bonica JJ (1973) Causalgia and other reflex sympathetic dystrophies. Postgrad Med 53: 143-148.
- Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR (2007) Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med 8: 326-331.
- Oaklander AL, Rissmiller JG, Gelman LB, Zheng L,Chang Y, et al. (2006) Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain 120: 235-243.
- Goebel A, et al. (2012) Complex regional pain syndrome in adults: UK guidelines for diagnosis, referral and management in primary and secondary care. RCP, London.
- JBJS (Journal of Bone and Joint Surgery). Instructions for authors. Levels of evidence for primary research question.
- Harbour R, Miller J (2001) A new system for grading recommendations in evidence based guidelines. BMJ 323: 334-336.
- Burton AW, Bruehl S, Harden RN (2005) Current diagnosis and therapy of complex regional pain syndrome: refining diagnostic criteria and therapeutic options. Expert Rev Neurother 5: 43-51.
- Haddox JD, Van Alstine D (1996) Pharmacolgic therapy for reflex sympathetic dystrophy. Phys Med Rehabil 10: 297-307.
- Harden RN, Rudin NJ, Bruehl S, Kee W, Parikh DK, et al. (2004) Increased systemic catecholamines in complex regional pain syndrome and relationship to psychological factors: a pilot study. Anesth Analg 99: 1478-1485.
- Galer B, Garden R (2001) Motor abnormalities in CRPS: A neglected bu key component, in Complex Regional Pain Syndrome. WA: IASP Press, Seattle.
- Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, et al. (2002) Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain 95: 119-124.
- Harden RN, Baron R, Janig W (2001) Preface, in Complex Regional Pain Syndrome. IASP Press: Seattle.
- Arner S (1991) Intravenous phentolamine test: diagnostic and prognostic use in reflex sympathetic dystrophy. Pain 46: 17-22.
- Raja SN, Davis KD, Campbell JN (1992) The adrenergic pharmacology of sympathetically-maintained pain. J Reconstr Microsurg 8: 63-69.
- Raja SN, Treede RD, Davis KD, Campbell JN (1991) Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology 74: 691-698.
- Kingery WS (1997) A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain 73: 123-39.
- Muizelaar JP, Kleyer M, Hertogs IA, DeLange DC (1997) Complex regional pain syndrome (reflex sympathetic dystrophy and causalgia): management with the calcium channel blocker nifedipine and/or the alpha-sympathetic blocker phenoxybenzamine in 59 patients. Clin Neurol Neurosurg 99: 26-30.
- Prough DS, McLeskey CH, Poehling GG, Koman LA, Weeks DB, et al. (1985) Efficacy of oral nifedipine in the treatment of reflex sympathetic dystrophy. Anesthesiology 62: 796-799.
- de Mos M, Huygen FJ, Stricker BH, Dieleman JP, Sturkenboom MC (2009) The association between ACE inhibitors and the complex regional pain syndrome: Suggestions for a neuro-inflammatory pathogenesis of CRPS. Pain 142: 218-224.
- Braus DF, Krauss JK, Strobel J (1994) The shoulder-hand syndrome after stroke: a prospective clinical trial. Ann Neurol 36: 728-733.
- Christensen K, Jensen EM, Noer I (1982) The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand 148: 653-655.
- Rico H, Merono E, Gomez-Castresana F, Torrubiano J, Espinos D, et al. (1987) Scintigraphic evaluation of reflex sympathetic dystrophy: comparative study of the course of the disease under two therapeutic regimens. Clin Rheumatol 6: 233-237.
- Pappagallo M, Rosenberg AD (2001) Epidemiology, pathophysiology, and management of complex regional pain syndrome. Pain Pract 1: 11-20.
- Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL (2004) Successful treatment of CRPS 1 with anti-TNF. J Pain Symptom Manage 27: 101-103.
- Ching DW, McClintock A, Beswick F (2003) Successful treatment with low-dose thalidomide in a patient with both Behcet's disease and complex regional pain syndrome type I: case report. J Clin Rheumatol 9: 96-98.
- Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS (1999) Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet 354: 2025-2028.
- Zuurmond WW, Langendijk PN, Bezemer PD, Brink HE, de Lange JJ, et al. (1996) Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream. Acta Anaesthesiol Scand 40: 364-367.
- Braga PC (1994) Calcitonin and its antinociceptive activity: animal and human investigations 1975-1992. Agents Actions 41: 121-131.
- Azria M (2002) Possible mechanisms of the analgesic action of calcitonin. Bone 30: 80S-83S.
- Lyritis GP, Trovas G (2002) Analgesic effects of calcitonin. Bone 30: 71S-74S.
- Perez RS, Kwakkel G, Zuurmond WW, de Lange JJ (2001) Treatment of reflex sympathetic dystrophy (CRPS type 1): a research synthesis of 21 randomized clinical trials. J Pain Symptom Manage 21: 511-526.
- Kozin F, McCarty DJ, Sims J, Genant H (1976) The reflex sympathetic dystrophy syndrome. I. Clinical and histologic studies: evidence for bilaterality, response to corticosteroids and articular involvement. Am J Med 60: 321-331.
- Varenna M, Zucchi F, Ghiringhelli D, Binelli L, Bevilacqua M, et al. (2000) Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol 27: 1477-1483.
- Adami S, Fossaluzza V, Gatti D, Fracassi E, Braga V (1997) Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis 56: 201-204.
- Manicourt DH, Brasseur JP, Boutsen Y, Depreseux G, Devogelaer JP (2004) Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum 50: 3690-3697.
- Robinson JN, Sandom J, Chapman PT (2004) Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med 5: 276-280.
- Kubalek I, Fain O, Paries J, Kettaneh A, Thomas M (2001) Treatment of reflex sympathetic dystrophy with pamidronate: 29 cases. Rheumatology (Oxford) 40: 1394-1397.
- Kohr D, Tschernatsch M, Schmitz K, Singh P, Kaps M, et al. (2009) Autoantibodies in complex regional pain syndrome bind to a differentiation-dependent neuronal surface autoantigen. Pain 143: 246-251.
- Goebel A, Baranowski A, Maurer K, Ghiai A, McCabe C, et al. (2010) Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med 152: 152-158.
- Harden RN (2005) The rationale for integrated functional restoration, in CRPS: Current Diganosis and Therapy. WA: IASP Press, Seattle.
- Stanton-Hicks M, Baron R, Boas R, Gordh T, Harden N, et al. (1998) Complex Regional Pain Syndromes: guidelines for therapy. Clin J Pain 14: 155-166.
- Stanton-Hicks MD, Burton AW, Bruehl SP, Carr DB, Harden RN, et al. (2002) An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract 2: 1-16.
- Baron R, Wasner G (2001) Complex regional pain syndromes. Curr Pain Headache Rep 5: 114-123.
- Oerlemans HM, Oostendorp RA, de Boo T, Goris RJ (1999) Pain and reduced mobility in complex regional pain syndrome I: outcome of a prospective randomised controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain 83: 77-83.
- Oerlemans HM, Oostendorp RA, de Boo T, van der Laan L, Severens JL, et al. (2000) Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Arch Phys Med Rehabil 81: 49-56.
- Oerlemans HM, et al. (2002) Favourable effect of adjuvant physical therapy (and to a lesser extent occupational therapy) compared with social work in reflex sympathetic dystrophy of one upper limb: A randomised controlled clinical trial. Nederlands Tijdshrift voor Geneeskunde 146: 895-902.
- Oerlemans HM, Goris JA, de Boo T, Oostendorp RA (1999) Do physical therapy and occupational therapy reduce the impairment percentage in reflex sympathetic dystrophy? Am J Phys Med Rehabil 78: 533-539.
- Perez RS, Zollinger PE, Dijkstra PU, Thomassen-Hilgersom IL, Zuurmond WW, et al. (2010) Evidence based guidelines for complex regional pain syndrome type 1. BMC Neurol 10: 20.
- Schreuders TA, Selles RW, Roebroeck ME, Stam HJ (2006) Strength measurements of the intrinsic hand muscles: a review of the development and evaluation of the Rotterdam intrinsic hand myometer. J Hand Ther 19: 393-401.
- Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, et al. (2013) Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med 14: 180-229.
- Ramachandran VS, Rogers-Ramachandran D (1996) Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci 263: 377-386.
- McCabe CS, Haigh RC, Ring EF, Halligan PW, Wall PD, et al. (2003) A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology (Oxford) 42: 97-101.
- Ramachandran VS, Altschuler EL, Hillyer S (1997) Mirror agnosia. Proc Biol Sci 264: 645-647.
- Moseley GL (2004) Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 108: 192-198.
- Harris AJ (1999) Cortical origin of pathological pain. Lancet 354: 1464-1466.
- Maihofner C, Handwerker HO, Neundörfer B, Birklein F (2004) Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63: 693-701.
- Maihofner C, Handwerker HO, Neundörfer B, Birklein F (2003) Patterns of cortical reorganization in complex regional pain syndrome. Neurology 61: 1707-1715.
- Giraux P, Sirigu A (2003) Illusory movements of the paralyzed limb restore motor cortex activity. Neuroimage 20: S107-111.
- Filius A, Korstanje JW, Selles RW, Hovius SE, Slijper HP (2013) Dynamic sonographic measurements at the carpal tunnel inlet: reliability and reference values in healthy wrists. Muscle Nerve 48: 525-531.
- Karmarkar A, Lieberman I (2006) Mirror box therapy for complex regional pain syndrome. Anaesthesia 61: 412-413.
- Selles RW, Schreuders TA, Stam HJ (2008) Mirror therapy in patients with causalgia (complex regional pain syndrome type II) following peripheral nerve injury: two cases. J Rehabil Med 40: 312-314.
- Rothgangel AS, Braun SM, Beurskens AJ, Seitz RJ, Wade DT (2011) The clinical aspects of mirror therapy in rehabilitation: a systematic review of the literature. Int J Rehabil Res 34: 1-13.
- Moseley GL (2005) Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. Pain 114: 54-61.
- Swart CM, Stins JF, Beek PJ (2009) Cortical changes in complex regional pain syndrome (CRPS). Eur J Pain 13: 902-907.
- Priganc VW, Stralka SW (2011) Graded motor imagery. J Hand Ther 24: 164-168.
- McCabe CS, Haigh RC, Shenker NG, Lewis J, Blake DR (2004) Phantoms in rheumatology. Novartis Found Symp 260: 154-174.
- Moseley GL (2006) Graded motor imagery for pathologic pain: a randomized controlled trial. Neurology 67: 2129-2134.
- Lagueux E, Charest J, Lefrançois-Caron E, Mauger ME, Mercier E, et al. (2012) Modified graded motor imagery for complex regional pain syndrome type 1 of the upper extremity in the acute phase: a patient series. Int J Rehabil Res 35: 138-145.
- Nelson DV, Stacey BR (2006) Interventional therapies in the management of complex regional pain syndrome. Clin J Pain 22: 438-442.
- Robertson DP, Simpson RK, Rose JE, Garza JS (1993) Video-assisted endoscopic thoracic ganglionectomy. J Neurosurg 79: 238-240.
- Janig W, Habler HJ (2000) Sympathetic nervous system: contribution to chronic pain. Prog Brain Res 129: 451-468.
- Schurmann M, et al. (2001) Clinical and physiologic evaluation of stellate ganglion blockade for complex regional pain syndrome type I. Clin J Pain 17: 94-100.
- Price DD, Long S, Wilsey B, Rafii A (1998) Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clin J Pain 14: 216-226.
- Bonelli S, Conoscente F, Movilia PG, Restelli L, Francucci B, et al. (1983) Regional intravenous guanethidine vs. stellate ganglion block in reflex sympathetic dystrophies: a randomized trial. Pain 16: 297-307.
- Malmqvist EL, Bengtsson M, Sorensen J (1992) Efficacy of stellate ganglion block: a clinical study with bupivacaine. Reg Anesth 17: 340-347.
- Cepeda MS, Lau J, Carr DB (2002) Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: a narrative and systematic review. Clin J Pain 18: 216-233.
- Burton AW (2006) Internetional therapies in Complex Regional Pain Syndrome: Treatment guidelines, Reflex Sympathetic Dystrophy Syndrome Association.
- Ramamurthy S, Hoffman J (1995) Intravenous regional guanethidine in the treatment of reflex sympathetic dystrophy/causalgia: a randomized, double-blind study. Guanethidine Study Group. Anesth Analg 81: 718-723.
- Blanchard J, Ramamurthy S, Walsh N, Hoffman J, Schoenfeld L (1990) Intravenous regional sympatholysis: a double-blind comparison of guanethidine, reserpine, and normal saline. J Pain Symptom Manage 5: 357-361.
- Jadad AR, Carroll D, Glynn CJ, McQuay HJ (1995) Intravenous regional sympathetic blockade for pain relief in reflex sympathetic dystrophy: a systematic review and a randomized, double-blind crossover study. J Pain Symptom Manage 10: 13-20.
- Rocco AG, Kaul AF, Reisman RM, Gallo JP, Lief PA (1989) A comparison of regional intravenous guanethidine and reserpine in reflex sympathetic dystrophy. A controlled, randomized, double-blind crossover study. Clin J Pain 5: 205-209.
- Hord AH, Rooks MD, Stephens BO, Rogers HG, Fleming LL (1992) Intravenous regional bretylium and lidocaine for treatment of reflex sympathetic dystrophy: a randomized, double-blind study. Anesth Analg 74: 818-821.
- Ribbers GM, Geurts AC, Rijken RA, Kerkkamp HE (1997) Axillary brachial plexus blockade for the reflex sympathetic dystrophy syndrome. Int J Rehabil Res 20: 371-380.
- Raj PP, Montgomery SJ, Nettles D, Jenkins MT (1973) Infraclavicular brachial plexus block--a new approach. Anesth Analg 52: 897-904.
- Raj PP (2000) Nerve blocks: Continuous regional analgesia, in Practical Managment of Pain. Mosby, St Louis, MO.
- Rauck RL, Eisenach JC, Jackson K, Young LD, Southern J (1993) Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology 79: 1163-1169.
- Cooper DE, DeLee JC, Ramamurthy S (1989) Reflex sympathetic dystrophy of the knee. Treatment using continuous epidural anesthesia. J Bone Joint Surg Am 71: 365-369.
- Koning H, et al. (1995) Cervical epidural blockade and reflex sympathetic dystrophy. Pain clin 8: 239-244.
- van Hilten BJ, van de Beek WJ, Hoff JI, Voormolen JH, Delhaas EM (2000) Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med 343: 625-630.
- Ranoux D, Attal N, Morain F, Bouhassira D (2008) Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol 64: 274-283.
- Safarpour D, Salardini A, Richardson D, Jabbari B (2010) Botulinum toxin A for treatment of allodynia of complex regional pain syndrome: a pilot study. Pain Med 11: 1411-1414.
- Carroll I, Clark JD, Mackey S (2009) Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Ann Neurol 65: 348-351.
- O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL (2013) Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev 4: CD009416.
- Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M (2004) The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years' follow-up of the randomized controlled trial. Ann Neurol 55: 13-18.
- Forouzanfar T, Kemler MA, Weber WE, Kessels AG, van Kleef M (2004) Spinal cord stimulation in complex regional pain syndrome: cervical and lumbar devices are comparably effective. Br J Anaesth 92: 348-353.
- Calvillo O, Racz G, Didie J, Smith K (1998) Neuroaugmentation in the treatment of complex regional pain syndrome of the upper extremity. Acta Orthop Belg 64: 57-63.
- Evans JA (1946) Sympathectomy for reflex sympathetic dystrophy; report of twenty-nine cases. J Am Med Assoc 132: 620-623.
- Kim K, et al. (2002) Sympathectomy: Open and thoracoscopic, in Surgical Management of Pain. Thieme Publishers, New York.
- Wilkinson HA (1996) Percutaneous radiofrequency upper thoracic sympathectomy. Neurosurgery 38: 715-725.
- Mockus MB, Rutherford RB, Rosales C, Pearce WH (1987) Sympathectomy for causalgia. Patient selection and long-term results. Arch Surg 122: 668-672.
- Kumar K, Nath RK, Toth C (1997) Spinal cord stimulation is effective in the management of reflex sympathetic dystrophy. Neurosurgery 40: 503-508.
- Del Pinal F (2013) I have a dream ... reflex sympathetic dystrophy (RSD or Complex Regional Pain Syndrome - CRPS I) does not exist. J Hand Surg Eur 38: 595-597.
- Pawelka S, Fialka V, Ernst E (1993) Reflex sympathetic dystrophy and cigarette smoking. J Hand Surg Am 18: 168-169.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 16703
- [From(publication date):
February-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12113
- PDF downloads : 4590
