Complementary Nutritional Treatment for IBD
Received: 28-Feb-2018 / Accepted Date: 01-Mar-2018 / Published Date: 05-Mar-2018
Editorial
Inflammatory bowel diseases (IBD) are multifactorial disorders, characterized by chronic and degenerative inflammation of the gastrointestinal tract including Crohn Disease (CD) and Ulcerative Colitis (UC). These two diseases, affecting about three million people worldwide, share common symptoms including rectal bleeding, abdominal pain, and diarrheal episodes. The dysregulated secretion of inflammatory cytokines, including IL-1, IL- 6 and TNF-α is a hallmark of IBDs likely triggered by a defect in the intestinal barrier function that becomes more permissive to antigen trafficking from the intestinal lumen to the basolateral compartment. In the last decades, the introduction of monoclonal antibodies against crucial mediators of the inflammatory cascade represented an important alternative/ complementary strategy to immune-suppressants, corticosteroids and antibiotics. Unfortunately, even using biological agents, a significant percentage of patients fail to achieve prolonged clinical remission [1,2].
Besides pharmacological therapies, IBD patients often require nutritional management of the disease with the intent to avoid/ postpone disease relapse. Unfortunately, patient’s nutritional management is a complicated path. Various nutritional supplements may be administered, even if vitamin D deficiency seems to be associated with the pathogenesis of IBD [3]. Iron deficiency is equally common in IBD patients with active disease, although dietary supplementation is sometimes associated with worsening disease symptoms and thus, intravenous infusion has to be preferred to oral administration. Fecal therapy has been recently used for the clinical treatment of refractory Clostridium difficile infection suggesting a crucial role for the intestinal microbiota transplant. Probiotics and prebiotics administration has been studied in the last decade, but more should be done to clarify the role of diets that favour growth of pathogenic bacteria that, in turn, compromise the intestinal barrier integrity [4].
Likely, the best example of efficacy for the treatment of CD patients is represented by fibre-free enteral nutrition, although in different studies the induction of remission varied from 20 to 84% [5]. It is known that, especially for Crohn’s, the composition of normal microbiota is changed and enteral nutritional therapies may prevent deleterious proliferation of intestinal bacterial colonies [6,7]. Similarly, the known anti-inflammatory effects of polyphenols were long associated with their anti-inflammatory properties [8-10]. Only recently, Gallegiante at al. associated polyphenol iron chelating properties with their anti-inflammatory effects [11]. This innovative prospective may be used as strategy to sequestrate luminal iron, a limiting factor for bacterial growth.
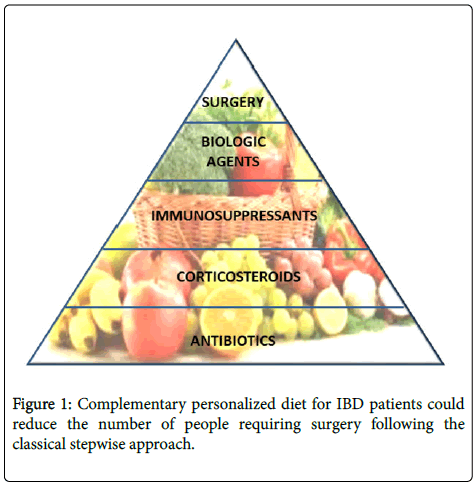
We are still working to understand the complex inter-relations between food intake, microbiota proliferation, intestinal permeability and inflammation. All together, these aspects can decide the balance between intestinal inflammation and tolerance, but much has to be clarified before the definition of a definitive nutritional strategy to sustain IBD remission (Figure 1). Currently, much of the nutritional suggestions are speculative or observational.
References
- Flamant M and Roblin X (2018) Inflammatory bowel disease: towards a personalized medicine. Therap Adv Gastroenterol 11: 1756283X17745029.
- Lu Y, Li X, Liu S, Zhang Y, and Zhang D et al. (2018) Toll-like Receptors and Inflammatory Bowel Disease. Front Immunol 9: 72.
- Halmos EP and Gibson PR (2015) Dietary management of IBD--insights and advice. Nat Rev Gastroenterol Hepatol 12: 133-146.
- De Santis S, Cavalcanti E, Mastronardi M, Jirillo E and Chieppa M et al. (2015) Nutritional Keys for Intestinal Barrier Modulation. Front Immunol 6: 612.
- Zachos M, Tondeur M and Griffiths AM (2007) Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database Syst Rev 24: CD000542.
- Gatti S, Galeazzi T, Franceschini E, Annibali R and Albano V et al. (2017) Effects of the Exclusive Enteral Nutrition on the Microbiota Profile of Patients with Crohn's Disease: A Systematic Review. Nutrients 9 pii: E832.
- Hunter J (2015) Elemental diet and the nutritional treatment of Crohn's disease.
- Cavalcanti E, Vadrucci E, Delvecchio FR, Addabbo F and Bettini S et al. (2014) Administration of reconstituted polyphenol oil bodies efficiently suppresses dendritic cell inflammatory pathways and acute intestinal inflammation. PLoS One 9: e88898.
- Delvecchio FR, Vadrucci E, Cavalcanti E, De Santis S, Kunde D et al. (2015) Polyphenol administration impairs T-cell proliferation by imprinting a distinct dendritic cell maturational profile. Eur J Immunol 45: 2638-2649.
- Scarano A, Butelli E, De Santi S, Cavalcanti E, Hill L, De Angelis M, Chieppa M, Martin C.
- Galleggiante V, De Santis S, Cavalcanti E1, Scarano A2, De Benedictis M et al. (2017) Dendritic Cells Modulate Iron Homeostasis and Inflammatory Abilities Following Quercetin Exposure. Curr Pharm Des 23: 2139-2146.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3343
- [From(publication date): 0-2018 - Mar 14, 2025]
- Breakdown by view type
- HTML page views: 2625
- PDF downloads: 718