Research Article Open Access
Comparison of Various Detection Systems Coupled to High Performance Liquid Chromatography for Determination of Tocopherols in Meat. The Influence and Comparison of the Most Popular Sample Preparation Method
Szterk A1 *, Roszko M2 , Najman K1 , Kruk M1 , Mroczek E1 , Zarodkiewicz M1 , Rogalski M1 and Waszkiewicz-Robak B11Department of Functional Food, Organic Food and Commodities Faculty of Human Nutrition and Consumer Sciences, Warsaw University of Life Sciences, Poland
2Department of Food Analysis, Institute of Agricultural and Food Biotechnology, Poland
- *Corresponding Author:
- Arkadiusz Szterk
Department of Functional Food
Organic Food and Commodities Faculty of Human Nutrition and Consumer Sciences
Warsaw University of Life Sciences
159 c Nowoursynowska, 02-776 Warsaw, Poland
Tel: +48 22 59 37057
Fax: +48 22 59 37040
E-mail:szterkarkadiusz@gmail.com
Received date: December 04, 2013; Accepted date: December 28, 2013; Published date: December 30, 2013
Citation: Szterk A, Roszko M, Najman K, Kruk M, Mroczek E, et al. (2013) Comparison of Various Detection Systems Coupled to High Performance Liquid Chromatography for Determination of Tocopherols in Meat. The Influence and Comparison of the Most Popular Sample Preparation Method. J Anal Bioanal Tech S2: 005. doi: 10.4172/2155-9872.S2-005
Copyright: © 2013 Szterk A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The presented data are of practical importance in terms of vitamin E determination in meat and other foodstuffs. Selection of a proper separation and detection method as well as sample preparation protocol might affect the reliability of results. Application of a sensitive method and such sample preparation procedures that do not cause significant degradation of the analyte ensures the reliability of the results. The conducted research has proved that the fluorescence detector (FLD) is the most sensitive of the detection systems tested and provides the LOD values in 3.8-38.5 ng ml-1 range. Ion trap was proved to be less a sensitive detector against vitamin E when compared to FLD. When atmospheric pressure chemical ionisation (APCI) was utilised the LOD values observed for γ- and α- tocopherole were 0.2 and 0.5 μg ml-1, respectively. However when atmospheric pressure photo/chemical ionisation (APPI/APCI) was used a significant increase in the ionization efficiency of δ-tocopherol was observed, which caused increases in the instrument sensitivity (LOD=1.0 μg ml-1). Sample preparation protocol based on extraction of the biological sample with ethanol and hexane with simultaneous change in partitioning coefficient, results in significantly higher recovery rates when compared to methods based on sample saponification and extraction with other solvents. Vitamin E contents in the tested pork samples were found to be in the 0.0-6.0 mg kg-1 range, depending on the determination method utilised.
Keywords
Tocopherol; Vitamin E; Separation technique; Liquid chromatography; Comparison methods; Mass spectrometry; APCI; APPI; LCMS
Abbreviations
LOQ: Limit of Quantivication; LOD: Limit of Detection; ESI: Electrospray Ionization; APCI: Atmospheric Pressure Chemical Ionization; APPI: Atmospheric Pressure Photoionization; APCI/APPI: Atmospheric Pressure Photochemical Ionization; FLD: Fluorescence Detector; PAD: Photodiode Array Detector; LC: Liquid Chromatography; TOF: Time of Flight Detector; MS: Mass Spectrometry; IT: Ion Trap
Introduction
Vitamin E was first discovered by Evans and Bishop in 1923 and covers all tocol derivatives. Vitamin E is a group of compounds that include 4 tocopherols and 4 tocotrienols. Among others the α-tocoferol shows the highest biological activity. β- and γ-tocopherol shows, respectively 40 and 8% of the α-tocopherol activity, while α-tocotrienol approximately 20% activity. Other compounds show little biological activity. Tocopherols are known as natural antioxidants that protect vitamin A, polyunsaturated fatty acids and other labile compounds from oxidation. In addition it was observed that vitamin E in the human body stabilises the cell membranes and might decrease the risk of cancer. There is data available which indicates that vitamin E might have a positive impact on the process of the body ageing, function of the heart and it is also known as a factor responsible for the restoration and strengthening of sexual potency.
α-tocopherol has the highest biological activity but the γ-tocopherol is consumed in the largest quantities. The latter is commonly found in various types of products of plant origin. It is also significantly much more abundant than α-tocopherol in meat products. It should be noted that γ-tocopherol has a significantly higher potency to scavenge the reactive nitrogen dioxide compared to the α- form [1,2]. γ-tocopherol rather than α-tocopherol is reported as an anti-cancer and heartprotecting agent [1]. It was recently reported that a mixture of α, γ, δ tocopherols (5:2:1 w/w/w) shows stronger anti-oxidative potency than α-tocopherole in the same quantity alone [3]. This report suggests that the tocopherol profile shall be determined besides total vitamin E contents to assess the food nutritional value. This however implies the necessity to apply non-destructive sample preparation procedures prior to determination that do not cause drop in individual tocopherol concentrations and changes in vitamin E profile.
The most commonly utilised methods of tocopherol determination employ liquid chromatography separation and UV-VIS or fluorometric detection [4]. Normal type column phases are frequently used to separate β- and γ-tocopherols, which could not be done on a typical reversed C18 phase [5-7]. However β-tocopherol is present in food at very low concentrations compared to other homologues, it also does not practically occur in products of animal origin. That is why reversed phase HPLC is considered as a very convenient technique for determination of δ-γ- and α-tocopherols in foodstuffs. The main advantage resulting from the application of the reversed phase is a high phase stability, reproducibility of retention times, and the possibility to avoid the use of highly volatile solvents compared to the normal phase [4-7].
Due to strongly diversified concentrations of tocopherols in various food products (in meat products <1 mg kg-1, especially after thermal treatment) it is necessary to utilise sensitive analytical methods that enable proper identification of the most important homologues (δ-, γ- and α-tocopherols).
GC-MS based methods are also commonly used for determination of tocopherols in food; this however implies a derivatisation step in the sample preparation procedure [8,9]. LC-APCI-MS, LC-ESI-MS using both normal and reversed are also commonly used techniques [9-21]. There are also numerous reports on application of more sophisticated detectors such as LC-APCI/ESI-MS/MS (triple quadrupole analysers) [9,16,17,22,23] or LC-APCI/ESI-TOF-MS [20].
There is a scarcity of data on the application of the LC-IT-MS technique coupled with photoionisation or photo/chemical ionisation for determination of tocopherols available. The applied sample preparation procedure prior to chromatography is also of great importance in the determination process. The most commonly applied sample preparation procedures involve a saponification step of the fatty fraction or the direct determination of tocopherols in the fatty extract.
It is well known that the stability of tocopherols in basic media is limited leading to rapid chemical degradation. To slow down the degradation process antioxidants such as ascorbic acid, pirogallol, butylated hydroxytoluene and its mixtures are used. There are many methods of sample preparation for determination of vitamin E available. This causes a problem of the proper selection of the method of vitamin E determination in a meat sample. The contents of tocopherols in meat are strongly diversified, which is directly related to the differences in the animal feeding (the use of vitamin E supplemented feed or tocopherols rich vegetable oils etc.) method. In addition the meat processing (especially heat treatment) in most cases is expected to cause some loss in the tocopherol contents and may also strongly affect its profile. In such a case there is a need to apply such sample preparation methods that would induce changes in the tocopherol concentration/profiles to a minimal extent. Subsequently an appropriate tocopherol separation and detection technique enabling quantification of its individual form must be utilised.
The aim of this work was to assess the applicability of the reversed phase high performance liquid chromatography coupled to programmable diode array (PDA) detector, fluorometer (FLD), and ion trap mass spectrometer operated in atmospheric pressure chemical ionisation (APCI), atmospheric pressure photo ionisation (APPI) and combined atmospheric pressure photochemical ionisation (APPIAPCI) for determination of δ-, γ- and α-tocopherols. The sensitivity of the applied detection systems against studied compounds was evaluated. The usefulness of fifteen the most commonly reported in the literature sample preparation procedures for determination of vitamin E in meat were evaluated. Soma additional modifications of the applied methods enabling achievement of a minimal loss of tocopherol have been reported.
Experimental
Materials
Experimental material was composed of 18 samples of pork ham obtained from the Polish White Landrace breed (9 samples) and hybrid Polish White Landrace×Duroc (9 samples). The animals were fed with standard, balanced feed supplied by INNTAKER Polska. After reaching the assumed mass the animals were slaughtered. The samples were taken 24 hours after ripening. Hams were processed as a complete culinary element by grinding. Samples were then packed into a 50 ml Falcon centrifuge tubes, frozen in liquid nitrogen and stored at -80°C. Samples were then analysed with regard to the method recovery and the vitamin E contents suing 15 different sample preparation procedures.
Reagents
Analytical standards of δ-, γ- and α-tocopherols, dimethyl ether, iso-propyl ether, ethanethiol, dimethyl sulphide, acetone, cyclohexanone, ethyl amine, n-butylamine, N,N-dimethylformamide, toluene, benzonitrile, and phenol (purity>99.5%) were supplied by Sigma Aldrich (Poland), Ethanol, isooctane, n-hexane, acetonitrile, acetic acid 50%, methyl-tert- butyl ether (MTBE), all of HPLC grade as well as potassium hydroxide, ascorbic acid, pirogallol, hydrochloric acid (36%), sodium chloride, ethylene diamine tetra sodium acetate of p.a. grade were obtained from POCh (Gliwice, Poland).
Preparation of standard solutions
Analytical standards (100 mg) were dissolved in 4 ml of n-hexane (Solution A: 25 mg ml-1). 50 μl of the first solution was transferred into a 50 ml measuring flask and diluted with n-hexane (Solution B: 0.025 mg ml-1).
To evaluate the relative fluorescence response factors were pipetted into a 50 ml round bottom flask 10 ml of δ-tocopherol (M=402.65 g mol-1) B solution, 10.4 ml of λ-tocopherol (M=416.68 g mol-1) B solution and 10.7 ml α-tocopherol (M=430.71 g mol-1) B solution. The mixture was then evaporated using a rotary evaporator in the absence of light at 30°C. The dry residue was quantitatively transferred with n-hexane into a 10 ml measuring flask (Solution C: 62.1 nmol ml-1). 20 μl of the C solution was transferred into a 2 ml vial and dried under a gentle stream of nitrogen. Residues were re-dissolved in MTBE and 5 μl (6.2 pmol of each tocopherol) were injected into the HPLC. Tocopherols were separated and the response factors were estimated as the relative area under the chromatographic peaks registered at λem=290 nm and λex=330 nm.
For determination of the relative absorption and ionisation coefficients 1 ml of the C solution was evaporated under a nitrogen stream and re-dissolved in 1ml of MTBE and used for experiments. 5 μl (310.5 pmol of each tocopherol) were injected into HPLC, analytes were separated. As in the previous case peaks area was used to assess the response factors. All experiments were conducted in 12 replications.
Quantification and methods evaluation
Tocopherols in the evaluated meat samples were quantified (HPLC/ FLD) using internal standard method. δ-tocopherol was used as an internal standard in concordance with the method previously reported [24]. Previously determined fluorescence response factors were used for calculations.
Response linearity was evaluated using individual tocopherol standards at concentrations in 0.03-8.3 μg ml-1 (FLD) and 0.1-66.4 μg ml-1 (other detectors) range. The lowest tested concentration was always below the limit of detection while the highest overloaded the detector. Each concentration level used for the calibration curve plotting was analysed in 12 replicates. The limits of detection and quantification were calculated as a theoretical concentration producing a signal to noise of a chromatographic peak equal to 3 and 10, respectively [21].
The recovery method was determined for the 16 tested methods of sample preparation according to Cho et al. [25] with minor modifications. 3 μg g-1 of δ-, γ- and α-tocopherol addition to the tested meat sample was used. Tocopherols were determined using HPLC coupled to fluorometer. Method recovery was evaluated in 6 separate replicates for each method of sample preparation.
Sample preparation
Sixteen various sample preparation procedures (the most commonly described in the literature) reported by He et al. [26]; Pfalzgraf et al. [27]; Cort et al. [28]; Schuep and Rettenmaier [29]; Kanazawa et al. [24]; Hewavitharana et al. [30]; Arnold et al. [31]; Gaal et al. [32]; Jenson et al. [33]; McMurray and Blanchflower [34]; Berlin et al. [35]; Hatam and Kayden [36]; Zaspel and Csallany [37]; Liu et al. [38]; Miller et al. [39] and modified Hewavitharana et al. [30] were used.
To the all utilised procedures a final step involving quantitative sample transfer into a round bottom flask, evaporation (in the dark) at 30°C using rotary evaporator and reconstitution in 8 ml of MTBE was added.
In the method reported by Hewavitharana et al. [30], the water at each stage of the sample preparation procedure was replaced by the saturated NaCl aqueous solution.
Apparatus
Accela Thermo Scientific chromatographic system composed of auto injector, PDA detector, quaternary Accela 600 pump was used for separation and quantifications. In addition Dionex FLD3400RS fluorometer and Thermo Scientific LCQ Fleet ion trap mass spectrometer were used for quantifications.
HPLC condition
Chromatographic separations were performed using a reversed phase Thermo Hypersyl GOLD C18 150 mm×2.1 mm×1.8 μm column. The following mobile phase gradient was utilised: A-H2O:ACN 1:9, BMTBE: 0 min 100% A, 5 min 100% A, 8 min 60% A, 9 min 10% A, 13 min 10% A from 14 up to 16 min return to initial conditions. Mobile phase flow rate was set at 450 μl min-1, column was thermostated at 50°C.
Detectors and ion source optimisation
Tocopherols were quantified using fluorometer (FLD) operated at λem=290 nm and λex=330 nm and PDA detector (λ=290 nm). Ion trap mass spectrometer operated in atmospheric pressure chemical-, photoand photochemical- ionisation modes was also used in the experiments. Ion trap operating parameters were optimised individually for the studied compounds–max injection time-200 ms, microscans-3, parent mass (m/z)–δ-tocopherol: 403, γ-tocopherol: 417 α-tocopherol 431, act type–CID, isolation width (m/z)–4, normalized collision energy–30%, activation Q–0.45, activation time (ms)–50, helium was used as a dumping gas, scan range (m/z)–250-435.
Optimisation of the atmospheric pressure chemical ionisation conditions was performed according to the reports of Lanina et al. [21] and Bustamante-Rangel et al. [40]. Acetonitrile: water solution (90:10) containing 0.04% of acetic acid was used as a mobile phase. Lanina et al. [21] have reported that significantly better tocopherol ionisation yields were observed when mobile phase composed of methanol:water (95:5) was used. The results of our experiments did not confirm those findings. The use of methanol did not provide improvement in sensitivity but unnecessarily prolonged the total separation time. Due to the above circumstances a mobile phase composed of acetonitrile and water was utilised with an addition of acetic acid, which considerably improved the ionisation yield and sensitivity.
Acetic acid is commonly reported in the literature as an additive used to improve the productivity of the ionisation process in the mass spectrometer ion source [21,25,40-42].
The observed yield of the positive ionisation was higher than negative ionisation and those findings are in concordance with the literature data [25,40,42]. After the conducted experiments the following APCI parameters were considered as optimal: Vaporiser temperature 400°C, capillary voltage 3 kV, corona current 5 μA, sheath gas flow rate 0.75 L min-1, aux gas flow rate 0.075 L min-1, sweep gas flow rate 0 L min-1, capillary temperature 150°C. Those parameters were close to those reported by other authors [25,40,42]. The same ionisation conditions were used in the APPI experiments but with corona discharge current turned off. When combination of APPIAPCI was used the krypton lamp was turned on during regular APCI operation.
In addition the influence of the post column addition of modifiers to the mobile phase such as dimethyl ether (IP=9.53 eV), iso-propyl ether (IP=9.2 eV), ethanethiol (IP=9.2 eV), dimethyl sulphide (IP=8.7 eV), acetone (IP=9.69 eV), cyclohexanone (IP=914 eV), ethyl amine (IP=8.86 eV), n-butylamine (IP=8.72 eV), N,N-dimethylformamide (IP=9.12 eV), toluene (IP=8.82 eV), benzonitrile (IP=9.71 eV) or phenol (IP=8.22 eV) on the ionisation process was assessed. The substances with ionisation potentials lower than 10 eV were selected for experiments due to ionisation energy of the krypton lamp used in the APPI source (10,3 eV–catalogue data). The two flow rates 10 and 50 μl min-1 of the mobile phase modifier was used. The aim of these experiments was to increase the efficiency of the photoionisation by indirect ionisation of tocopherol molecules.
Statistical assessment
The results were statistically assessed using analysis of variance (ANOVA) and hierarchical and non-hierarchical cluster analysis methods according with Czarniecka-Skubina E et al. [43]. STATISTICA StatSoft package was used for analysis.
Results
The method limits of detection, linearity ranges and the relative response factors of FLD, PDA and mass spectrometer (with different ionisation techniques) calculated for δ-, γ- and α-tocopherols separated with reversed phase HPLC are shown in Tables 1-5.
| LOD | Linearity range | r2 | Relative response factors (F) λex = 290 nm/λem = 330 nm n = 6,21 pmol |
|||
| mg kg-1 (ng ml-1) |
Equation | mg kg-1 (ng ml-1) |
F | RSD | ||
| δ | 0,005 (3,8) |
y=1,54.108x | 0,007 - 0,97 (5,4 – 749,4) |
0,983 | 1 | 0 |
| γ | 0,01 (7,7) |
y=-1,380106+1,15.108x | 0,012 - 1,7 (9,2 – 1282,0) |
0,981 | 0,79 | 0,67 |
| α | 0,05 (38,5) |
y=-653491+2,88.107x | 0,053 - 5,0 (40,8 – 3846,2) |
0,986 | 0,18 | 0,76 |
Table 1: Limit of detection, linearity range and relative response factors calculated for studied tocopherols determined by LC-FLD.
| LOD | Linearity range | r2 | Relative response factors (A) λ = 290 nm n = 310,5 pmol |
|||
| mg kg-1 (µg ml-1) |
Equation | mg kg-1 (µg ml-1) |
A | RSD | ||
| δ | 1,45 (1,1) |
y=23121,4+3337,9x | 1,6 - 62,5 (1,2 – 48,0) |
0.985 | 1 | 0 |
| γ | 1,25 (0,96) |
y=34373,4+3844,0x | 1,4 - 62,5 (1,1 – 48,0) |
0.987 | 1,3 | 3,21 |
| α | 1,45 (1,1) |
y=27470,2+3693,6x | 1,6 - 62,5 (1,2 – 48,0) |
0.991 | 1,2 | 1,82 |
Table 2: Limit of detection, linearity range and relative response factors calculated for studied ocopherols determined by LC-PDA.
| LOD | Linearity range | r2 | Relative response factors (I) n = 310,5 pmol |
|||
| mg kg-1 (µg ml-1) |
Equation | mg kg-1 (μg ml-1) |
I | RSD | ||
| δ | 2,4 (1,8) |
y=-3506,3+985,7x | 2,5 - 62,5 (1,9 – 48,0) |
0.984 | 1 | 0 |
| γ | 0,6 (0,5) |
y=-9570,8+2319,9x | 0,7 - 62,5 (0,54 – 48,0) |
0.983 | 2,55 | 4,15 |
| α | 0,3 (0,2) |
y=-34869,7+4976,1x | 0,4 - 62,5 (0,3 – 48,0) |
0.980 | 4,45 | 2,48 |
Table 3: Limit of detection, linearity range and relative response factors calculated for studied tocopherols determined by LC-APCI/MS/MS.
| LOD | Linearity range | r2 | Relative response factors (I) n = 310,5 pmol |
|||
| mg kg-1 (µg ml-1) |
Equation | mg kg-1 (µg ml-1) |
I | RSD | ||
| δ | 2,9 (2,2) |
y=-423,7+367,3x | 3,0 – 62,5 (2,3 – 48,0) |
0,996 | 1 | - |
| γ | 1,3 (1,0) |
y=351,3+375,6x | 1,4 – 62,5 (1,1 – 48,0) |
0,994 | 2,4 | 3,6 |
| α | 0,8 (0,6) |
y=-102342429,3x | 0,9 – 62,5 (0,7 – 48,0) |
0,998 | 4,3 | 2,9 |
Table 4: Limit of detection, linearity range and relative response factors calculated for studied tocopherols determined by LC-APPI/MS/MS.
| LOD | Linearity range | r2 | Relative response factors (I) n = 310,5 pmol |
|||
| mg kg-1 (μg ml-1) |
Equation | mg kg-1 (μg ml-1) |
I | RSD | ||
| δ | 1,0 (0,8) |
y= 9372,0+902,2x | 1,1 – 62,5 (0,85 – 48,0) |
0,991 | 1 | - |
| γ | 0,8 (0,6) |
y= 12220,710443x | 0,9 – 62,5 (0,7 – 48,0) |
0,998 | 2,1 | 3,1 |
| α | 0,4 (0,3) |
y= 33655,2+39991,3x | 0,5 – 62,5 (0,4 – 48,0) |
0,992 | 5,0 | 5,0 |
Table 5: Limit of detection, linearity range and relative response factors calculated for studied tocopherols determined by LC-APPI-APCI/MS/MS.
The results of this study have proved that fluorometer has the highest sensitivity against tocopherols of the detectors evaluated. The PDA detector had the lowest sensitivity.
δ-tocopherol shows the highest intensity of fluorescence hence the lowest LOD values were observed for this compound. The presence of additional methyl- groups on the tocopherol molecules (γ- and α-) significantly reduces the efficiency of fluorescence therefore the highest LOD was calculated for α-tocopherol. Comparison of the evaluated detection techniques have proved that FLD shows the lowest dynamic linearity range.
The relative response values calculated when the PDA detector was utilised was similar to those observed for PDA. δ-, γ- and α- tocopherols show similar light absorption hence the calculated LOD values were similar. The dynamic linearity range of the PDA detector was significantly wider than those observed for FLD.
The application of the mass spectrometer gave a significant increase in the method sensitivity comparing to the PDA detection. However the LOD values calculated for individual tocopherol were influenced by the ionisation technique applied. In all studied cases (APCI, APPI, APCI-APPI) the ionisation yield was affected by the presence of methyl-substituents on the tocopherol molecule. That is why the highest sensitivity was observed for the α-tocopherol. Regardless of the technique of ionization tocopherols ionized as (M+H)+ protocol during positive ionization module. The full mass scan range was m/z 100-500 (1 s/scan) and the target ions generated by tocopherols were in positive ion mode (M+H)+: m/z 431.3 for α-tocopherol; m/z 417.3 for γ-tocopherol and m/z 403.3 for δ-tocopherol. In positive ion mode provided the formation of protonated molecular ions [M+H]+ as well as the abundant characteristic fragment ions of the studied analytes (m/z: 165.3 α-tocopherol; 151.3 γ-tocopherol and 137.3 δ-tocopherol) due to elimination of the phytyl chain (RDA process).The lowest LOD values were observed for α- and γ-tocopherol when APCI ionisation was applied. Photochemical ionisation (APPI-APCI) was the most effective against δ-tocopherol and relatively less efficient against α- and γ-homologues.
Direct photoionisation of tocopherols (in the acetonitrile: water medium) was found to be relatively ineffective. An attempt to increase efficiency by the indirect ionisation of tocopherols with easily photoionisating compounds (with ionisation potential below 10 eV) was made. Through a tee joint a solution of various compounds were introduced to the post column. In any case a significant increase in the ionisation efficiency of the studied tocopherols was observed. In many cases the dopant addition reduced the photoionisation efficiency; this phenomenon was particularly evident in case of nitrogen containing additives.
Table 6 shows the tocopherols recovery values calculated for the different meat sample preparation methods. The most commonly applied sample preparation procedures were evaluated. The conducted experiments have demonstrated that the tocopherol recovery was strongly affected by the sample preparation procedure applied (p<0.01). A significantly higher recovery was observed for most sample preparation procedures that do not involve meat sample saponification but only a simple solvent extraction and direct determination. However the method reported by Liu et al. [38] involving sample saponification showed the best recovery of all methods tested. The method reported by Hewavitharana et al. [30] where the water was replaced by the saturated sodium chloride solution during the tocopherols re-extraction out of the ethanolic solution step was considered as the best tested method.
| Method | d-tocopherol | γ-tocopherol | α-tocopherol | |||
 |
SD |  |
SD | 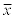 |
SD | |
| He et al. [26] | 1,8a | 0,8 | 7,2a | 1,3 | 1,6a | 0,9 |
| Pfalzgraf et al. [27] | 16,8b | 4,2 | 8,7ab | 1,3 | 13,2b | 1,1 |
| Cort et al. [28] | 17,2b | 3,2 | 13,9bc | 4,4 | 18,1bc | 1,9 |
| Schuep et al. [29] | 22,7b | 1,5 | 17,6c | 0,6 | 20,2cd | 1,2 |
| Kanazawa et al. [24] | 30,7c | 3,8 | 16,0c | 1,2 | 21,1cd | 3,4 |
| Arnold et al. [31] | 34,7c | 5,4 | 16,8c | 3,5 | 25,2d | 2,1 |
| Gaal et al. [32] | 79,5g | 5,0 | 46,8e | 1,0 | 41,0e | 1,3 |
| Jenson et al. [33] | 59,5f | 4,7 | 47,1e | 3,9 | 51,2f | 0,3 |
| McMurray and Blanchflower [34] | 51,4d | 0,9 | 37,8d | 1,2 | 53,8f | 0,5 |
| Berlin et al. [35] | 58,5ef | 4,6 | 44,5e | 7,5 | 57,3fg | 7,2 |
| Hatam and Kayden [36] | 52,4de | 3,4 | 61,1g | 2,6 | 61,5g | 1,7 |
| Hewavitharana et al. [30] - without saponification | 63,5f | 2,5 | 68,1h | 3,9 | 71,1h | 0,9 |
| Zaspel and Csallany [37] - without saponification | 49,4d | 2,1 | 60,0g | 2,6 | 72,9hi | 1,5 |
| Liu et al. [38] | 63,6f | 6,2 | 52,5f | 5,5 | 78,1i | 3,1 |
| Miller et al. [39] - without saponification | 88,4h | 3,7 | 89,3i | 0,7 | 88,7j | 2,6 |
| Hewavitharana et al. [30] with modification and without saponification | 91,1h | 0,9 | 90,2i | 1,4 | 93,5j | 0,4 |
Table 6: Average recovery of the individual tocopherols depending on the sample preparation method applied [%] n=96.
Table 7 shows the vitamin E concentrations determined with HPLCFLD in samples processed with various sample preparation procedures. The concentration of γ-tocopherol in meat samples was only assessed with the procedures showing the highest recovery values. The methods showing low recovery did not enable determination of γ-tocopherol in meat; also it was not possible to determine α-tocopherol in pork with use of those methods.
| Method | γ-tocopherol | α-tocopherol | ||
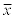 |
SD | 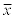 |
SD | |
| He et al. [26] | nd | - | nd | - |
| Pfalzgraf et al. [27] | nd | - | 0,3 | 0,08 |
| Cort et al. [28] | nd | - | 0,5 | 0,04 |
| Schuep et al. [29] | nd | - | 0,7 | 0,07 |
| Kanazawa et al. [24] | nd | - | 0,7 | 0,04 |
| Arnold et al. [31] | nd | - | 0,7 | 0,03 |
| Gaal et al. [32] | 0,013 | 0,001 | 2,2 | 0,06 |
| Jenson et al. [33] | 0,012 | 0,001 | 3,0 | 0,03 |
| McMurray and Blanchflower [34] | nd | - | 3,0 | 0,13 |
| Berlin et al. [35] | 0,011 | 0,001 | 3,0 | 0,16 |
| Hatam and Kayden [36] | 0,016 | 0,001 | 3,6 | 0,21 |
| Hewavitharana et al. [30] - without saponification | 0,045 | 0,002 | 4,6 | 0,43 |
| Zaspel and Csallany [37] - without saponification | 0,017 | 0,001 | 4,1 | 0,27 |
| Liu et al. [38] | 0,020 | 0,001 | 4,4 | 0,13 |
| Miller et al. [39] - without saponification | 0,045 | 0,001 | 5,2 | 0,15 |
| Hewavitharana et al. [30] with modification and without saponification | 0,051 | 0,001 | 6,0 | 0,68 |
Table 7: Concentration of tocopherols determined in pork ham with various sample preparation methods [mg kg-1]. n=288.
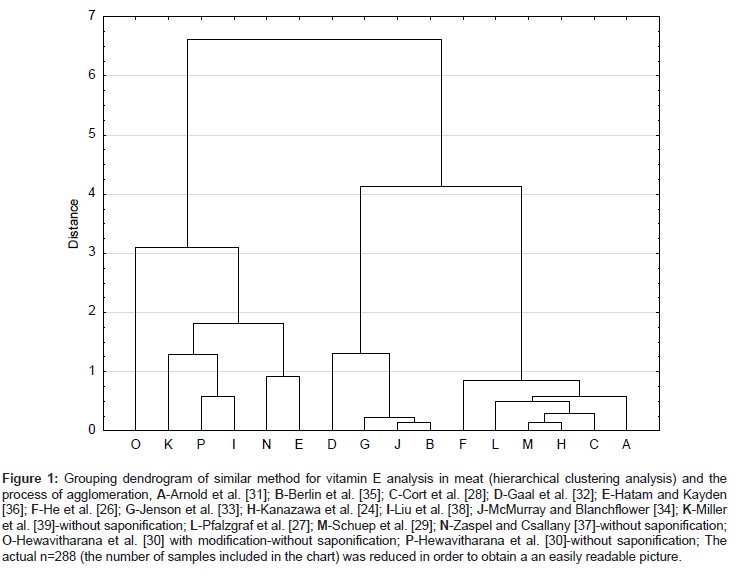
The results of the vitamin E determination in meat obtained with various methods were statistically assessed with hierarchical and nonhierarchical methods (Figure 1 and Table 8) to identify the methods that give similar results.
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
| Vitamine E content [mg kg-1] |
4,1 - 6,0 | 2,2 - 3,6 | 0,5 - 0,7 | 0,0 - 0,3 |
| Methods | Zaspel & Csallany [37]; Miller et al. [39]; Liu et al. [38]; Hewavitharana et al. [30] with modification; Hewavitharana et al. [30] | Hatam & Kayden [36]; McMurray & Blanchflower [34]; Berlin et al., [35]; Gaal et al., [32] Jenson et al., [33] | Cort et al. [28]; Arnold et al. [331]; Schuep et al. [29]; Kanazawa et al. [24] | Pfalzgraf et al. [27]; He et al. [26] |
Table 8: Non-hierarchicla statistical analysis of the results similarity n=288.
Figure 1: Grouping dendrogram of similar method for vitamin E analysis in meat (hierarchical clustering analysis) and the process of agglomeration, A-Arnold et al. [31]; B-Berlin et al. [35]; C-Cort et al. [28]; D-Gaal et al. [32]; E-Hatam and Kayden [36]; F-He et al. [26]; G-Jenson et al. [33]; H-Kanazawa et al. [24]; I-Liu et al. [38]; J-McMurray and Blanchflower [34]; K-Miller et al. [39]-without saponification; L-Pfalzgraf et al. [27]; M-Schuep et al. [29]; N-Zaspel and Csallany [37]-without saponification; O-Hewavitharana et al. [30] with modification-without saponification; P-Hewavitharana et al. [30]-without saponification; The actual n=288 (the number of samples included in the chart) was reduced in order to obtain a an easily readable picture.
The results obtained with modified and unmodified method reported by Hewavitharana et al. [30], as well as methods proposed by Liu et al. [38], Miller et al. [39] and Zaspel and Csallany [37] gave the highest concentrations of the vitamin E in pork. The concentration of the vitamin determined with the latter methods was in the 4.1-6.0 mg kg-1 range. The remaining methods depending on the quantity and type of the added antioxidant, concentration of the aqueous KOH solution used, and the duration and the temperature of the saponification process gave significantly lower results.
Methods that bypass the saponification step significantly reduce vitamin E, therefore the concentration of vitamin E in pork determined with such methods gave higher results.
Discussion
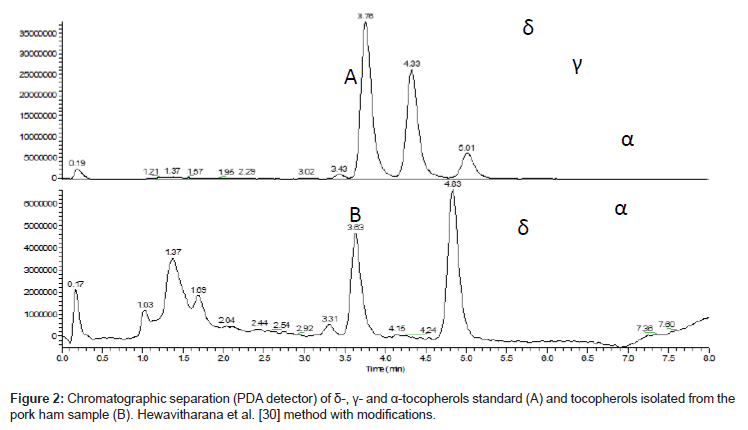
Reversed phase high performance liquid chromatography is a commonly applied technique for the determination of vitamin E in various types of biological samples. Most frequently octadecylsilica (C18) based beds are used for separation. Such phases do not however enable separation of γ and β homologues of tocopherols and tocotrienols [44-48]. The use of more sophisticated beds for example C30 phase solves the separation problem of tocopherols and tocotrienols [10,49]. The aim of this study was only to assess the levels of γ- and α- tocopherols that are predominantly present in meat. Obviously δ-tocopherol concentration, which was used as an internal standard was always assessed. Chromatographic separation of the studied compounds is shown on Figure 2. The use of a reversed phase chromatography enabled a significant reduction in the total analysis time of up to 8 minutes (Figure 2). Some additional time was however necessary to bring the column to initial conditions. Retention time of standard of tocopherols are little difference in meat sample, because it is matrix effect, and retention time in all tocopherol are little smaller.
The sensitivity of the tocopherols (as well as other compounds) determination method is influenced mainly by the detection system used. The most commonly used detector type for determination of vitamin E is UV-Vis and FLD [50-54]. Some reports on the application of an electrochemical detector for this purpose are also available in the literature [50]. Various types of mass spectrometers in connection to HPLC are being increasingly used for assessment of tocopherols concentrations and profiles [9-21]. The atmospheric pressure chemical ionisation (APCI) method matches compounds primarily for such groups [15,21,41,42,49,54,55]. Some reports on the application of the electrospray ionisation method (ESI) are also available [21,40]. Results of this study have demonstrated that fluorometry is the most sensitive method for determination of tocopherols. The limits of detection obtained with this technique were in 3.8 to 38.5 ng ml-1 range (0.005- 0.05 mg kg-1) depending from the tocopherols chemical structure. With increasing numbers of methyl substituents on the tocopherol molecule a decrease in the detector response was observed. This was directly related with the calculated LOD and LOQ values for the individual tocopherols. These results are in concordance with the literature data. The reported average limit of quantification for various vitamin E forms (γ- and α-tocopherols mainly) is approximately 10 ng ml-1 [15,53]. The limits of detection obtained when PDA detector is utilised are about 60 times higher and are on average 1 μg ml-1 and do not depend as strongly as in the case of fluorescence on the chemical structure of tocopherols. Absorbances observed for the studied compounds were similar. Those results are partly consistent with the literature. Gimeno et al. [52] and Cho et al. [25] have reported similar limits of quantification (1 μg ml-1) values to those reported in this study while Sanchez-Machado et al. [53] and Zhao et al. [51] have reported significantly lower values of 0.1 and 0.25 μg ml-1, respectively. Those differences are most probably related to the differences in the design of the detection systems used.
The limits of detection for the studied tocopherols when mass spectrometric (ion trap) detection was used were in the 0.2 to 2.2 μg ml-1 range and were strongly affected by the compound chemical structure and the ionisation technique applied. Due to relatively low sensitivity the application of an ion trap based mass spectrometer for determination of tocopherols in meat is not fully justified. The limits of detection reported in this study were however significantly lower when compared to the literature data. For example, Lanina et al. [21] have reported limits of determination of approximately 3 ng ml-1 for various tocopherols for a single quadrupole mass spectrometer operated in a negative polarity atmospheric pressure chemical ionisation mode. For a positive ionisation 2 ng ml-1 and 0.8 ng ml-1 detection limits were reported by Liang et al. [41] and Vaule et al. [15], respectively. When electrospray ionisation was used prior to single quadrupole mass analyser the limits of detection were in 5-20 ng ml-1 range [21]. Kamao et al. [54] have reported a similar limit of detection value (2 ng ml-1) for α-tocopherol when triple quadrupole mass spectrometer operated in positive ion chemical ionisation was used. Results of this study concerning the obtained limit of detection values when atmospheric pressure chemical ionisation (APCI) was applied were in concordance with results reported by Zarrouka et al. [42]. The latter authors have reported the limits of detection at 3.5, 0.6 and 0.23 mg kg-1 for δ-, γ- and α-tocopherols, respectively. The authors have also found that with the increasing number of methyl groups in the tocopherol structure an increase in ionisation efficiency is observed. On the basis of the results of this study and the available literature data it might be concluded that an ion trap based mass spectrometer is not a detector system suited for the analysis of tocopherols. To lower the tocopherols detection limits several experiments with application of photoionisation and photochemical ion sources were conducted. However the application of such ionisation types did not cause any significant improvement in the method sensitivity. In the following experiments indirect ionisation of the tocopherols was tried in in situ reactions with photoionised excipients (modifiers with an ionisation potential below 10 eV). Those experiments did not however result in a satisfactory effect, most probably due to low efficiency of photoionisation of the tocopherols and the insufficient energy of the ionised intermediate molecules. The use of photochemical ionisation resulted in a significant increase in the ionisation efficiency comparing to the pure photoionisation process. In this case the lowest limits of detection at 1 mg kg-1 (0.8 ng ml-1) for δ-tocopherol were observed.
In general it might be concluded that the fluorometer is the most sensitive detector against tocopherols. The ion trap shows a significantly lower sensitivity. Chemical ionisation is a good ionisation technique with respect to γ- and α- tocopherols while the application of photochemical ionisation significantly improves the ionisation efficiency of δ-tocopherol (providing its good detectability).
Reversed phase liquid chromatography with florometric detection was used to determine the vitamin E in pork. A comparison of 15+1, the most commonly applied sample preparation procedures used for the tocopherol determination with respect to the recovery yields obtained, was conducted. The conducted study has revealed that the investigated methods are very different in terms of the analyte recovery. It was demonstrated that methods, which do not include the saponification or the tissue hydrolysis steps but only a simple extraction using an ethanol or hexane had the highest recovery found. The concentrations of vitamin E in meat samples were the highest when those methods were used for determinations. Tocopherols are present in meat in freenon bonded form [56] and are located in various biological membranes (cell membranes and the membranes of organelles) in liquid bilayer of phospholipids. Tocopherols are bonded to phospholipids due to presence of Van der Waals interactions occurring between lipid molecules. Hence the extraction process conducted with a solvent, which is able to break those interactions, is able to provide good tocopherols recovery. The results have proved that the combined use of the ethanol (mid-polarity solvent) could effectively disperse the lipid– lipid type interactions of tocoptherols and phospholipids. This enables its easy extraction from the meat tissue. Tocopherols are relatively easily soluble in ethanol and mixtures of ethanol with water. The application of a single step extraction with isooctane referring to the method proposed by Hewavitharana et al. [30] resulted in recoveries in 50-73% range.
δ-tocopherol has the highest polarity of the compounds investigated. With the increasing number of methyl groups the decrease in the molecule polarity is observed. This resulted in lower recoveries of δ-tocopherol in this method, because it remained in the ethanolic solution.
The modification of the Hewavitharana et al. [30] method when the saturated NaCl solution was used, resulted in a significant increase in the partitioning coefficients of the tocopherols relative to the isooctane phase. This improved the recovery yield of the method.
Results revealed that the saponification step led to chemical degradation of the analytes to a significant degree. That is why the application of a solvent extraction solely produces significantly better recovery yields in vitamin E determination. Triacyloglicerydes are extracted together with the tocopherols and are subsequently introduced onto the chromatographic column. This causes the need for its elution from the chromatographic column after tocopherols are separated. This could be simply achieved by applying a gradient mobile phase composed of acetonitrile: water and methyl-tert-butylether (MTBE) or isopropanol:hexane mixtures [57]. In this work MTBE was used due to the significantly lower viscosity of this solvent and resulting higher maximum mobile phase velocities leading to reduced analysis time. In the method proposed by Hewavitharana et al. [30] (and its modification) no significant matrix chromatographic enhancements/extinction effects were observed. This was probably associated with a high dilution of the sample and a small injection volume (5 μl). The saponification process significantly simplifies the sample matrix, degrades the triacyloglicerides fraction but causes also release of tocopherols. The method reported by Liu et al. [38] was found to be the best and gave recovery values in 52-78% range (depending on the tocopherol). The method involves the use of diluted aqueous potassium hydroxide (11%) solution and a vast addition of ascorbic acid (500 mg) acting as an antioxidant, which protects tocopherols against an aggressive alkaline medium. Other sample preparation methods involving saponification with concentrated hydroxide solutions or lower antioxidants (ascorbic acid, pirogalol) concentrations gave significantly worse results. The extension of the saponification process duration also caused higher losses of the tocopherols. The concentration of potassium hydroxide, the quantity of ascorbic acid (or pirogallol) added and the duration of the saponification process must be precisely optimised if minimal tocopherol degradation rate is to be obtained. It was also observed that individual tocopherols have different durability in basic medium. α-tocopherol is the most stable homologue while γ- and δ- shows lower stability. These results are in concordance with the literature data.
The average vitamin E contents in meat of animals feed with regular feed was in 0.2 mg kg-1 up to 5.6 mg kg-1 range. Tocopherols concentration might be influenced by the animal breed, type of feed used, chemical form of the naturally occurring vitamin E compounds in feed (α-, β-, γ-, δ-tocopherols, its optical isomers and esters such as tocopherol acetate), the type of vegetable oil used as feed additive (influencing the tocopherols bioavailability and gastrointestinal absorption rate) [58-60].
Conclusion
The results of this study are considered to be of practical significance in terms of the vitamin E analysis in meat and other food products. The proper selection of the separation method (the need to determine particular vitamin forms), detection method, and the sample preparation procedure affects the method performance and the accuracy of results.
The application of a sensitive method and such sample preparation procedure that does not cause a significant degradation of tocopherols enables determination of the vitamin E contents in a very precise manner.
Results of this study have proved that the florometer is the most sensitive detector for tocopherols determination. The ion trap shows a significantly lower sensitivity against tocopherols when compared with a florometer. Chemical ionisation is a good ionisation technique for γ- and α-tocopherols while application of photochemical ionisation significantly improves the ionisation of δ-tocopherol. The sample preparation procedure based on extraction of tocopherols from the biological sample with ethanol and hexane, involving change in the partitioning coefficients shows significantly better recovery yields when compared to methods that involve saponification or extraction with other solvents. Depending on the sample preparation method applied the results of the vitamin E determination were in the 0-6 mg kg-1 range.
Acknowledgements
This work was financially supported by “BIOZYWNOSC” (BIOFOOD)- innovative, functional products of animal origin No POIG.01.01.02-014-090/09 co-financed by the European Union through the European Regional Development Fund under the Innovative Economy Operational Programme 2007-2013.
References
- Wagner KH, Kamal-Eldin A, Elmadfa I (2004) Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab 48: 169-188.
- Saldeen K, Saldeen T (2005) Importance of tocopherols beyond a-tocopherol:evidence from animal and human studies. Nutr Res 25: 877- 889.
- Stocker P, Lesgards JF, Vidal N, Chalier F, Prost M (2003) ESR study of a biological assay on whole blood: antioxidant efficiency of various vitamins. Biochim Biophys Acta 1621: 1-8.
- Abidi SL (2000) Chromatographic analysis of tocol-derived lipid antioxidants. J Chromatogr A 881: 197-216.
- Abidi SL, Mounts TL (1996) Normal Phase High-Performance Liquid Chromatography of Tocopherols on Polar Phases. J Liq Chromatogr Relat Technol 19: 509 - 520
- Kamal-Eldi A, Görgen S, Pettersson J, Lampi AM (2000) Normal-phase high-performance liquid chromatography of tocopherols and tocotrienols. Comparison of different chromatographic columns. J Chromatogr A 881: 217-227.
- Rupérez FJ, Martín D, Herrera E, Barbas C (2001) Chromatographic analysis of alpha-tocopherol and related compounds in various matrices. J Chromatogr A 935: 45-69.
- Liebler DC, Burr JA, Philips L, Ham AJ (1996) Gas chromatography-mass spectrometry analysis of vitamin E and its oxidation products. Anal Biochem 236: 27-34.
- Mottier P, Gremaud E, Guy PA, Turesky RJ (2002) Comparison of gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry methods to quantify alpha-tocopherol and alpha-tocopherolquinone levels in human plasma. Anal Biochem 301: 128-135.
- Rentel C, Strohschein S, Albert K, Bayer E (1998) Silver-plated vitamins: a method of detecting tocopherols and carotenoids in LC/ESI-MS coupling. Anal Chem 70: 4394-4400.
- Hao Z, Parker B, Knapp M, Yu L (2005) Simultaneous quantification of alpha-tocopherol and four major carotenoids in botanical materials by normal phase liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Chromatogr A 1094: 83-90.
- Heudi O, Trisconi MJ, Blake CJ (2004) Simultaneous quantification of vitamins A, D3 and E in fortified infant formulae by liquid chromatography-mass spectrometry. J Chromatogr A 1022: 115-123.
- Kalman A, Mujahid C, Mottier P, Heudi O (2003) Determination of alpha-tocopherol in infant foods by liquid chromatography combined with atmospheric pressure chemical ionisation mass spectrometry. Rapid Commun Mass Spectrom 17: 723-727.
- Stöggl W, Huck C, Wongyai S, Scherz H, Bonn G (2005) Simultaneous determination of carotenoids, tocopherols, and gamma-oryzanol in crude rice bran oil by liquid chromatography coupled to diode array and mass spectrometric detection employing silica C30 stationary phases. J Sep Sci 28: 1712-1718.
- Vaule H, Leonard SW, Traber MG (2004) Vitamin E delivery to human skin: studies using deuterated alpha-tocopherol measured by APCI LC-MS. Free Radic Biol Med 36: 456-463.
- Al-Talla ZA, Tolley LT (2005) Analysis of vitamin E derivatives in serum using coordinated ion spray mass spectrometry. Rapid Commun Mass Spectrom 19: 2337-2342.
- Lee MR, Lin CY, Li ZG, Tsai TF (2006) Simultaneous analysis of antioxidants and preservatives in cosmetics by supercritical fluid extraction combined with liquid chromatography-mass spectrometry. J Chromatogr A 1120: 244-251.
- Krucker M, Lienau A, Putzbach K, Grynbaum MD, Schuler P, et al. (2004) Hyphenation of capillary HPLC to microcoil (1)H NMR spectroscopy for the determination of tocopherol homologues. Anal Chem 76: 2623-2628.
- Lienau A, Glaser T, Krucker M, Zeeb D, Ley F, et al. (2002) Qualitative and quantitative analysis of tocopherols in toothpastes and gingival tissue employing HPLC NMR and HPLC MS coupling. Anal Chem 74: 5192-5198.
- Hall WL, Jeanes YM, Pugh J, Lodge JK (2003) Development of a liquid chromatographic time-of-flight mass spectrometric method for the determination of unlabelled and deuterium-labelled alpha-tocopherol in blood components. Rapid Commun Mass Spectrom 17: 2797-2803.
- Lanina SA, Toledo P, Sampels S, Kamal-Eldin A, Jastrebova JA (2007) Comparison of reversed-phase liquid chromatography-mass spectrometry with electrospray and atmospheric pressure chemical ionization for analysis of dietary tocopherols. J Chromatogr A 1157: 159-170.
- Perri E, Mazzotti F, Raffaelli A, Sindona G (2000) High-throughput screening of tocopherols in natural extracts. J Mass Spectrom 35: 1360-1361.
- Lauridsen C, Leonard SW, Griffin DA, Liebler DC, McClure TD, et al. (2001) Quantitative analysis by liquid chromatography-tandem mass spectrometry of deuterium-labeled and unlabeled vitamin E in biological samples. Anal Biochem 289: 89-95.
- Kanazawa H, Miyata C, Nagata Y, Urano S, Matsushima Y (2000) Determination of alpha-tocopherol and alpha-tocopherylquinone in rat tissues and plasma by high-performance liquid chromatography with electrochemical detection. Chem Pharm Bull (Tokyo) 48: 1462-1466.
- Cho K, Rima J, Chang CL, Li QX (2007) Spectrofluorometric and high-performance liquid chromatographic determination of all-rac-a-tocopheryl acetate in virgin olive oil. Journal of Food Composition and Analysis 20: 57-62.
- He Y, Wang K, Wang L (2010) Effect of a-Tocopherol and ß-Carotene Supplementation on Meat Quality and Antioxidant Capacity of Pigs Fed High-Linseed Oil Diet. The Journal of Animal & Plant Sciences 20: 180-188.
- Pfalzgraf A, Steinhart H, Frigg M (1995) Rapid determination of alpha-tocopherol in muscle and adipose tissues of pork. Z Lebensm Unters Forsch 200: 190-193.
- Cort WMs, Vicente TS, Waysek EH, Williams BD (1983) Vitamin E content of feedstuffs determined by high-performance liquid chromatographic fluorescence. J Agric Food Chem 31: 1330-1333.
- Schüep W, Rettenmaier R (1994) Analysis of vitamin E homologs in plasma and tissue: high-performance liquid chromatography. Methods Enzymol 234: 294-302.
- Hewavitharana AK, Lanari MC, Becu C (2004) Simultaneous determination of vitamin E homologs in chicken meat by liquid chromatography with fluorescence detection. J Chromatogr A 1025: 313-317.
- Arnold RN, Scheller KK, Arp SC, Williams SN, Schaefer DM (1993) Dietary a-Tocopheryl Acetate Enhances Beef Quality in Holstein and Beef Breed Steers. Journal of Food Science 58: 28-33.
- Gaal T, Mezes M, Noble RC, Dixon J, Speake BK (1995) Development of antioxidant capacity in tissues of the chick embryo. Comp Biochem Physiol 112B: 711-716.
- Jensen SK, Jensen C, Jakobsen K, Engberg RM, Andersen JO, et al. (1998) Supplementation of broiler diets with retinol acetate, ß-carotene or canthaxanthin: Effect on vitamin status and oxidative status of broilers in vivo and on meat stability. Acta Agric Scand Sect A Animal Sci 48: 28-37.
- McMurray CH, Blanchflower WJ (1979) Application of a high-performance liquid chromatographic fluorescence method for the rapid determination of alpha-tocopherol in the plasma of cattle and pigs and its comparison with direct fluorescence and high-performance liquid chromatography-ultraviolet detection methods. J Chromatogr 178: 525-531.
- Berlin E, McClure D, Banks MA, Peters RC (1994) Heart and liver fatty acid composition and vitamin E content in miniature swine fed diets containing corn and menhaden oils. Comp Biochem Physiol Physiol 109: 53-61.
- Hatam LJ, Kayden HJ (1979) A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res 20: 639-645.
- Zaspel BJ, Csallany AS (1983) Determination of alpha-tocopherol in tissues and plasma by high-performance liquid chromatography. Anal Biochem 130: 146-150.
- Liu Q, Scheller KK, Schaefer DM (1996) Technical note: a simplified procedure for vitamin E determination in beef muscle. J Anim Sci 74: 2406-2410.
- Miller KW, Lorr NA, Yang CS (1984) Simultaneous determination of plasma retinol, alpha-tocopherol, lycopene, alpha-carotene, and beta-carotene by high-performance liquid chromatography. Anal Biochem 138: 340-345.
- Bustamante-Rangel M, Delgado-Zamarreño MM, Sánchez-Pérez A, Carabias-Martínez R (2007) Determination of tocopherols and tocotrienols in cereals by pressurized liquid extraction-liquid chromatography-mass spectrometry. Anal Chim Acta 587: 216-221.
- Liang Y, Kang A, Xie Y, X-d. Liu X-d, Xie L, et al. (2008) Selective, sensitive and simple LC–APCI–MS method for the quantitation of Alpha-Tocopheryl Nicotinate in human plasma. International Journal of Mass Spectrometry 273: 132-137.
- Zarrouk W, Carrasco-Pancorbo A, Zarrouk M, Segura-Carretero A, Fernández-Gutiérrez A (2009) Multi-component analysis (sterols, tocopherols and triterpenic dialcohols) of the unsaponifiable fraction of vegetable oils by liquid chromatography-atmospheric pressure chemical ionization-ion trap mass spectrometry. Talanta 80: 924-934.
- Czarniecka-Skubina E, Przybylski W, Kajak-Siemaszko K, Jaworska D, Wachowicz I (2010) Effect of pH24 and Intramuscular Fat Content on Technological and Sensory Quality of Pork. Pol J Food Nutr Sci 60: 43-49.
- Nelis HJCF, De Leenheer AP (1983) Isocratic nonaqueous reversed-phase liquid chromatography of carotenoids. Anal Chem 55: 270-275.
- Kaplan LA, Miller JA, Stein EA (1987) Simultaneous measurement of serum retinol, tocopherols, carotenes, and carotenoids by high performance liquid chromatography. J Clin Lab Anal 1: 147 - 152.
- Cavina G, Gallinella B, Porrà R, Pecora P, Suraci C (1988) Carotenoids, retinoids and alpha-tocopherol in human serum: Identification and determination by reversed-phase HPLC. J Pharm Biomed Anal 6: 259-269.
- Handelman GJ, Shen B, Krinsky NI (1992) High resolution analysis of carotenoids in human plasma by high-performance liquid chromatography. Methods Enzymol 213: 336-346.
- Khachik F, Beecher GR, Smith JC Jr (1995) Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem Suppl 22: 236-246.
- Strohschein S, Rentel C, Lacker T, Bayer E, Albert K (1999) Separation and Identification of Tocotrienol Isomers by HPLC-MS and HPLC-NMR Coupling. Anal Chem 71: 1780-1785.
- Gaziano JM, Johnson EJ, Russell RM, Manson JE, Stampfer MJ, et al. (1995) Discrimination in absorption or transport of beta-carotene isomers after oral supplementation with either all-trans- or 9-cis-beta-carotene. Am J Clin Nutr 161: 1248-1252.
- Zhao B, Tham SY, Lu J, Lai MH, Lee LK, et al. (2004) Simultaneous determination of vitamins C, E and beta-carotene in human plasma by high-performance liquid chromatography with photodiode-array detection. J Pharm Pharm Sci 7: 200-204.
- Gimeno E, Castellote AI, Lamuela-Raventós RM, de la Torre MC, López-Sabater MC (2000) Rapid determination of vitamin E in vegetable oils by reversed-phase high-performance liquid chromatography. J Chromatogr A 881: 251-254.
- Sánchez-Machado DI, López-Hernández J, Paseiro-Losada P (2002) High-performance liquid chromatographic determination of alpha-tocopherol in macroalgae. J Chromatogr A 976: 277-284.
- Kamao M, Tsugawa N, Suhara Y, Wada A, Mori T, et al. (2007) Quantification of fat-soluble vitamins in human breast milk by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 859: 192-200.
- Di Stefano V, Avellone G, Bongiorno D, Cunsolo V, Muccilli V, et al. (2012) Applications of liquid chromatography-mass spectrometry for food analysis. J Chromatogr A 1259: 74-85.
- Berges E (1999) Importance of vitamin E in the oxidative stability of meat: Organoleptic qualities and consequences. In Brufau J, Tacon A edition, Feed manufacturing in the Mediterranean region: Recent advances in research and technology. Zaragoza : CIHEAM 347-363.
- Szterk A, Roszko M, Sosinska E, Derewiaka D, Lewicki PP (2010) Chemical Composition and Oxidative Stability of Selected Plant Oils. J Am Oil Chem Soc 87: 637-645.
- Anderson LE Sr, Myer RO, Brendemuhl JH, McDowell LR (1995) The effect of excessive dietary vitamin A on performance and vitamin E status in swine fed diets varying in dietary vitamin E. J Anim Sci 73: 1093-1098.
- Monahan FJ, Buckley DJ, Morrissey PA, Lynch PB, Gray JI (1992) Influence of dietary fat and α-tocopherol supplementation on lipid oxidation in pork. Meat Sci 31: 229-241.
- Rey AI, Kerry JP, Lynch PB, López-Bote CJ, Buckley DJ, et al. (2001) Effect of dietary oils and alpha-tocopheryl acetate supplementation on lipid (TBARS) and cholesterol oxidation in cooked pork. J Anim Sci 79: 1201-1208.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16057
- [From(publication date):
specialissue-2014 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 11436
- PDF downloads : 4621