Comparison of the Oxidative Stress and Histopathological Change Inducing Capacities of Cycas circinalis Leaf Powder and the Ethanolic Extract in Rats
Received: 05-May-2021 / Accepted Date: 19-May-2021 / Published Date: 26-May-2021 DOI: 10.4172/2476-2067.1000156
Abstract
This study was carried out to investigate the oxidative stress status of colorectal cells of rats exposed to Cycas circinalis leaf powder and the ethanol extract separately. Seven (07) groups of five (5) rats each were used for this study. Group 1 rats were maintained on normal rat feed without cycas leaf powder or the extract (control), group 2 rats were exposed to the leaf powder tainted feed (5% w/w) continuously for six weeks. Group 3 rats were placed on normal feed but administered ethanol extract of cycas leaf (10 mg/kg body weight) while groups 4,5,6 and 7 were exposed to the extract at a dose of 30,50,80 and 100 mg/kg body weight respectively once a week for 6 weeks by gavage. At the end of the treatment period each rat was sacrificed and the colon excised and sections obtained for histological examination and biochemical assessment of colon homogenate supernatant for oxidative stress indices. Results showed that there were significant (p≤0.05) decreases in the total protein level as well as superoxide dismutase, catalase and gluthatione peroxidase activities but an increase in malondialdehyde level in the colon homogenate supernatant of rats placed on the powder tainted feed and the ethanol extract treated ones when compared to the control. Histopathological examination of stained colon sections revealed that cycas powder and the extract at 80 and 100 mg/kg body weight caused ultrastructural changes in the colon amongst which are inflammation, reduced number of globlet cells with nuclear polarization and nuclear atypia which are early events in the onset of colorectal cancer. The results suggest that the leaf powder and the ethanolic extract of C.circinalis at higher doses are capable of inducing oxidative stress and early ultrastructural changes in colorectal cells, the later extent being indices of early events in colorectal carcinogenesis.
Keywords: Cycas circinalis; Ethanolic extract; Oxidative stress;Colorectal cancer
Introduction
There is a growing understanding and acceptance of the concept that Reactive Oxygen Species (ROS), which are aetiological agents in the development of a range of diseases. Among these diseases is colorectal cancer [1]. Oxidative stress is an imbalance between production of ROS and the ability of cellular defense system to diminish ROS level and repair the damage caused by them [2].
Colorectal cancer (CRC) is the third most common cancer worldwide and the fourth most common cause of death [3,4]. Almost invariably it presents as pathology caused by persistent oxidative stress and inflammation [5].
The plant families Cycadaceae are palm-like plants which have survived the Mesozoic era with little change [6]. Cycads are widely distributed in tropical and subtropical regions [7]. Evidence that crude cycad material was carcinogenic was obtained early in 1962 when rats fed cycas seed meal diet were killed because of palpable abdominal tumour masses, ascites, and a rapidly developing anaemia [8]. Previous studies by [9] found that rats fed crude cycad meal developed hepatocellular carcinomas as well as kidney and intestinal tumours. It was later found that the carcinogen in cycad is cycasin [10], which was first isolated from the seeds of C.revoluta Thumb, identified by [11] and later found by [12] in C. circinalis Linn.
Concentrations of cycasin are highest in the seeds and roots, but present in all parts of the plant [9]. Cycasin is toxic only when given orally, and its toxicity appears after a period of about 12 hours [8]. There are reports on the carcinogenicity of cycasin in rats[13-15] in mice [16-18] hamsters [19] guinea pigs [20], rabbits [21] and aquarium fish [22].
Cycasin is synthesized from Methylazoxymethanol (MAM) by the enzyme UDP-glucosyltransferase [23]. It is metabolized by plant, bacterial and mammalian B-glucosidases to liberate the aglycone, MAM. MAM is the active toxic metabolite of cycasin [13]. It spontaneously breaks down into reactive molecules such as methydiazonium ions and carbon-centered free radicals which can methylate DNA [24]. The accumulation of lesions in DNA in form of methylated sites is responsible for the teratogenic, mutagenic, hepatotoxic, carcinogenic and neurotoic properties of MAM [24].
Though, there is evidence of the carcinogenic capacity of cyacs leaf powder, when incorporated in diet (5% w/w) [25], there is dearth of information on the ability of the extract to induce oxidative stress and colon cancer in experimental animals. The present study was therefore designed to compare the oxidative stress and histopathological change-inducing capacity of C.circinalis leaf powder and the ethanolic leaf extract in rats.
Materials and Methods
Preparation of the ethanolic leaf extracts of Cycas circinalis
C.circinalis leaf powder (150 g) was soaked in 1.5 liters of absolute ethanol (99% w/v) for 72 hours with regular stirring. The extract was then filtered through a sintered funnel and Whatman No. 1 filter paper. The filtrate obtained was concentrated in a hot water bath at 70°C. The resultant greenish paste deposited at the bottom of the flask was then dried in an oven at 45°C. The dry extracts was then scraped with a stainless spatula, crushed in a mortar and then kept in glass bottle until required. The weight of extract obtained from 150 g of the pulverized dry leaf was estimated.
LD50 determination
The oral LD50 of the ethanolic leaf extract of C.circinalis was determined using the method of Lorke [26].
Animals grouping and treatment
The rats were randomly divided into seven (07) groups of 5 rats per each. Members of each group were housed separately in a clean, disinfected cage in a room with a 12-hour light/dark cycle. The rats were maintained on growers mash (Bendel Feed and Flour Mill (BFFM), Ewu, Nigeria) and water ad libitium for one week prior to commencement of the experiment. When the experiment started, group 1 rats were maintained on normal feed, growers mash without cycas leaf powder or the extract (control).
Group 2 rats were exposed to the leaf powder tainted growers mash (5% w/w) continuously for six weeks. Group 3 rats were placed on normal feed but administered ethanol extract of cycas leaf (10 mg/kg body weight) while groups 4,5,6 and 7 were exposed to the extract at a dose of 30,50,80 and 100 mg/kg body weight respectively once a week for 6 weeks by gavage. Cycas circinalis leaf extract was dissolved in 25% ethanol prior to administration. All groups were kept on their respective diet for six weeks.
Animal sacrifice
This study was carried out in strict compliance with the ethics in Guidelines and Specification on Experimental Animal Care [27]. At the end of the treatment period each rat was anesthetized using halothane saturated chamber.
Sample preparation
While under anaesthesia the abdominal region was opened and the colon was excised and sections obtained for histological examination and biochemical assessment of colon homogenate supernatant for oxidative stress indices.
Sections for histology were immediately fixed in 10% formol-saline prior to processing. The colon homogenate of each rat was prepared by grinding 0.5 g in ice-cold mortar with acid-washed sand in 5 ml of normal saline. Each homogenate was centrifuged at 3500 rpm for 5 minutes and the resultant supernatant was separated and left frozen at -20°C until required.
Biochemical assays
Superoxide dismutase activity was assayed by the method of [28] which involved the following of the auto-oxidation of adrenaline to adrenochrome at 420 nm. Catalase activity was assayed by using the method of [29], in which the decomposition of hydrogen peroxide was monitored at 480 nm. Glutathione peroxidase activity was determined by measuring the production of purpurogallen from pyrogallol at 420 nm [30].
Total protein was estimated using that involves the formation of biuride, commonly called “Biuret” method as described in Randox test kit assay leaflet. Malondialdehyde levels were measured in a colorimetric reaction with thiobarbituric acid as described by Buege and Aust.
Histology
Colon sections fixed in formol-saline were processed for light microscopy examination at the Department of Morid Anatomy and Histopathology, University of Benin Teaching Hospital (UBTH). The slides were examined and interpreted by a Consultant Pathologist.
Statistics
The results from biochemical assays are expressed as mean ± SEM. In order to establish whether the mean values were statistically significantly different from each other, Analysis of Variance (ANOVA) was done using SPSS software.
In order to know which means have differences that are significantly different, LSD multiple range tests was done by employing SPSS computer software. Values were considered significantly different at p ≤ 0.05.
Results
The yield from ethanol extraction of Cycas circinalis leaf powder was 103.07/g residue.
Biochemical findings
The effects of the consumption of C. circinalis powder tainted feed and administered ethanolic extract of the powder on colon total protein, SOD, catalase and gluthatione peroxidase as well as MDA levels of the rats in various groups as shown in Table 1. Group B rats (cyacas tainted diet) had significantly (p ≤ 0.05) higher MDA level than any other group. However the MDA level of all the treatment groups increased significantly (p ≤ 0.05) compared to the control group. There was also a significant (p ≤ 0.05) decrease in the colon total protein level in all the treatment groups when compared to the control. The enzymes superoxide dismutase, catalase and glathatione peroxidase also decreased significantly (p ≤ 0.05) relative to the group.
| Groups | TP (g/dL) | SOD(units/g tissue) ×10-2 | CAT (K/min) | GPx (Units/mg fresh weight) | MDA (mmoles/g tissue) ×10-3 |
|---|---|---|---|---|---|
| A | 1.42 ± 0.06 | 30.61 ± 0.61 | 1.96 ± 0.01 | 2.04 ± 0.10 | 17.60 ± 0.20 |
| B | 0.91 ± 0.01a | 19.4 ± 0.52a | 0.82 ± 0.05a | 0.87 ± 0.01a | 65.85 ± 0.37a |
| C | 1.02 ± 0.01b | 27.86 ± 0.74a,b | 1.64 ± 0.25a,b | 1.42 ± 0.02a,b | 45.31 ± 0.48a,b |
| D | 0.98 ± 0.01c | 27.03 ± 0.02a,c | 1.62 ± 0.15a,c | 1.39 ± 0.01a | 46.01 ± 0.01a,b,c |
| E | 0.94 ± 0.03d | 26.75 ± 0.23a | 1.60 ± 0.05a,d | 1.35 ± 0.03a | 46.31 ± 0.10a,b,d |
| F | 0.92 ± 0 .01e | 26.11 ± 0.03a,b,e,f | 0.84 ±0.02b,c,d | 1.15 ± 0.52e | 47.45 ± 0.26a,b,e |
| G | 0.90 ± 0 .02f | 21.10 ± 0.15a,b,c,d,e,f | 0.80 ± 0.05b,c,d,f | 1.05 ± 0.05b,c,d,f | 48.45 ± 045a,b,f |
Values with a given superscript a, b ,c ,e or f singly or combined within a column are significantly (p ≤ 0.05) different relative to the value of the group with the corresponding uppercase letters, A, B, C, D, E, or F.
Oral LD50 of ethanolic leaf extract of Cycas circinalis
No death was recorded at any of the doses administered. Oral LD50 was therefore determined to be over 5000 mg/kg body weight.
Histopathological observations
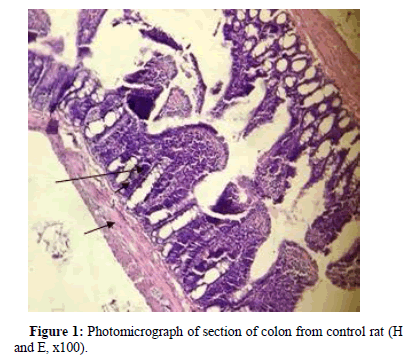
Stained section of control rat colon, revealed prominent lamina propria lined by distinct columnar epithelium and crypt of lieberkuhn (long arrow) bound by muscularis mucosa (short arrow), Figure 1.
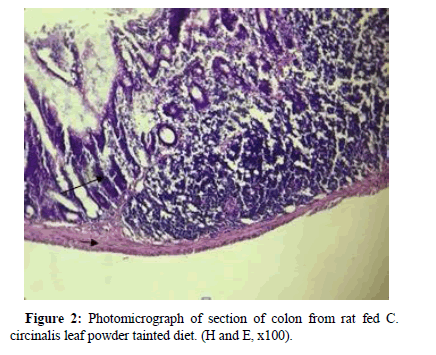
Section of colon from rat exposed to cycas tainted feed showed shorter and narrower lamina propria (Figure 2), (Long arrow) surrounded by inflammatory exudates. Columnar epithelium was deeply stained with marked nuclear atypia, reduced goblet cells with nuclear polarization.
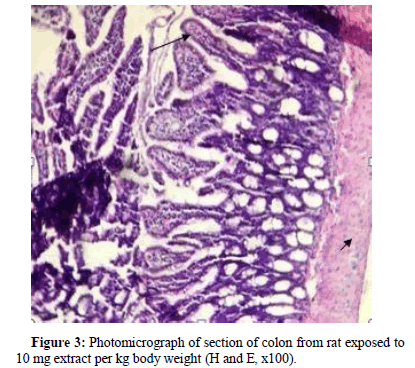
Section of rat exposed to 10 mg extract/kg body weight revealed the presence of increased of goblet cells indicated by long arrow (Figure 3).
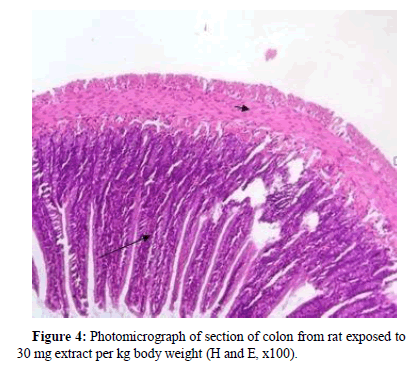
Besides other normal histological features of the colon epithelium, colon section for rat exposed to 30 mg extract per kg body weight, revealed the presence of pyknotic nucleus (Figure 4).
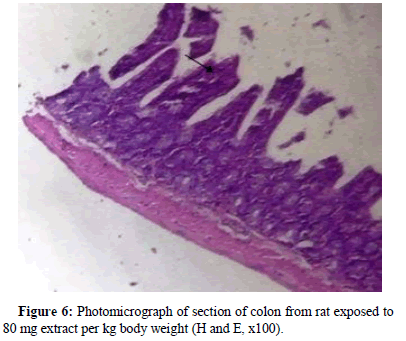
Rat from the group exposed to 50 mg extract per kg body weight had colon with densely stained pyknotic nuclei. Also evident was nuclear stratification and mild fibrosis (long arrow), (Figure 5). Rat exposed to 80 mg extract per kg body weight had colon section revealed columnar epithelium that is deeply stained with marked nuclear atypia (Figure 6).
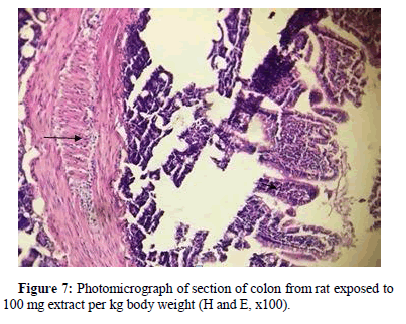
Section of colon from rat exposed to 100 mg extract per kg body weight is presented in Figure 7 . Columnar epithelium and crypt of lieberkuhn are clearly evident and have pyknotic nuclei that is densely
Discussion
Cycas, the only currently known genus of the family cycadaceae, are considered as fossil plants though they have evolved only about 12 million years ago [31]. The most studied cycas are Cycas revoluta and Cycas circinalis, which contains the carcinogenic toxin cycasin [9,11]. Cycasin is metabolized by plant, bacterial and mammalian β-glucosidases to liberate the aglycone, methylazoxylmethanol (MAM), which is the active metabolite of cycasin [23]. Since researchers became aware of the potent carcinogenic properties of cycasin and MAM, these agents have been employed as reliable animal models for inducing a wide range of intestinal cancers, including colon cancer [9].In the present study, the abnormal changes seen in the colon ultra-structure of rats given cycas-tainted diet (5% w/w) as well as 80 and 100 mg ethanol extract of the leaf per kg body weight which include inflammation, reduced number of goblet cells with nuclear polarization and nuclear atypia, are some of the early events in the onset of colorectal cancer [5,25,32]. Results from this study also show a significant increase in lipid peroxidation level evidenced by high MDA levels in the colon homogenate supernatant of rats fed cycas-tainted diet and those administered ethanolic extract of Cycas circinalis leaf. The most deleterious impact of oxidative stress is lipid peroxidation, which has been implicated in the pathogenesis of numerous diseases including cancer [33]. Lipid peroxidation changes the fluidity of cell membranes, reduces capacity to maintain and equilibrated concentration gradients, and increases membrane permeability and inflammation [34].
There was also depletion in colon total protein in the test groups as compared to the control group. The cycas-induced decrease in colon tissue protein is in agreement with colon tissue protein loss usually associated with most cancers [25]. The protein loss has been attributed to increased protein degradation and/or decreased protein synthesis [35, 36]. Proteins are also susceptible to ROS induced damage and are frequent target of increased production of free radicals [37]. ROS oxidizes structural proteins and inhibits proteolytic system. Such reactions leads to alteration of structural proteins or alteration of enzyme functions, which can result to a wide range of downstream functional consequences, such as inhibition of enzymatic and binding activities, increased or decreased uptake by cells, inactivation of DNA repair enzymes, and loss of fidelity of damaged DNA polymerases in replicating DNA [38].
Antioxidant enzymes, catalase, superoxide dismutase and glutathione peroxidase activities also decreased significantly in the rats fed cycas-tainted diet and the ethanolic leaf extract. There is an overwhelming evidence to indicate that oxidative stress, defined as an imbalance between oxidants and antioxidants in favour of the former, leads to many biochemical changes. The depletion of the antioxidant enzymes could be attributed to the primary defense system by these enzymes against the ROS generated by the increased lipid peroxidation. It is well known that SOD, catalase and GPx play important role as protective defense enzymes against lipid peroxidation in tissues [39]. GPx, an oxidative stress inducible enzyme plays a role in the peroxyl scavenging mechanism and in maintaining functional integration of cell membranes [40].The decreased catalase activities could be attributed to the fact that catalase is used by cells to defend against the toxic effect of hydrogen peroxide, which is generated by various reactions and/or environmental agents and by the action of superoxide dismutase enzymes while detoxifying superoxide anions [41]. The general decrease in the antioxidant defense system could be attributed to depletion of the enzymes in attempt to reduce the impact of cycasin-induced oxidative stress on the colorectal cells. Decreased antioxidant enzymes and increased oxidative stress suggest a strong relationship between oxidative stress and colorectal cancer [42]."
Conclusion
The results obtained in this study suggests evidence that Cyacs circinalis powder (5% w/w) and higher doses of the ethanolic leaf extract inducedss oxidative stress in the colon of rats and some ultrastructural changes in the colon which are indicators of early events in the development of colorectal cancer.
References
- Valko M, Rhodes CJ, Moncol J, Izakovic M,Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160: 1-40.
- Ozougwu JC (2016) The Role of Reactive Oxygen species and antioxidants in oxidative stress. Int J Res Pharm Biosci 3: 1-8.
- Parkin DM, Bray F, Ferlay J, Pisani P (2006) Global Cancer Statistics, 2002.CA Cancer J Clin 55: 74-108
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C,et al. (2008) Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893-2917.
- Terzic J, Grivennikov S, Karin E, Karin M (2010) Inflammation and Colon Cancer. Gastroenterology 138: 2101-2114.
- Hirono I (1993) Edible Plants containing natural ocurring carcinogens in japan. Jpn J Cancer Res 84: 997-1006.
- Lanqueur GL,Spatz M (1968) Toxicology of Cycasin.Cancer Res 28:2262-2267.
- Lanqueur GL, Michelson O, Whiting MG, Kurlan J (1963) Carcinogenic properties of nuts from cycas circinalis l. Indigenous to guam. J Natl Cancer Inst 31: 1919-1951.
- Lanqueur GL (1964) Carcinogenic effects of cycad meal and cycasin, methylaxoxymethanol glycosides in rats and effect of cycasin in germfree rats. Fed Proc 23 :1386-1387.
- Nishida K, Kobayashi A, Nagahama T (1955) Studies on Cycasin as new glycoside of Cycas revolute Thumb. I. Isolation and structure of cycasin. Bull Agric Chem Soc Japan 19: 77-84.
- Riggs NV (1956) Glucosyloxyazoxymethane, a constituent of the seeds of Cycas circinalis L. Chem Ind 92: 1-4.
- Â Lanqueur GL (1965) The induction of intestinal neoplasm in rats with cycasin and its aglycone. Virchows Arch 340: 151-163.
- Â Hirono I,Lanqueur GL,Spatz M (1968) Tumor induction in Fischer and Osborne-Mendel Rats by a Single Adminstration of Cycasin. J Natl Cancer Inst 40: 1003-1010.
- Â FukunishiR, Terashi S, Watanabe K, Kawaji K (1972) High yield of hepatic tumor in rats by cycasin. Gann 63:575-578.
- Hirono I, Shibuya C,Fushimi K (1969) Tumor Induction in C57BL/6 mice by a single administration of cycasin. Cancer Res 29:1 658-1662.
- HironoI, Shibuya C (1970) High incidence of pulmonary tumors in dd mice by a single injection of cycasin.Gann 61: 403-407.
- Shibuya C, Hirono I (1973) Relations between postnatal days of mice and carcinogenic effect of cycasin. Gann. 64: 109-110.
- Hirono I, Hayashi K, Mori H, Miwa T (1971) Carcinogenic effect of cycasin in Syrian golden hamsters and the transplantability of induced tumors. Cancer Res 31: 283-287.
- Spatz M (1964) Carcinogenic effect of cycad meal in guinea pigs. Fed Proc 23: 1384-1385.
- Watanabe K, Iwashita H , Muta K, Hamada Y, Hamada K (1975) Hepatic tumors of rabbits induced by cyacad extracts.Gann 66: 335-339.
- Stanton MF (1966) Hepatic neoplasm in aquarium fish exposed to Cycas circinalis. 25:661.
- Lanqueur GL, McDaniel EG, Matsumoto H (1967) Tumor Induction in Germfree Rats with Methylazoxymethanol (MAM) and synthetic MAM Acetate. J Natl Cancer Inst 39: 355-371.
- SpencerPS, Garner CE, Palmer VS, Kisby G.E (2015) Environmental Factors in Neurodevelopmental and Neurodegenerative Disorder. Elsevier Inc.
- Okolie NP, Agu K, Eze GI (2013) Proctective effect of ethanolic leaf extract of Annona muricata Linn on some early events in Cycas-induced colorectal carcinogenesis in rats. J Pharm Sci Innov 2: 14-21.
- Lorke D (1983) A new approach of practical acute toxicity testing.Arch Toxicol 54: 275-287.
- Animal Care and Use program (2011) In, Guidelines for Care and Use of Animals for Scientific Research. (8th edn),National Academic Press,Washigton.
- Misra HP, Fridovic I (1972) The role of superoxide ion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247: 3170-3175.
- Cohen G, Dembiee D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34: 30-38.
- Flohe L, Gu?zler WA (1984) Assay of glutathione peroxidise. Methods Enzymol 105: 114-121.
- Nagalingum NS, Marshal CR, Quental TB, Tai HS, Little DP, et al (2011) Recent synchronous radiation of a living fossil. Science 334: 796-799
- Rose C, Wu H (2010) Morphologic Criteria of Invasive Adenocarcinoma in Biopsy Specimens. Int J Pathol 12: 1.
- Spiteller G (2007) The important role of lipid peroxidation in aging and age-dependent diseases. Mol Biotechnol 37: 5-12.
- Finaud J, Lac G, Filaire E (2006) Oxidative stress: relationship with exercise and training. Sports Med 36: 327-358.
- Evans WJ , Morly JE, AgrilesJ, Bales C, Baracos V, et al (2008) Cachexia: A new definition.Clin Nutr 27: 793-799.
- Perse M (2013) Oxidative Stress in the Pathogenesis of Colorectal Cancer: Cause or Consequence?.BioMed Res Int 2013: 1-4.
- Shringarpure R, Davies KJA (2002) Protein turnover by the proteasome in aging and diseases. Free Radic Biol Med 32: 1084-1089.
- Aziza SH, Abdel A, Mady HA (2014) Chemopreventive Effect of Curcumin on Oxidative Stress, Antioxidant Status, DNA Fragmentation and Caspase-9 Gene Expression in 1,2-dimethylhydrazine-induced Colon Cancer in Rats. Am J Biochem Mol Biol 4: 22-34.
- Gilad E, Zingarelli B, O’Connor M, Salzman AL, Bertok L, et al (1996) Effects of radiodetoxified endotoxin on nitric oxide production in J774 macrophages and in endotoxin shock.J Endotoxin Res 3: 513-519.
- Michiels C, Raes M, Toussaint O,Remacle J (1994) Importance of Se-glutathione peroxidase, catalase and Cu-Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17: 235-248.
- Ahmet D, Nurhayat A, Halit D, Erkan D, Tulay G, et al (2017) Investigation of levels of oxidative stress and antioxidant enzymes in colon cancers. J Clin Ana Med 8: 469-473.
Citation: Isoje Abigail, Obi Frederick, Usifo Ruth and ObadoniIsabel (2021) Comparison of the Oxidative Stress and Histopathological Change Inducing Capacities of Cycas circinalis Leaf Powder and the Ethanolic Extract in Rats. Toxicol: Open Access 7:156. DOI: 10.4172/2476-2067.1000156
Copyright: © 2021 Abigail I,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3180
- [From(publication date): 0-2021 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2400
- PDF downloads: 780