Research Article Open Access
Comparison of Sampling Probe and Thermal Desorber in HAPSITE ER for Analysis of TO-15 Compounds
Jae Kwak1*, Maomian Fan2, Brain A Geier3, Claude C Grigsby2 and Darrin K Ott41The Henry M Jackson Foundation for the Advancement of Military Medicine, Air Force Research Laboratory, 711th Human Performance Wing, Wright-Patterson AFB, USA
2Air Force Research Laboratory, 711th Human Performance Wing, Wright-Patterson AFB, USA
3Infoscitek Corporation, Air Force Research Laboratory, 711th Human Performance Wing, Wright-Patterson AFB, USA
4Air Force Research Laboratory, 711th Human Performance Wing, U.S. Air Force School of Aerospace Medicine, Wright-Patterson AFB, USA
- *Corresponding Author:
- Jae Kwak
The Henry M Jackson Foundation for the Advancement of Military Medicine
Air Force Research Laboratory, 711th Human Performance Wing
Wright-Patterson AFB, OH 45433, USA
Tel: +1-937-938-3790
Fax: +1-937-656-6898
E-mail: jae_hyock.kwak.ctr.kr@us.af.mil
Received date: January 23, 2014; Accepted date: February 07, 2014; Published date: February 10, 2014
Citation: Kwak J, Fan M, Geier BA, Grigsby CC, Ott DK (2014) Comparison of Sampling Probe and Thermal Desorber in HAPSITE ER for Analysis of TO-15 Compounds. J Anal Bioanal Tech S2:008. doi: 10.4172/2155-9872.S2-008
Copyright: © 2014 Kwak J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The Hazardous Air Pollutants on Site (HAPSITE), a portable Gas Chromatograph-Mass Spectrometer (GCMS), has been used to detect, identify, and quantify Volatile Organic Compounds (VOCs) from environmental samples, providing on-site analysis to aid in operational risk management. HAPSITE is equipped with a hand-held sampling probe in which an air sample is delivered into a concentrator, and the VOCs collected in the concentrator are transferred, separated, and identified in the GC-MS. An upgraded version, HAPSITE ER, has recently been introduced with additional sampling capability for solid phase micro extraction and Thermal Desorption (TD). To our knowledge, however, no study has yet evaluated the performance of the thermal desorber accommodated in HAPSITE ER. In this study, therefore, we analyzed EPA Method TO-15 compounds with two different sampling methods (probe and thermal desorber for TD tubes) in a HAPSITE ER, and compared their results against each other. A major finding was that the peak intensities of the TO-15 compounds, particularly those with high Boiling Point (BP), were substantially higher in the results obtained with the thermal desorber than in those with the sampling probe. The lower peak intensities of the compounds observed in the probe analysis are likely due to the condensation of the VOCs in the probe (transfer) line that is 6 feet long and maintained at 40°C as they are delivered from the probe to the concentrator, whereas the thermal desorber is directly connected to the HAPSITE (no transfer line is used), thereby eliminating the condensation of VOCs. In conclusion, our study suggests that for the analysis of VOCs with high up to 220°C, the use of TD tubes followed by desorption in the thermal desorber offered by the newer version of HAPSITE is recommended.
Keywords
Air Pollutants; Gas chromatograph-mass spectrometer; Thermal desorber
Introduction
The INFICON Hazardous Air Pollutants on Site (HAPSITE®), a portable Gas Chromatograph-Mass Spectrometer (GC-MS), has been used to detect, identify, and quantify unknown hazardous materials (e.g. chemical warfare agents, volatile toxic industry chemicals, etc.) in an operational environment [1-6], providing on-site analysis to aid in operational risk management. HAPSITE is equipped with a hand-held sampling probe via which an air sample is delivered into a concentrator in the HAPSITE system. The Volatile Organic Compounds (VOCs) collected in the concentrator are transferred and separated through a GC column. The GC effluents then pass through a membrane maintained at 80°C, where volatile analytes move to the MS while inorganic gases (e.g. nitrogen and oxygen) are discarded [2]. A quadrupole mass spectrometric detector is operated under vacuum provided by a Non Evaporative Getter (NEG) and an ion sputter pump [7].
While the probe method allows near real time analysis of an air sample, it limits the volume of the sample collected, i.e. the sensitivity. In addition, VOCs with high Boiling Point (BP) are more likely condensed in the probe (transfer) line that is 6 feet long and maintained at 40°C, when they are delivered from the probe to the concentrator. To our surprise, however, this potential condensation issue has never been considered in the studies conducted previously. An upgraded version, HAPSITE ER, has recently been introduced with additional sampling capability for Solid Phase Micro Extraction (SPME) and Thermal Desorption (TD). Since the desorbers for a SPME fiber and a TD sorbent tube are directly connected to the HAPSITE ER (no transfer line is used), the condensation of VOCs with high BP in the probe line that possibly occurs when the sampling probe is used can be minimized. To our knowledge, however, no study has yet evaluated the performance of the new thermal desorbers. In this study, therefore, we analyzed EPA Method TO-15 compounds with two different sampling methods (probe and thermal desorber for TD tubes) in a HAPSITE ER, and compared the performance of the methods.
Materials and Methods
Thermal desorption sorbent tubes
Stainless Steel (SS) TD tubes containing a single component sorbent Tenax® TA purchased from Markes International (South Wales, UK) was used in this study. All tubes were conditioned prior to use based on the manufacturer’s instruction.
Preparation of TO-15 compounds in a bag
To prepare 20 ppbv TO-15 compounds in a 5 L ALTEF polypropylene bag (Jensen Inert Products, Coral Springs, FL, USA), 100 mL was taken from a cylinder of the TO-15 65 component mix (1 ppm concentration) purchased from Restek (Bellefonte, PA, USA) using a 100 mL gas-tight syringe and then spiked into the 5L bag filled with nitrogen. The bag was left overnight for equilibration prior to sampling. Then, 100 mL was taken from the bag with the HAPSITE sampling probe at 130 mL/ min or transferred to a Tenax sorbent tube with a 100 mL gas-tight syringe for the thermal desorber analysis. The sampling and analysis of the TO-15 compounds were performed 3 times with each sampling method (probe and thermal desorber) in the HAPSITE.
HAPSITE
A HAPSITE® ER system obtained from INFICON (East Syracuse, NY, USA) was used for analysis of the TO-15 mix in this study. A nonpolar column (100% polydimethylsiloxane; 15 m×0.25 mm ID×1.0 μm df) was equipped into the HAPSITE. For both probe and thermal
desorber analyses, the temperatures of membrane, valve oven and heated lines were 80, 70 and 70°C, respectively. The GC temperature program and parameters in the mass spectrometer were identical as well. The GC temperature program started at 50°C for 2 min, increased at 3°C/min to 80°C, at 12°C/min to 120°C, and at 26°C/min to 200°C where the final temperature was held for 5.6 min. The GC analysis time was 24 min. Nitrogen was used as the carrier gas at a constant pressure of 85 kPa. The mass spectrometer was operated in the electron impact ionization mode at 70 eV. The mass scan range was m/z 41 to m/z 300, and the scan time was 0.78 sec. The only difference between probe and thermal desorber methods was that the TO-15 mix captured by the probe was delivered to the concentrator at 40°C, whereas the mix adsorbed in a Tenax tube was desorbed in the thermal desorber at 330°C for 10 min and then delivered to the concentrator. Note that the actual desorption temperature of the SS TD tube doesnot reach the set temperature. The HAPSITE thermal desorber accessory was designed for glass TD tubes. INFICON measured the actual temperatures of glass and SS TD tubes placed in a thermal desorber by inserting a thermocouple into each type of tubes. They noted that while the glass tubes reached the standard method set temperature (300°C), the SS tubes only reached around 200°C. The temperature difference between different types of TD tubes is likely due to the difference in the thermal conductivity of the TD tube materials. The HAPSITE ER injects known volumes of internal standards 1,3,5-tris(trifluoromethyl)benzene [TRIS] and bromopentafluorobenzene [BPFB] (10.7 ppm and 5.5 ppm, respectively) for each analysis from the internal standard canister purchased from INFICON.
Statistical analysis
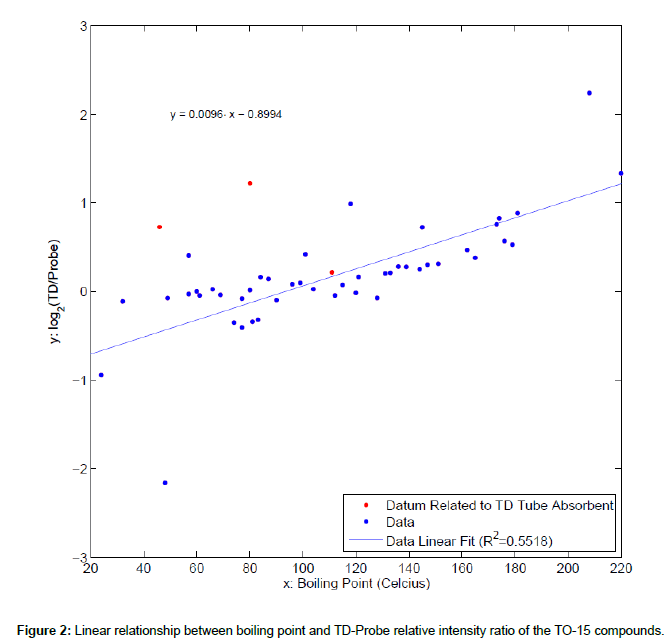
Least squares regression analysis was executed within Matlab© computing environment. Diagnostics, such as residual analysis, was performed using Matlab and Matlab Statistics Toolbox. The difference of the peak intensity for each TO-15 compound obtained by TD vs. probe methods was summarized by log2 ratio; triplicates from TD and probe were summarized by average. A simple linear equation between boiling point and TD-Probe relative intensity ratio of the TO-15 compounds was derived.
Results and Discussion
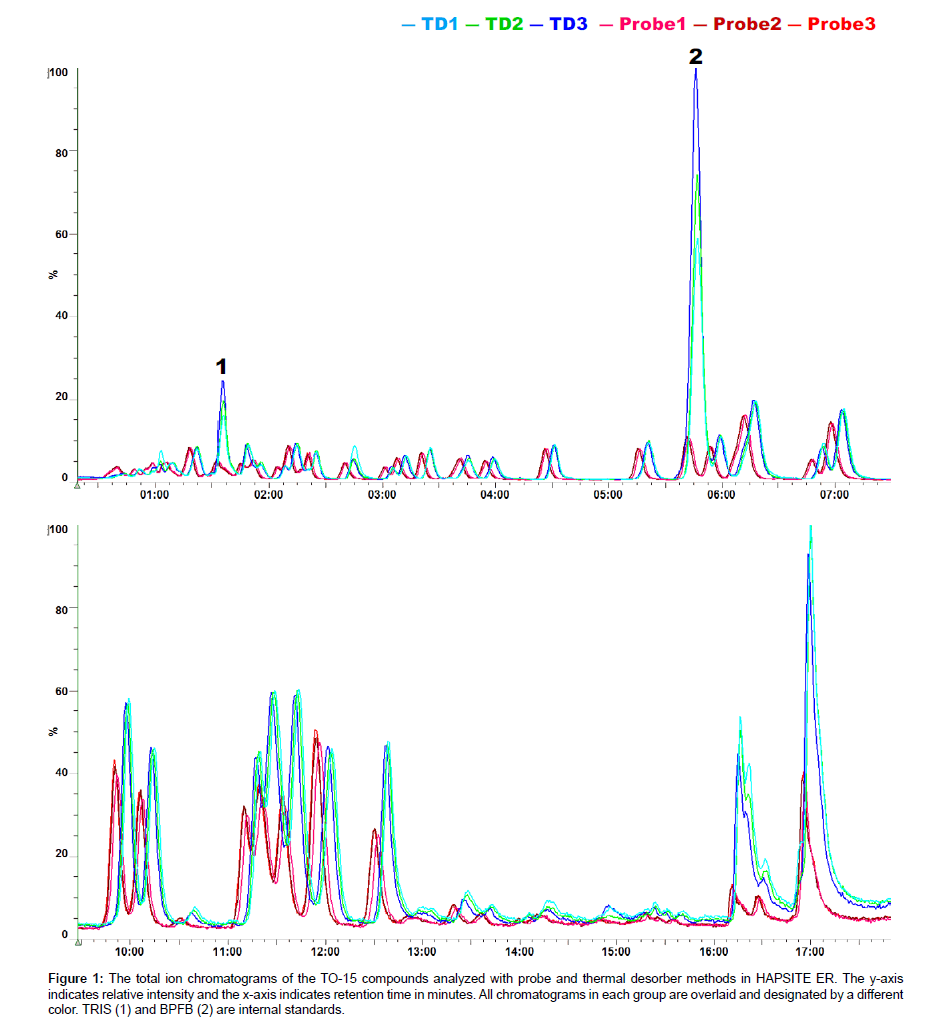
Figure 1 shows the total ion chromatograms of the TO-15 compounds obtained with probe and thermal desorber methods in the HAPSITE ER. Forty nine TO-15 compounds were detected with both sampling methods and no compound detected exclusively with one method was observed. The names, ions for quantification (Q-Ions), Retention Times (RTs), Boiling Points (BPs) and peak intensities for the compounds are listed in Table 1. We noticed several differences in the chromatograms obtained with the different methods. First, the retention time for each TO-15 compound differed slightly. Although the GC temperature program was identical between the two methods, the TO-15 compounds eluted up to 5 seconds later in the thermal desorber analysis than in the probe analysis (Figure 1). We speculate that the carrier gas (nitrogen) pressure in the GC column in the TD analysis may be slightly altered since the GC carrier gas and the thermal desorber purging gas are from the same nitrogen tank. In addition, the difference in the valve events between different sampling methods that control the carrier gas supply, internal standard supply, nitrogen purge in the thermal desorber, sample delivery into the GC, etc., may also result in the column pressure differences. Second, the peak intensities of the internal standards TRIS and BPFB were much higher in the chromatograms obtained with the thermal desorber than in those with the sampling probe (Figure 1 and Table 1). In the probe method, the standards are injected during the line purge event that occurs prior to sampling and that requires a purging with an air sample for 1 min (Personal communication with INFICON). Consequently, the internal standards are diluted with the sample even before the sample is actually being collected and delivered to the concentrator. While dilution of the internal standards occurs in the thermal desorber analysis, the degree of dilution is less than that in the probe analysis. The dilution is caused by the nitrogen flow in the desorber, but the flow is lower than the air sampling flow (130 mL/min) in the probe analysis. As a result, the peak intensities of the internal standards were higher in the results obtained with the TD method. Finally, the peak intensities of the TO-15 compounds, particularly those with high BP, were substantially higher in the results obtained with the thermal desorber than in those with the sampling probe (Table 1). As shown in Figure 2, 55.2% of the variation in the TD to psrobe difference was explained by bp, indicating a linear relationship between BP and the TD-probe relative intensity ratio of the TO-15 compounds. Three chemicals, i.e. carbon disulfide, benzene, and toluene, were excluded from the analysis because their substantial portions are derived from the Tenax TD tube absorbent; residual diagnostics also supported exclusion of these chemicals that are marked with red symbols in Figure 2. Note that the concentrations of the TO- 15 compounds taken by the probe and the Tenax TD tubes should be same (100 mL was taken from the 5 L bag containing 20 ppbv TO-15 mix each for the probe and the TD tubes). The lower peak intensities of the compounds observed in the probe analysis are likely due to the condensation in the probe line that is 6 feet long and maintained at 40°C as they are delivered from the probe to the concentrator. Also, it is noteworthy that the TO-15 compounds adsorbed in the Tenax tubes are almost completely desorbed in the thermal desorber and then delivered to the concentrator (Data not shown). In conclusion, our study suggests that for the analysis of VOCs with high BP up to 220°C, the use of TD tubes followed by desorption in the thermal desorber offered by the newer version of HAPSITE is recommended.
| Analyte Name | Q-Ion | RT | BP(°C) | Intensity of Q-ion @ 20 ppb | Log2(TD/Probe) | |||
|---|---|---|---|---|---|---|---|---|
| TD | Probe | |||||||
| Average | %RSD | Average | %RSD | |||||
| Acetone | 58 | 0.80 | 57 | 162,000 | 21 | 122,333 | 4 | 0.4052 |
| Isopropanol | 45 | 0.84 | 83 | 79,067 | 5 | 98,633 | 9 | -0.3190 |
| Trichloromonofluoromethane | 101 | 0.83 | 24 | 85,533 | 12 | 164,667 | 2 | -0.9450 |
| 1,1-Dichloroethene | 61 | 0.92 | 32 | 191,333 | 4 | 207,000 | 3 | -0.1135 |
| 1,1,2-Trichloro-1,2,2-trifluoroethane | 151 | 0.97 | 48 | 12,667 | 3 | 56,733 | 7 | -2.1632 |
| Carbon disulfide | 76 | 0.99 | 46 | 1,493,333 | 48 | 901,667 | 3 | 0.7279 |
| (E)-1,2-Dichloroethene | 61 | 1.06 | 49 | 376,667 | 6 | 397,000 | 3 | -0.0759 |
| 1,1-Dichloroethane | 63 | 1.10 | 57 | 367,000 | 4 | 374,667 | 5 | -0.0298 |
| 2-Butanone (MEK) | 72 | 1.18 | 80 | 103,200 | 7 | 102,167 | 4 | 0.0145 |
| (Z)-1,2-Dichloroethene | 61 | 1.27 | 60 | 413,667 | 1 | 413,333 | 3 | 0.0012 |
| Ethyl acetate | 88 | 1.31 | 77 | 34,800 | 10 | 36,833 | 8 | -0.0819 |
| Hexane | 57 | 1.31 | 69 | 455,000 | 6 | 467,333 | 2 | -0.0386 |
| Chloroform | 83 | 1.34 | 61 | 494,667 | 2 | 510,333 | 2 | -0.0450 |
| Tetrahydrofuran | 72 | 1.46 | 66 | 97,667 | 7 | 96,067 | 2 | 0.0238 |
| 1,3,5-Tris(trifluoromethyl)benzene (TRIS; an IS) | 213 | 1.56 | 1,048,000 | 29 | 190,667 | 7 | ||
| 1,2-Dichloroethane | 62 | 1.54 | 84 | 512,000 | 3 | 457,333 | 3 | 0.1629 |
| 1,1,1-Trichloroethane | 97 | 1.61 | 74 | 201,667 | 8 | 257,333 | 2 | -0.3517 |
| Benzene | 78 | 1.76 | 80 | 1,936,667 | 5 | 829,667 | 7 | 1.2230 |
| Carbon Tetrachloride | 117 | 1.81 | 77 | 191,667 | 16 | 254,333 | 3 | -0.4081 |
| Cyclohexane | 84 | 1.88 | 81 | 359,667 | 12 | 456,000 | 5 | -0.3424 |
| 1,2-Dichloropropane | 63 | 2.08 | 96 | 307,333 | 1 | 290,333 | 5 | 0.0821 |
| Bromodichloromethane | 83 | 2.17 | 90 | 628,000 | 5 | 671,667 | 4 | -0.0970 |
| 1,4-dioxane | 88 | 2.20 | 101 | 150,333 | 8 | 112,333 | 6 | 0.4204 |
| Trichloroethylene | 130 | 2.20 | 87 | 410,000 | 7 | 371,667 | 3 | 0.1416 |
| Heptane | 71 | 2.36 | 99 | 517,000 | 6 | 483,667 | 1 | 0.0962 |
| (Z)-1,3-Dichloro-1-propene | 75 | 2.67 | 104 | 592,667 | 8 | 582,667 | 6 | 0.0246 |
| Methyl Isobutyl Ketone | 43 | 2.68 | 118 | 650,667 | 54 | 328,000 | 3 | 0.9882 |
| (E)-1,3-Dichloro-1-propene | 75 | 3.04 | 112 | 600,000 | 7 | 619,333 | 6 | -0.0458 |
| 1,1,2-Trichloroethane | 97 | 3.15 | 115 | 396,667 | 2 | 376,667 | 2 | 0.0746 |
| Toluene | 91 | 3.37 | 111 | 1,316,667 | 5 | 1,133,333 | 1 | 0.2163 |
| Methyl Butyl Ketone (2-Hexanone) | 43 | 3.64 | 128 | 293,000 | 2 | 308,667 | 5 | -0.0751 |
| Dibromochloromethane | 129 | 3.70 | 120 | 547,667 | 15 | 554,000 | 5 | -0.0166 |
| 1,2-Dibromoethane | 107 | 3.92 | 133 | 892,333 | 5 | 771,333 | 2 | 0.2102 |
| Tetrachloroethylene | 166 | 4.45 | 121 | 607,667 | 2 | 542,000 | 0 | 0.1650 |
| Chlorobenzene | 112 | 5.28 | 131 | 1,213,333 | 3 | 1,053,333 | 3 | 0.2040 |
| Bromopentafluorobenzene (BPFB; an IS) | 117 | 5.70 | 8,876,667 | 27 | 1,203,333 | 1 | ||
| Ethylbenzene | 91 | 5.91 | 136 | 1,933,333 | 2 | 1,593,333 | 1 | 0.2790 |
| p/m-Xylene | 91 | 6.21 | 139 | 2,826,667 | 3 | 2,333,333 | 1 | 0.2767 |
| Tribromomethane | 173 | 6.13 | 151 | 639,333 | 11 | 515,000 | 1 | 0.3120 |
| Styrene | 104 | 6.81 | 145 | 1,103,333 | 5 | 668,000 | 1 | 0.7239 |
| o-Xylene | 91 | 6.97 | 144 | 1,393,333 | 1 | 1,170,000 | 3 | 0.2520 |
| 1,1,2,2-Tetrachloroethane | 83 | 6.98 | 147 | 1,163,333 | 1 | 943,333 | 1 | 0.3024 |
| 1-Ethyl-4-methylbenzene (4-Ethyltoluene) | 105 | 9.87 | 162 | 1,946,667 | 3 | 1,406,667 | 4 | 0.4687 |
| 1,3,5-Trimethylbenzene (Mesitylene) | 105 | 10.12 | 165 | 1,276,667 | 2 | 980,667 | 3 | 0.3805 |
| 1,2,3-Trimethylbenzene (Hemimellitene) | 105 | 11.20 | 176 | 1,276,667 | 1 | 859,667 | 4 | 0.5705 |
| Benzyl chloride | 126 | 11.44 | 179 | 111,000 | 5 | 76,933 | 12 | 0.5289 |
| 1,3-Dichlorobenzene | 146 | 11.36 | 173 | 1,015,667 | 2 | 601,000 | 5 | 0.7570 |
| 1,4-Dichlorobenzene | 146 | 11.59 | 174 | 1,116,667 | 1 | 629,000 | 7 | 0.8281 |
| 1,2-Dichlorobenzene | 146 | 12.54 | 181 | 860,000 | 2 | 466,000 | 4 | 0.8840 |
| 1,3,5-Trichlorobenzene | 180 | 16.21 | 208 | 666,667 | 9 | 141,000 | 14 | 2.2413 |
| 1,1,2,3,4,4-Hexachloro-1,3-butadiene | 225 | 16.96 | 220 | 613,333 | 4 | 243,667 | 6 | 1.3318 |
Table 1: The ions used for quantification, retention times, boiling points of TO-15 compounds detected in HAPSITE ER, the peak intensities of the ions obtained by the probe and thermal desorber methods, and their logged intensity ratios. N=3 for each sampling method.
Figure 1: The total ion chromatograms of the TO-15 compounds analyzed with probe and thermal desorber methods in HAPSITE ER. The y-axis indicates relative intensity and the x-axis indicates retention time in minutes. All chromatograms in each group are overlaid and designated by a different color. TRIS (1) and BPFB (2) are internal standards.
Acknowledgements
The authors are grateful to Dr. Lindsay Harrington for her technical support on HAPSITE and Dr. Jennifer A Martin for her useful suggestions on an earlier version of this manuscript.
References
- Smith PA, Koch D, Hook GL, Erickson RP (2004) Detection of gas-phase chemical warfare agents using field-portable gas chromatography–mass spectrometry systems: instrument and sampling strategy considerations. TrAC Trends in Analytical Chemistry 23: 296-306.
- Sekiguchi H, Matsushita K, Yamashiro S, Sano Y, Seto Y, et al. (2006) On-site determination of nerve and mustard gases using a field-portable gas chromatograph-mass spectrometer. Forensic Toxicology 24: 17-22.
- Fair JD, Bailey WF, Felty RA, Gifford AE, Shultes B, et al. (2009) Method for rapid on-site identification of VOCs. J Environ Sci (China) 21: 1005-1008.
- Fair JD, Bailey WF, Felty RA, Gifford AE, Shultes B, et al. (2010) Quantitation by Portable Gas Chromatography: Mass Spectrometry of VOCs Associated with Vapor Intrusion. Int J Anal Chem .
- Gorder KA, Dettenmaier EM (2011) Portable GC/MS methods to evaluate sources of cVOC contamination in indoor air. Groundwater Monitoring & Remediation 31: 113-119.
- Johnston JE, Gibson JM (2013) Spatiotemporal variability of tetrachloroethylene in residential indoor air due to vapor intrusion: a longitudinal, community-based study. J Expo Sci Environ Epidemiol.
- Smith PA (2012) Person-portable gas chromatography: rapid temperature program operation through resistive heating of columns with inherently low thermal mass properties. J Chromatogr A 1261: 37-45.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14344
- [From(publication date):
specialissue-2014 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 9895
- PDF downloads : 4449