Comparison of Salivary PH Changes with Tap Water and Mineral Water Rinse after 50% Sucrose Solution Rinse: A Cross-Over Trial
Received: 16-Jul-2017 / Accepted Date: 23-Aug-2017 / Published Date: 29-Aug-2017
Abstract
Aim: To compare the effect on salivary pH following mouth rinse using tap water and various brands of mineral water after rinsing with 50% sucrose solution.
Introduction: Oral hygiene is the primordial level of prevention of dental caries. Since mouth rinsing habit is a daily oral hygiene habit, modification with pH modifying agents can greatly help in the prevention of dental caries.
Methodology: A cross-over trial was done among 60 children in the age group of 3-6 years. The salivary pH was measured at 3 intervals at baseline, after rinsing with 50% sucrose solution and after rinsing with tap water. After 30 min the similar protocol is followed for measuring pH after rinsing with mineral water. Randomisation was followed in selecting the brand of mineral water to be used. The results obtained were compared with Wilcoxon Signed Ranks Test the salivary pH after tap water and mineral water rinse and with Kruskal-Wallis Test in between the three brands of mineral water.
Results: There was a significant difference in salivary pH after the tap water and mineral water rinse with a higher pH after mineral water rinse. However, there was no significant difference in between the three different brands of mineral water.
Conclusion: Rinsing mouth with a solution having higher alkaline pH leads to neutralization of acid production, thereby preventing the caries process.
Keywords: Demineralization; Mineral water; Mouth rinse; Salivary pH; Tap water
Introduction
Although preventable, dental caries is considered to be the most common condition of childhood [1]. Consumption of more sugary food [2] and less frequency of oral hygiene practice [3] is a major determinant of the same. Early childhood caries, a more common condition of childhood is a multifactorial disorder with improper dietary pattern and oral hygiene habits being a major determinant. Children experiencing caries in the early childhood phase are more of development of caries in the permanent dentition.
Caries can not only be prevented by controlling dietary habits, but also by optimal oral hygiene habits [4]. Studies have shown that children with good oral hygiene practice have a lower incidence of caries than children with poor oral hygiene practice [5,6].
Brushing and flossing are the oral hygiene practices most commonly practised in day to day life [7]. Tooth brushing with a fluoridated toothpaste is effective in reducing the prevalence of caries [6]. Flossing tend to remove plaque from the proximal tooth surface, thereby preventing dental caries [6]. The method of cleaning and frequency are important determinants in caries prevention [7]. Mouth rinsing with fluoride mouth rinses was a mass prophylactic measure in the prevention of dental caries as recommended by FDI (expansion) [8]. Other type of mouth rinses like chlorhexideine mouth rinse [9] and salt water mouth rinse [10] also aids in caries prevention.
According to Key’s triad, salivary bacteria play an important role in development of caries along with dietary habit (substrate) and host [4]. The oral biofilm mainly consist of predominant bacterial species from coccoid or straight rod bacteria [11]. The role of biofilm has a major role in the caries initiation and progression. Streptococcus mutans, Enterococcus faecium, Aerococcus viridans, Actinomyces meyeri, Lactobacillus acidophilus and Eubacterium limosum isolated from oral biofilm are known have increased titers in the presence of caries [12]. Study by Santos et al. has shown a relationship between the frequency and type of oral hygiene practice on the oral biofilm. Oral hygiene practice has shown to decrease the accumulation of biofilm, indirectly halting the caries process [13].
Salivary pH is another important determinant in causation of carries. Hence Modification of salivary pH to alkalinity plays an important role in prevention of dental caries [14]. Increase in the clearance rate changes the salivary pH there by modifying the pH of the plaque and promoting remineralization [15]. For relative protection against dental caries, flow rate, buffering capacity, calcium phosphate and fluoride concentration in the saliva are essential [14].
According to Watt in 2005, people tend to imply oral hygiene practice that takes less time and effort. With this purpose, a cross over trial was conducted in Chennai to evaluate and compare the effect on salivary pH after tap water and mineral water mouth rinse following rinsing with 50% sucrose.
Methodology
A cross-over randomised control trial was done in preschool children. The study design protocol and informed consent was approved by the ethical committee board of the institution with the ethical number STP/SDMDS16PED3. The procedure was followed in accordance with the Helsinki Declaration of 1975 that was revised in 2000. The inclusion criteria were that children should be between 2-6 years of age, they were medically healthy, had full primary dentition with or without the eruption of first permanent molar and the parent or guardian provided informed consent for the child’s participation. Children with intra oral or extra oral abscess and parents who were not willing to participate in the study were excluded. These children were enrolled from the preschools in Chennai as well as randomly selected patients visiting Saveetha Dental College and Hospital for dental treatment. Based on the sample size calculated block randomization was followed.
A pre screening was done to obtain a dmft score for each patient. This was done to include equal number of caries free children and children with ECC for comparison of the result between the two groups.
Sample size calculation
The sample size was calculated by a pilot study done among 15 participants with all the participants rinsing with tap water after 50% sucrose solution, and 5 participants rinsing with the individual brand of mineral water. The sample size calculation was done with G power using a priori where the difference between two dependent matched pairs was compared. The α error was taken as 0.05 and (1-β) as 0.95 with the effect size of 0.5574. The sample size obtained was 44 in total. This sample size was over estimated to compensate for attrition and salivary sample was collected from 60 preschool children.
Data collection
All children were asked to swish mouth with tap water and any one of the three brands of mineral water (Bisleri, Aquafina, Kinley) after a 50% sugar rinse. The three different brands of mineral water were maintained confidential to the patients by labelling them as A,B and C respectively. The observer and patient blinding was thus carried out. The three brands of mineral water were checked for their brands after the completion of the study and were labelled as A, B and C throughout the study .In the first attempt, un stimulated saliva samples was taken and pH was measured. After this, all participants were asked to swish and swift a 50% sugar solution for 1 min. 15 min later saliva sample is obtained and pH is measured by electrical pH meter. Immediately after this they rinsed the mouth with tap water for 1 min. A third sample was taken after another 15 min and pH was measured. Based on the block randomization, the chit allocation method was followed where the brand of mineral water to be given as chosen. After 30 min , again un stimulated saliva was taken after which , all participants are asked to swish with 50% sugar solution for 1 min and saliva sample was collect after 15 min and pH measured. Immediately after this, they rinsed the mouth with any one of the three brands of mineral water for 1 min and third saliva sample was obtained after 15 min and pH was measured to avoid diurnal variation of the pH, all the samples were collected during the morning time in between 9 am till 12 pm.
pH data recorded was grouped as pre pH, pH after 50% sucrose rinse, pH after tap water rinse and pH after individual mineral water rinse. The change in the pH of saliva after tap water rinse and mineral water rinse were compared. As well change in pH after using various brands of mineral water was also analysed In addition, the difference in the pH between the caries free and children with ECC was compared.
Statistical analysis
The data obtained were analysed using SPSS software version 22. The Normality tests Kolmogorov-Smirnov and Shapiro-Wilks tests results reveal that data obtained from individual brand of mineral water did not follow Normal distribution. Wilcoxon Signed Ranks Test was used to compare pH levels between Tap water and Mineral water rinse. Kruskal-Wallis Test was used to compare pH levels between the three brands of mineral water. Significance level is fixed as 5% (α=0.05). The drop in the pH after the 50% sucrose rinse and rinse in pH after tap water rinse between caries free children and children with ECC were compared using Mann- Whitneys test (p<0.05).
Results
Of the total children recruited in the study 45% were boys and 55% were girls (Table 1) in the age group of 3 years to 6 years with the mean age of 4 years and 9 months. 13.3% belong to the 3 years age group, 20% belong to 4 years, 30% belong to 5 years and 36.7% belong to 6 years age group (Tables 2a and 2b).
| Gender | N | % |
|---|---|---|
| Male | 27 | 45.0 |
| Female | 33 | 55.0 |
| Total | 60 | 100.0 |
Table 1: Frequency Table for Gender.
| Statistic | Value |
|---|---|
| N | 60 |
| Mean | 4.90 |
| Std. Deviation | 1.053 |
| Minimum | 3.0 |
| Maximum | 6.0 |
| 1st Quartile | 4.0 |
| Median | 5.0 |
| 3rd Quartile | 6.0 |
Table 2a: Descriptive statistics for Age.
| Age | N | % |
|---|---|---|
| 3 | 8 | 13.3 |
| 4 | 12 | 20.0 |
| 5 | 18 | 30.0 |
| 6 | 22 | 36.7 |
| Total | 60 | 100.0 |
Table 2b: Descriptive statistics for Age.
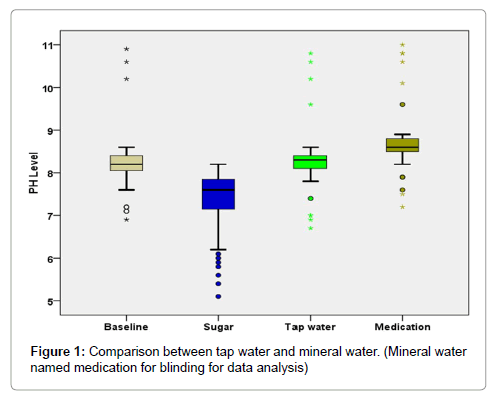
The mean pH of the saliva after tap water rinse in caries free children was 8.25 (SD-0.276) and median (interquartile range) of 8.20(8.30- 8.40). The mean pH of saliva after tap water rinse in children with ECC was 8.34 (SD-0.925) and median (interquartile range) of 8.30 (8.00- 8.40). The mean pH of the saliva after mineral water rinse in caries free children was 8.63 (SD-0.229) and median (interquartile range) of 8.60 (8.50-8.80). The mean pH of saliva after mineral water rinse in children with ECC was 8.83 (SD-0.950) and median (interquartile range) of 8.60(8.40-8.80) (Table 3).
| DMFS | |||
|---|---|---|---|
| Absent | Present | ||
| Tap water | N | 30 | 30 |
| Mean | 8.25 | 8.34 | |
| Std. Dev | .276 | .925 | |
| Median | 8.30 | 8.30 | |
| 1st Quartile | 8.20 | 8.00 | |
| 3rd Quartile | 8.40 | 8.40 | |
| Mineral water | N | 30 | 30 |
| Mean | 8.63 | 8.83 | |
| Std. Dev | .229 | .950 | |
| Median | 8.60 | 8.60 | |
| 1st Quartile | 8.50 | 8.40 | |
| 3rd Quartile | 8.80 | 8.80 | |
Table 3: Descriptive Statistics.
Comparative analysis of Wilcoxon signed Rank test (Table 4 and Figure 1) reveal that salivary pH is higher after rinsing with mineral water as compared to salivary pH after rinsing with tap water (P<0.001).
| Ranks | N | Mean Rank | Z-Value | P-Value | |
|---|---|---|---|---|---|
| Mineral water-Tap water | Negative | 1 | 58.00 | 6.267 | <0.001 |
| Positive | 58 | 29.52 |
Table 4: Wilcoxon Signed Ranks Test to compare PH levels between Tap water and Medication.
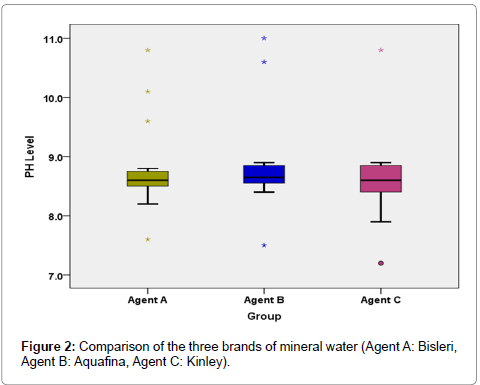
The mean (SD) pH after rinsing with individual A or B or C brand of mineral water is 8.76 (0.685), 8.82 (0.742), 8.61 (0.666) having a median (interquartile range) value of 8.60 (8.50-8.75), 8.65 (8.55-8.85), 8.60 (8.40-8.85) respectively (Table 5).
| Group | ||||
|---|---|---|---|---|
| Agent A | Agent B | Agent C | ||
| PH levels after mineral water | N | 20 | 20 | 20 |
| Mean | 8.76 | 8.82 | 8.61 | |
| Std. Dev | .685 | .742 | .666 | |
| Median | 8.60 | 8.65 | 8.60 | |
| 1st Quartile | 8.50 | 8.55 | 8.40 | |
| 3rd Quartile | 8.75 | 8.85 | 8.85 | |
Table 5: Descriptive Statistics.
Comparative analysis of Kruskal-Wallis Test (Table 6 and Figure 2) reveals no significant variation in the salivary pH after rinsing with the three different brands of mineral water (P-0.609).
| Group | N | Mean Rank | Chi-Square value | P-Value | |
|---|---|---|---|---|---|
| Mineral water | Agent A | 20 | 29.43 | 0.991 | 0.609 |
| Agent B | 20 | 33.60 | |||
| Agent C | 20 | 28.48 |
Table 6: Kruskal-Wallis Test to compare PH levels between medications.
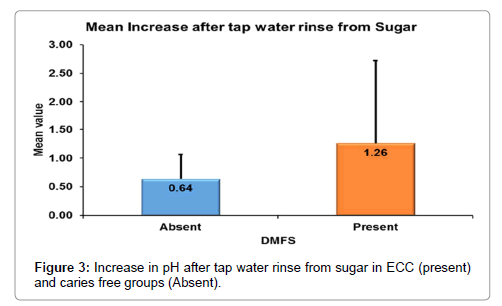
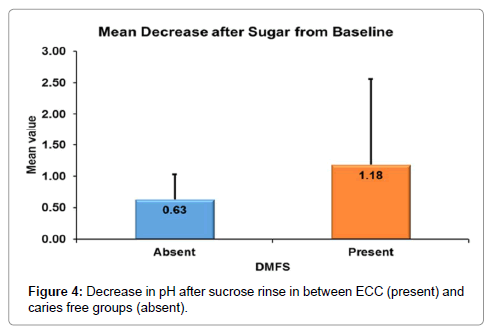
According to Mann-whitney’s (Tables 7 and 8 and Figure 3) test there was a significantly more increase in the salivary pH after tap water rinse in ECC as compared to caries free children. Similarly, there was more decrease in the salivary pH in ECC group as compared to caries free group (Figure 4).
| DMFS | |||
|---|---|---|---|
| Absent | Present | ||
| Increase after tap water rinse from Sugar | N | 30 | 30 |
| Mean | .64 | 1.26 | |
| Std. Dev | .425 | 1.461 | |
| Median | .60 | .60 | |
| 1st Quartile | .50 | .50 | |
| 3rd Quartile | .60 | 1.00 | |
| Percentage increase after tap water rinse from Sugar | N | 30 | 30 |
| Mean | 8.80 | 20.56 | |
| Std. Dev | 7.119 | 26.799 | |
| Median | 7.64 | 7.74 | |
| 1st Quartile | 6.25 | 6.33 | |
| 3rd Quartile | 8.00 | 15.63 | |
| Decrease after Sugar from Baseline | N | 30 | 30 |
| Mean | .63 | 1.18 | |
| Std. Dev | .397 | 1.379 | |
| Median | .50 | .60 | |
| 1st Quartile | .40 | .40 | |
| 3rd Quartile | .70 | .80 | |
| Percentage decrease after Sugar from Baseline | N | 30 | 30 |
| Mean | 7.61 | 13.33 | |
| Std. Dev | 4.826 | 13.459 | |
| Median | 6.17 | 7.14 | |
| 1st Quartile | 4.88 | 5.00 | |
| 3rd Quartile | 8.24 | 11.27 | |
Table 7: Descriptive Statistics.
| Changes | DMFS | N | Mean Rank | Z-Value | P-Value |
|---|---|---|---|---|---|
| Increase after tap water rinse from Sugar | Absent | 30 | 28.23 | 1.020 | 0.308 |
| Present | 30 | 32.77 | |||
| Percentage increase after tap water rinse from Sugar | Absent | 30 | 27.50 | 1.332 | 0.183 |
| Present | 30 | 33.50 | |||
| Decrease after Sugar from Baseline | Absent | 30 | 28.25 | 1.009 | 0.313 |
| Present | 30 | 32.75 | |||
| Percentage decrease after Sugar from Baseline | Absent | 30 | 27.55 | 1.309 | 0.190 |
| Present | 30 | 33.45 |
Table 8: Mann-Whitney Test to compare the differences in PH levels between DMFS groups.
The range of pH change is higher for children with ECC as compared to carried free children. The fall of the pH below the critical pH is more in children with ECC as compared to caries free group.
Discussion
Salivary pH is an important salivary parameter affecting carious process [16]. Demineralization and remineralisation happens depends on the pH of saliva. The alkaline pH of saliva neutralizes the acid produced by the plaque bacteria [16,17]. Further, a more basic pH of saliva aids in remineralisation by precipitation of bicarbonate ions.
Stephan’s curve describes increased demineralization on repeated exposure to low salivary pH [18]. Stephan reported that plaque pH fall after sugar exposure was less in caries free individual as compared to children with caries [19]. According to Leach, enamel solubility is largely dependent on the plaque pH and the acid production leading to fall in pH [20]. Agus et al. [21] and Van Houte et al. [22] have also done similar studies in animal and in vitro showing the decrease in the salivary pH as a determinant of dental caries.
This study shows a significantly greater rise in salivary pH with tap water and mineral water rinse from the pH after 50% sucrose solution. Rinsing with water thus results in dilution of the acid produced after the sugar consumption thereby causing neutralization and rise in pH. Study by Olivia lim et al. has concluded that pH of the saliva increase if the mouth rinse is done with a solution having alkaline pH [23]. Another study contradicted the fact and stated that tap water rinsing has no significant effect on the salivary pH and showed xylitol chewing gum having higher effect [17].
The composition of mineral water shows a more alkaline pH as compared to tap water which there by helps in more neutralization of the acid produced thereby raising the pH more as compared to tap water as found in this study [23]. However, no other study has been reported that has compared buffering capacity of saliva with solution of different alkalinity. A study has stated that rinsing mouth with mouthwash being more alkaline aids in neutralizing the acidity, which can be indirectly co related to the present study [16].
There were no significant statistical differences in the change in pH of salivary in between the three different brands of mineral water. The presence of increased bicarbonate in the saliva leads to a super saturated solution thereby increasing the pH [24]. The clearance rate and buffering capacity of saliva is a major determinant in the carious process [16]. Gopinath et al. stated that the clearance rate and the quantity of the secretion of saliva change the pH. In the present study, the protective effect of Tap water rinse can be attributed to increases in the clearance rate, there by altering the pH having a more protective effect towards caries prevention. Thus altering buffering capacity of saliva by altering salivary pH can aid as a preventive measure. Previous studies have also stated that salivary buffering capacity is an important determinant in caries progression and prevention [10]. This finding co related with the previous study by Johansen et al telling about the lower buffering capacity of saliva makes it more caries prone [25]. According to the present study, tap water rinse provides increase buffering capacity of saliva there by aiding as a preventive factor. The present study shows an increase in salivary pH with mineral water as compared to tap water, which can be attributed to the higher pH of the mineral water as compared to tap water, thus increasing the buffering capacity of saliva [23]. The present study does not show a significant difference in between the three brands of mineral water. This can be attributed to a less variation in between the pH of individual mineral water leading to insignificant changes.
The present study also shows a significantly higher change in the salivary pH in children with ECC as compared to caries free children. A similar finding has been noted by Stephan in his study [19]. which noted a higher fall in pH after sugar consumption in children with ECC.
The present study however does not explain the molecular level phenomenon involved in the plaque pH changes. Hence further studiers need to be carried out to study the molecular level changes occurring with the use of solutions having increased buffering capacity. Also, further studies need to be carried out to evaluate the influence of the rinsing and oral hygiene practice on the individual composition of the saliva to determine the correct causation relationship.
Conclusion
Water rinsing increases salivary pH after the reduction with 50% sucrose solution. Rinsing with a solution that is mineral water having a more alkaline pH leads to increase in salivary pH, which thereby aids as a stop in the process or demineralization by acid production. This indirectly enhances the caries prevention and a simple oral hygiene practice which can be easily used.
References
- Fejersjov O (2004) Changing paradigms in concept on dental caries: consequences for oral health care. Caries Res 38: 182-191.
- Pereira SM, Tagliaferro EP, Pardi V, Cenci MS, Cortelazzi KL, et al. (2010) Sugar consumption and dental health: is there a correlation? Gen Dent 58: 6-12.
- Freddo SL, Aerts DR, Abegg C, Davoglio R, Vieira RC, et al.( 2008) Oral hygiene habits and use of dental sevices among teenage students in a city in southern Brazil. Cad Saude Publica 24: 1991-2000.
- Tinanoff N, Palmer CA (2000) Dietary determinants of dental caries and dietary recommendation for preschool children. J Public Health Dent 60: 197-206.
- Moynihan P, Petersen PE (2004) Diet, nutrition and the prevention of dental diseases. Public Health Nutr 7: 201-226.
- Andlaw RJ (1978) Oral hygiene and dental caries –a review. Int Dent J 28: 1-6.
- Bellini HT, Arneberg P, von der Fehr FR (1981) Oral hygiene and caries. A review. Acta Odontol Scand 39: 257-265.
- FDI Commission (2002) Mouthrinses and dental caries. Int Dent J 52: 337- 345.
- Autio-Gold J (2008) The role of chlorhexidine in caries prevention. Oper Dent 33: 710-716.
- Robert EG (1978) The viability of micro organism in carious lesion five years after covering with a fissure sealant. JADA 97: 455-462.
- Germano F, Bramanti E, Arcuri C, Cecchetti F, Cicciu M, et al. (2013) Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study results. Eur J Dent 7: 152-158.
- Hoceini A, Khelil NK, Yelles IB, Mesli A, Ziouani S, et al. (2016) Caries related factors and bacteria composition of supragingival plaquein caries free and caries active Algerian Adults. Asian Pac J Trop Biomed 6: 720-726.
- Santos AP, Sellos MC, Ramos ME, Sovieo VM (2007) Oral hygiene frequency and presence of visible biofilm in the primary dentition. Braz Oral Res 21: 64-69.
- Abou EL-Yazeed M, Taha S, Shehaby EI F, Salem G (2009) Relationship between salivary composition and dental caries among Egyptian down syndrome children. Aust J Basic Appl Sci 3: 720-730.
- Pannunzio E, Amanico OM, Vitalle MS, Souza DN, Mendes FM, et al. ( 2010) Analysis of the stimulated whole saliva in overweight and obese school children. Rev Assoc Med Bras 56: 32-36.
- Dehghan M, Tantbirojn D, Kymer-Davis E, Stewart CW, Zhang YH, et al. (2015) Nutralizing salivary pH by mouthwashes after an acidic challenge. J Investig Clin Dent 8.
- Mirjalili N, Karbassi MHA, Farahman J (2014) comparing tap water mouth rinse with tooth brushing and sugar free chewing gum: investigating the validity of a popular belief. J Dent Oral Hyg 6: 22-25.
- Colak H, Dulgergil CT, Dalli M, Hamidi MM (2013) Early childhood caries update : A review of causes, diagnoses and treatments. J Nat Sci Biol Med 4: 29-38.
- Stephan RM (1940) Changes in the hydrogen ion concentration on tooth surface and in carious lesion. J Am Dent Assoc 27: 718-723.
- Leach SA (1960) Some notes on solubility of enamel and dentin in acid. Arch Oral Biol 1: 218-232.
- Agus HM, Un PSH, Cooper MH, Schamscula RG (1980) Ionised and bound fluoride in resting and fermenting dental plaque and individual human caries experience. Arch oral Biol 25: 517-522.
- Van Houte J, Lopman J, Kent R (1996) The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surface. J Dent Res 75: 1008-1014.
- Kulthanan K, Nuchkull P, Varothai S (2013) The pH of water from various sources : an overview for recommendation for patients with atopic dermatitis. Asia Pac Allergy 3: 155-160.
- Leone CW, Oppenheim FG (2001) Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. J Dent Educ 65: 1054-1062.
- Dehghan M, Tantbirojn D, Davis EK, Stewart CW, Â Zhang YH, et al. (2015) Neutralizing salivary pH by mouthwash after an acidic challenge. J Invest Clin Dent 8.
Citation: Panchal V, Gurunathan D (2017) Comparison of Salivary PH Changes with Tap Water and Mineral Water Rinse after 50% Sucrose Solution Rinse: A Cross-Over Trial. J Clin Diagn Res 5: 142.
Copyright: © 2017 Panchal V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 6663
- [From(publication date): 0-2018 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 5664
- PDF downloads: 999