Comparison of Microbial Fuel Cells and Anaerobic Digestion Technology in Nairobi Market and Slaughterhouse Waste Management
Received: 10-Oct-2022 / Manuscript No. EPCC-22-76803 / Editor assigned: 12-Oct-2022 / PreQC No. EPCC-22-76803 (PQ) / Reviewed: 27-Oct-2022 / QC No. EPCC-22-76803 / Revised: 29-Oct-2022 / Manuscript No. EPCC-22-76803 (R) / Published Date: 04-Nov-2022 DOI: 10.4172/2573-458X.1000303
Abstract
Population growth and urbanization has led to increased waste production in landfills. These landfills and slaughterhouses release greenhouse gases to the atmosphere and therefore, waste treatment and management is vital. In this study, Nairobi market wastes and bovine abattoir wastes were treated via anaerobic digestion and microbial fuel cell technologies to produce biogas and electricity, respectively. Proximate analysis was carried out on Nairobi market wastes before inoculating with rumen fluid from Dagoretti slaughterhouse waste at psychrophilic anaerobic digestion for a thirty days’ hydraulic retention time. Similarly, the waste was treated using microbial fuel cells inoculated with slaughterhouse waste in a dual chamber microbial fuel cell for thirty days. The daily cumulative biogas production was measured volumetrically while voltage and current from the cells was recorded using a multi-meter.
The results obtained showed that blank rumen fluid generated 1800 mL and 0.061 V of biogas and voltage, respectively. The biogas produced from the rumen-fluid inoculated fruit market wastes was in the range of 300 to 3500 mL while voltage ranged from 0.010 to 0.701 V. The amount of biogas and voltage generated was dependent on the proximate properties of the waste, operation conditions like pH, temperature and moisture content of the waste. This means that, using similar digester/anodic chamber, the maximum cumulative biogas generated of 3500mL translate to 21 V.A. Therefore, this study concluded that both anaerobic digestion and microbial fuel cell technologies are appropriate in conversion of waste to green energy.
keywords
Abattoir; Anaerobic; Biogas; Market; Microbial; Voltage
Introduction
High-energy demand with rapid industrialization and mechanization combined with environmental pollution due to the burning of fossil fuels has driven a shift toward renewable energy. Food waste (FW) related issues in developing countries is currently considered to be a major threatening factor for sustainable development and landfill solid waste management systems. FW is rich in organic matters (i.e. carbohydrate, protein, and lipid) [1]. Due to incomplete FW management systems, many developing countries are facing challenges, such as environmental and sanitary problems that are caused by FW [2]. The food waste treatment-based anaerobic digestion (AD) has been proven to play a primary role in electricity industry with high potentially economic benefits, which could reduce electricity prices in comparison with other renewable energy resources such as wind and solar power [3,4]. Biogas derived from biomass is a potential renewable energy source that can be used in different sectors such as transportation sector, electricity generation, heat production, combined heat and power systems, and fuel cells. Moreover, the upgraded biogas can be applied as transportation fuel via an internal combustion chamber (for internal combustion engine (ICE) vehicles), and electricity station (for electric vehicles) [5].
Biogas is produced after organic materials are broken down by bacteria in an oxygen-free environment, a process called anaerobic digestion (AD). Biogas systems use anaerobic digestion to recycle these organic materials, turning them into biogas, which contains both energy (gas), and bio-slurry [6]. The AD process already occurs in nature, landfills, and some livestock manure management systems, but can be optimized, controlled, and contained using an anaerobic digester [7]. Biogas contains roughly 50-70 percent methane, 30-40 percent carbon dioxide, and trace amounts of other gases. The liquid and solid digested material, called digestate, is frequently used as a soil amendment [8]. Some organic wastes are more difficult to break down in a digester than others. Food waste, fats, oils, and greases are the easiest organic wastes to break down, while livestock waste tends to be the most difficult. Mixing multiple wastes in the same digester, referred to as co-digestion, can help increase biogas yields. Warmer digesters, typically kept between 30 to 38 degrees Celsius, can also help wastes break down more quickly. Increasing the concentrations of proteins and lipids, and decreasing carbohydrate content in FW, led to high buffering capacity, reduction of proteins (52.7-65.0%) and lipids (57.4- 88.2%), and methane production (385-627 mLCH4/g volatile solid), while achieving a short retention time [9]. Suhartini et al., 2019, found that canteen food waste has higher organic material content, mainly carbohydrate, protein and lipid. This indicating it’s potential to be used as feedstock for biogas/methane production.
After biogas is captured, it can produce heat and electricity for use in engines, micro-turbines, and fuel cells [10]. Biogas can also be upgraded into bio-methane and injected into natural gas pipelines or used as a vehicle fuel. The average calorific value of biogas is about 21- 23.5 MJ/m³, so that 1 m³ of biogas corresponds to 0.5-0.6 l diesel fuel or about 6 kWh [11]. The biogas yield of a plant depends not only on the type of feedstock, but also on the plant design, fermentation temperature and retention time. In most cases, biogas is used as fuel for combustion engines, which convert it to mechanical energy, powering an electric generator to produce electricity [12].
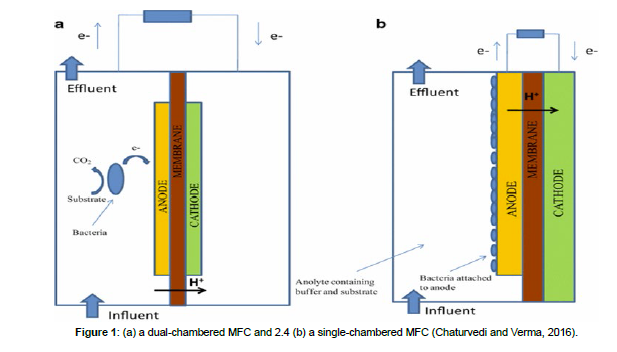
Microbial fuel cells (MFCs) are a framework in which microorganisms oxidize natural / inorganic mixes into adenosine triphosphate (ATP) by consecutive responses where electrons exchange at the terminal electron acceptor to produce electricity [13]. MFCs comprise of the anode and cathode with a cationic film in between. The degraders live in the anodic cell where they feed on glucose (decaying matter) that acts as a source of electrons. The metabolites produce both electrons and protons simultaneously. Exchange of electrons occurs on the surface of the anode. Thereafter, electrons move from anode to cathode via electrical circuit and protons is oxidized to water. Free oxygen has widely been used as an electron acceptor [14]. MFCs are categorized into mono and dual chamber. The MFCs that has separate cathodic and anodic chambers are known as double chambered MFCs while those having cathode and anode in a solitary chamber are called solitary chambered MFC. Figure 1 below indicates double chamber MFC and single chambered MFC (Figure 1).
In some previous studies, food waste and rumen waste have been employed in waste to electricity projects. For example Kamau et al ., 2018a, 2018b utilized fruit wastes from Nairobi markets inoculated with rumen fluid from slaughterhouses and generated voltage and current in the range of 0.023 – 0.768 V and 0.01 – 0.987mA, respectively. The studies concluded that, voltage and current generation was highly influenced by substrate properties and microbial community.
In this study, anaerobic digestion and microbial fuel cells technologies widely applied in organic solid wastes management were compared in generation of biogas and voltage, respectively.
Justification
In recent years, the escalating increase in energy consumption due to rapid industrial development has threatened the environmental balance. The generation of organic wastes, especially, food waste (FW) also results in environmental pollution problems if not well managed. The FW contains many biodegradable organic components and could be anaerobically digested to produce biogas as a green bio-energy.
Moreover, the approach of the FW as a source of bio-energy feedstock is expected to solve some issues of waste treatment and green energy generation and also overcome the controversy on using crops for fuel/ energy.
Methodology
Sampling
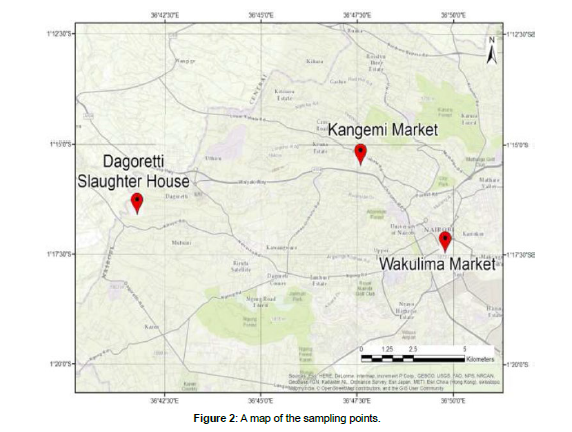
The rumen fluid used in this study was obtained from Dagoretti slaughterhouses (1°17’02.6”S 36°41’02.2”E) in Kiambu County, Kenya. The market wastes including vegetable and fruits wastes were obtained from Kangemi Market (1°15’52.9”S 36°44’55.6”E) and Wakulima Market (1°17’13.3”S 36°49’56.2”E) in Nairobi County, Kenya. A map of the sampling sites is shown in figure 2.
Proximate analysis
The proximate composition was done on homogenized samples of mango, avocado, melon, banana and tomato. The analysis included; energy, fat, nitrogen-free extract, ash, moisture content, protein, fiber, carbohydrates by the techniques of AOAC, 2003 as described by Kamau et al. (2020).
Bacteria Total Count
The spread plate technique was used to enumerate the total viable bacteria in rumen fluid samples (International Standards Organization (ISO-6579), 2002). All the media were prepared according to manufactures instructions. For enumeration of total viable count (TVC), nutrient agar media (NA) were used. From each dilution, 0.1 mL was inoculated on the center of the respective agar media by sterile pipette and spread by a sterile glass rod. After that, the plates were incubated at 37ºC for 24 h. Following incubation, colonies that appeared on NA were counted and calculated by multiplying the average number of colonies in particular dilution with dilution factors and recorded as colony-forming unit per gram of samples as described by Mbugua et al., 2022.
Anaerobic Digestion
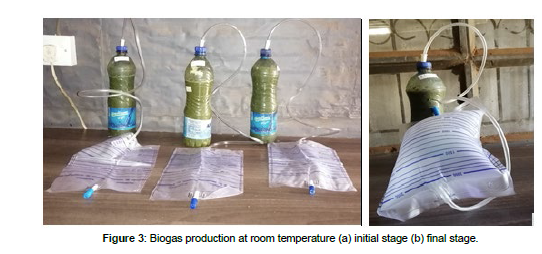
Banana, avocado, watermelon, tomato and mango fruits waste were collected from Kangemi/Wakulima market. They were separately reduced in size by chopping with a kitchen knife before blending. A blended mixture was also made using 250mL of all the fruits and mixed thoroughly. The blended market wastes and rumen fluid were loaded into 1000 mL plastic digester shown in figure 3 in the ratio of 1:1 and biogas produced measured daily using a graduated glass syringe or polythene bag for thirty days at psychrophilic condition.
Microbial Fuel Cell
The anodic chamber was loaded with homogenized 500 g of avocado, water melon, tomato, mango, banana and waste mixed with water in a ratio of 1:1. Graphite rods connected to a copper wire were inserted as electrodes and the chamber sealed to enhance anaerobic conditions. The cathodic chamber was loaded with distilled water. Both cells were connected via a salt bridge of 3% NaCl in agarose, solidified and passed through a PVC pipe as described by Kinyua et al., 2022 as shown in figure 4. The voltage and current were taken regularly via a multi-meter connected to copper wires joined to the carbon rods [15].
All the procedures were carried out in triplicates and the mean and standard deviation computed statistically. The plots were made using Qti-Plot software.
Results
The moisture levels were in the range of 74.31 – 95.16% for all the wastes. Low percentages of proteins and fats were observed at 0.57 -3.05% and 0.12 – 9.03%, respectively. Table 1 shows the percentage of proximate matter moisture content in fruits waste on an as-received basis.
| Sample | % Moisture | % Protein | % Fat | % Ash | % Fiber | % Carb. | % Nfe | Energy (Kcal/100g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tomato | 95.16±4.00 | 0.57±0.01 | 0.12±0.01 | 0.46±0.01 | 0.76±0.01 | 2.93±0.09 | 15.08±1.11 | 2.93±0.05 | |||||||
| Banana | 74.31±2.10 | 3.05±0.12 | 0.50±0.07 | 1.67±0.05 | 1.24±0.14 | 19.24±1.00 | 93.66±19.34 | 19.24±2.00 | |||||||
| Avocado | 82.83±3.00 | 1.32±0.14 | 9.03±1.36 | 0.84±0.02 | 2.61±0.98 | 3.37±0.55 | 100.03±12.90 | 3.37±1.11 | |||||||
| Mango | 86.82±3.89 | 0.87±0.07 | 0.68±0.08 | 0.44±0.02 | 1.28±0.21 | 9.91±1.00 | 49.24±2.88 | 9.91±1.00 | |||||||
| Water Melon | 92.85±4.55 | 0.90±0.09 | 0.33±0.04 | 0.74±0.04 | 0.76±0.09 | 4.42±0.88 | 24.18±2.45 | 4.42±0.78 | |||||||
Table 1: Percentage of proximate matter moisture content in fruits waste on an as-received basis.
The physical properties of the market wastes are show in table 2. Total solids were obtained by subtracting moisture content from 100. In tomato waste, the moisture content was 95.16%. Mohammed et al. (2017) reported 90.75% moisture levels in tomato fruits. The percentage of moisture levels obtained was in range with previous studies by Oko- Ibom et al., 2007, Adubofuor et al., 2010 and Hossain et al., 2010 who found moisture levels of 88.19 - 90.67% [16-19].
| Sample | % Moisture | Total Solids | % Ash | %Mineral Matter | %Volatile Matter | % Fixed Solids |
|---|---|---|---|---|---|---|
| Tomato | 95.16 | 4.84 | 0.46 | 0.506 | 4.38 | 3.92 |
| Banana | 74.30 | 25.70 | 1.67 | 1.837 | 24.03 | 22.36 |
| Avocado | 82.83 | 17.17 | 0.84 | 0.924 | 16.33 | 15.49 |
| Mango | 86.82 | 13.18 | 0.44 | 0.484 | 12.74 | 12.3 |
| Water Melon | 92.85 | 7.15 | 0.74 | 0.814 | 6.41 | 5.67 |
Table 2: Physical properties of various market wastes.
The ash content shows the minerals/non-degradable matter in a sample when water and degradable matter are removed. Lower ash matter was found in fruit wastes, for example, 0.46% in tomatoes. Watermelon, mango and avocado ash levels were 0.44, 0.84 and 0.74%, respectively. From table 1, the highest NFE was reported in sweet potato, avocado and banana wastes at 32.17, 100.03, 93.66%, respectively, with the lowest being recorded in leafy vegetables like kales, spinach and coriander at 4.03, 2.38 and 2.16%, respectively. Similar results had been reported by Mbugua et al., 2020 on tomato and avocado with a slight variation of 0.023 -0.043.
Microbial Counts
The results obtained for the bacteria counts from the rumen fluid was 3.15±0.01 * 1010 cfu/mL. These counts agree with what had been reported previously, for example, Deepanraj et al., (2018) observed highest bacterial colony counts in cow rumen fluid (434.33) followed by goat (262.67) and chicken (170.67) in a colony counts study of bacterial species from rumen fluids of different animals. Ozbayram et al., (2018) and Liu et al., (2016) observed twice as many microbes in rumen waste compared to manure. The standard of any manure employed in anaerobic degradation is determined by the total viable count [20]. Total cfu/g of bacteria of (1.78 – 2.84 ± 0.01x105 cfu/g) was reported in three samples of cow dung collected from different farm by Kiyasudeen et al., (2015). The serial dilution methods developed by [21,22] were used to assess the bacterial population. The total viable count (TVC) is a critical metric for determining the quality of dung for use as manure or as a biofuel source. Gagandeep’s, (2017) study enumerated TVC in three cow dung samples ranging from 1.9 * 106 to 2.8 *106 cfu/g. Ambar et al., (2017) reported TVC of 9.55* 108 and 1.32* 108 cfu/g, respectively in cow manure and cow rumen waste.
Biogas production
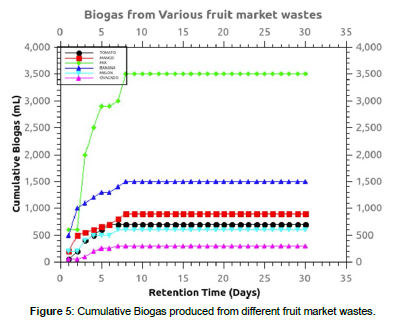
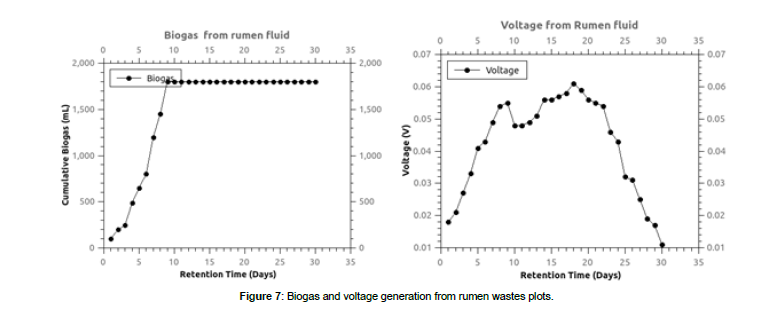
The biogas generated from the fruit market wastes inoculated with rumen fluid at a ratio of 1:1 without initial pH adjustments is shown in figure 5. The highest cumulative biogas was obtained from the fruit mixtures at 3500 mL. This is explained by the balance of proximate matter after mixing the wastes which further translate to pH balance during the anaerobic digestion. Mbugua et al., (2019) observed high biogas from avocado wastes co-digested with cow dung in all the wastes. High landfill gas generation rate was experienced at the initial stages which platooned on day seven. This is explained by the changes in the pH in the acidogenesis and acetogenesis AD phases leading to biogas production halt due to microbial death. This had been observed by Mbugua et al., 2021 who suggested a pH range of 6.5-8.0 as favorable for methane producing microbes. The pH of the digester becomes acidic with time and therefore, biogas production is slowed.
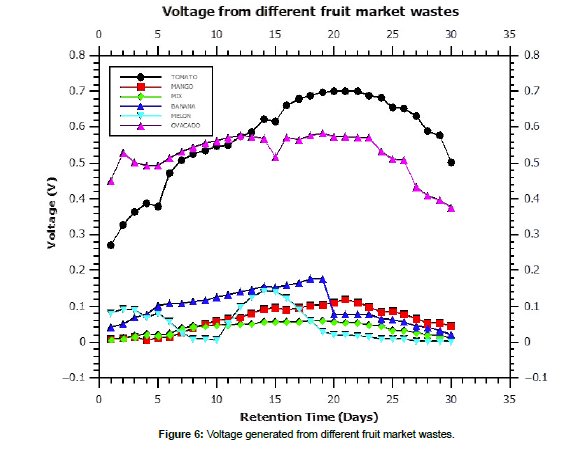
The voltage and power obtained from avocado and tomato amongst the other fruit wastes were as shown in figure 6. Tomato waste recorded the highest voltage at 0.701 V compared to 0.01 – 0.17 V in banana, melon, mango and fruit. Kamau et al., 2018 had observed a voltage range of 0.234 – 0.705 V in fruit wastes inoculated with rumen wastes. Similarly, Imwene et al., 2021 observed a voltage range of 0.567 – 0.689 V in tomato wastes inoculated with goat rumen microbes. Kinyua et al.,2022 recorded similar results in from tomatoes in similar experimental conditions. These findings are consistent with a study that found that the rate of microbial metabolism at the anode increased as the electrical potential of the anode increases; thus, the rate of microbial metabolism in response to electron concentration or electrical potential determines the amount of electricity produced in the MFC [23-27] [figure 7].
In figures 5 and 6, the biogas and voltage generated from blank rumen wastes is shown. It was observed that biogas generation followed a normal distribution generation curve with maximum generation being recorded on day seven at 1800 mL. The voltage generation rate was high on the initial stages of the experiment which slowed after day seven with constant production henceforth up to day 24 after which downward production was recorded. Kamau et al, 2018; Imwene et al.,2020 and Kinyua et al., 2022 similarly observed normal distribution trend in voltage generation from tomato wastes [28,29].
Discussions
The pH value provides an estimate of the fermentation process’s state. For AD, a pH range of 6.5 - 7.5 is ideal [30-32]. Some of the feeding materials tend to decrease the pH of the digestate. Cunfang Liu et al. (2008) reported that a lowering the pH can inhibit gas generation and results to accumulation of acids. Jayaraj et al., (2014) investigated influence of pH on biogas yield from food waste in reactors maintained at pH 5, 6, 7, 8 and 9 at mesophilic temperatures. If the pH value decreases below 6, methane production is strongly inhibited. The temperature of the reaction medium influences the pH value. While the temperature is increasing, the carbon dioxide solubility decrease;this is why in the case of thermophilic digesters the pH value is higher than in the mesophilic ones where the carbon dioxide will dissolve easy and will produce carbonic acid in reaction with the water, increasing the acidity [33-35]. During the digestion process, the pH value may increase because of the ammonia presence resulted either by the protein degradation or by its presence in the charging flux; also it can decrease if VFA will accumulate in the reaction medium. The reaction medium must provide sufficient buffering capacity to neutralize VFA accumulation [36-38]. Babel et al., (2004) noted that methanogens metabolic rates are affected by pH variation. Any changes outside their operations spectrum halts biogas generation.
The biogas produced in this study can be converted to electricity assuming that 1 m³ of biogas corresponds to about 6 kWh or 6000 V.A [39-41]. This means that, using similar digester/anodic chamber, the maximum cumulative biogas generated of 3500mL translate to 21 V.A. Though the two processes have limitations, the resultants products can be inter-converted depending on the product needed. Direct conversion of waste to bio-electricity eliminates the need to purify biogas to bio-methane for electricity production.
Conclusion
The proximate analysis showed moisture content of 74.31 – 95.86% for all the wastes. Low percentages of proteins and fats were observed at 0.52 -3.49 % and 0.09 – 1.54 %, respectively. The carbohydrate levels ranged from 1.99±0.12 to 32.17±2.31 % while the the crude fiber in this study was in the range of 0.54 – 2.61%. Anaerobic digestion of fruits wastes results in biogas generation with the rate of biogas formation reported highest in day 0-7 of AD which gradually reduced in the remaining retention time of AD. In microbial fuel cell technology, the tomato waste recorded a 0.0.701 V optimum voltage while avocado generated 0.584 V. This study recommends waste to energy conversion via anaerobic digestion and microbial fuel cells as a green method of landfill waste management.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Abanades S, Abbaspour H, Ahmadi A (2022) A conceptual review of sustainableelectrical power generation from biogas. Energy Sci Eng 10: 630-655.
- Ambar P, Endang S, Rochijan, Nanung AF, Yudistira S, et al. (2017) Potential test on utilization of cowʼs rumen fluid to increase biogas production rate and methane concentration in biogas. Asian J Anim Sci 11: 82-87.
- Babel S, Fukushi K, Sitanrassamee B (2004) Effect of acid speciation on solid waste liquefaction in an anaerobic acid digester. Water Res 38: 2416-2422.
- Chen P, Qinglong X, Addy M, Zhou W, Liu Y, et al. (2016) Utilization of municipal solid and liquid wastes for bioenergy and bioproducts production. Bioresource Technology 215: 163-172.
- Cun-fang Liu (2008) Prediction of Methane Yield at Optimum pH for anaerobic digestion of Organic Fraction of Municipal Solid Waste. Bioresource Technology 99: 882-888
- Deepanraj B, Sivasubramanian V, Jayaraj S (2015) Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and pH. J Renew Sustain Ener 7: 063-104.
- EESI (2017) Fact Sheet | Biogas: Converting Waste to Energy.
- EPA (2016) Municipal Solid Waste.
- ESMAP (2005) Advancing Bioenergy for Sustainable Development - Guideline for Policy-makers and Investors.
- Ezekoye VA, Ezekoye BA (2009) Characterization and storage of biogas produced from theanaerobic digestion of cowdung, spent grains/cow dung and cassava peels/rice husk. Pac J sci technol 10: 898-904
- Fachagentur Nachwachsende Rohstoffe EV (2009) Biogas Basisdaten Deutschland – Stand: Oktober 2008. Germany.
- Frazier WC, West off DC (1995) Food Microbiology 4th ed. New Delhi 384-396.
- Gagandeep K (2017) Isolation and Identification of Bacteria’s from Cattle Dung used in Microbial Fuel Cells to Generate Bioelectricity. Int J Revie & Res 5: 1-18.
- Ieropoulos IA, Greenman J, Melhuish C, Hart J (2006) Comparative study of three types of microbial fuel cell. Enzyme Microb Tech 37: 238-245.
- Imwene KO, Mbui DN, Mbugua JK, Kinyua AP, Kairigo PK, et al. (2021) Kinetic Modelling of Microbial Fuel Cell Voltage Data from Market Fruit Wastes in Nairobi, Kenya. IJSRCH 6: 25-37.
- International Standards Organization (ISO-6579) (2002) Microbiology of food and animal feeding stuffs—horizontal method for detection of Salmonella spp, 4th edition. Switzerland 1-27.
- Jayaraj S, Deepanraj B , Sivasubramanian V (2014) Study On the Effect of pH On Biogas Production from Food Waste by Anaerobic Digestion. 9th International Green Energy Conference 799-805.
- Kamau JM, Mbui DN, Mwaniki JM, Mwaura FB (2020) Influence of Substrate Proximate Properties on Voltage Production in Microbial Fuel Cells. IJEER 8: 12-21.
- Kamau JM, Mbui DN, Mwaniki JM, Mwaura FB (2020) Lab Scale Biogas Production from Market Wastes and Dagoretti Slaughterhouse Waste in Kenya. IJEER 8: 12-21.
- Kamau JM, Mbui DN, Mwaniki JM, Mwaura FB (2018) Characterization of voltage frombfood market waste: microbial fuel cells. Int J Biotech & Bioeng 4: 37-43.
- Kamau JM, Mbui DN, Mwaniki JM, Mwaura FB (2018) Utilization of rumen fluid in production of bio- energy from market waste using microbial fuel cells technology. J Appl Biotechnol Bioeng 5: 227‒231.
- Kamau JM, Mbui DN, Mwaniki JM, Mwaura FB (2020) Proximate analysis of fruits and vegetables wastes from Nairobi County, Kenya. J Food Nutr Res 5: 1-8.
- Kinyua A, Mbugua JK, Mbui DN, Kithure J, Michira I, et al. (2022) Voltage Recovery from Pesticides Doped Tomatoes, Cabbages and Loam Soil Inoculated with Rumen Waste: Microbial Fuel Cells. IJSRSET 9: 172-180.
- Kinyua A, Mbugua JK, Mbui DN, Kithure J, Michira I, et al. (2022) Voltage Recovery from Pesticides Doped Tomatoes, Cabbages and Loam Soil Inoculated with Rumen Waste: Microbial Fuel Cells. IJSRSET 9: 172-180.
- Kiyasudeen SK, Ibrahim MK, Ismail SA (2015) Characterization of Fresh Cattle Wastes Using Proximate, Microbial and Spectroscopic Principles. Am Eurasian J Agric Environ Sci 15: 1700-1709.
- Lazor M, Hutnan M, Sedlacek S, Koles N, Spalkova V (2010) Anaerobic codigestion.
- Li Y, Jin Y, Borrion A, Li H, Li J (2017) Effects of organic composition on the anaerobic biodegradability of food waste. Bioresour Technol 243: 836-845.
- Mbugua JK, Mbui DN, Waswa AG, Mwaniki JM (2022) Kinetic Studies and Simulation of Microbial Fuel Cells Voltage from Clostridium Spp. and Proteus. J Microb Biochem Technol 14: 483.
- Mbugua JK, Mbui DN, Mwaniki J, Mwaura F, Sheriff S (2020) Influence of Substrate Proximate Properties on Voltage Production in Microbial Fuel Cells. J Sustain Bioenergy Syst 10: 43-51.
- Neves L, Oliveira R, Alves M (2003) Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochem 39: 2019-2024.
- Ong KL, Kaur G, Pensupa N, Uisan K, Lin CSK (2017) Trends in food waste valorization for the production of chemicals, materials and fuels: Case study South and Southeast Asia. Bioresour Technol 248: 100-112.
- Ozbayram EG, Orhan I, Bahar I, Hauke H, Sabine K (2018) Comparison of Rumen and Manure Microbiomes and Implications for the Inoculation of Anaerobic Digesters. Microorganisms 6: 1-10.
- Park DH, Zeikus J (2000) Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl Environ Microbiol 66: 1292-1297.
- Pratima KC, Bhakta BA (2015) Production of Biogas from Slaughterhouse Waste In Lalitpur Sub-metropolitan City. In Proceedings of IOE Graduate Conference 143-149.
- SSCHE May, 24–28.
- Suhartini S, Lestari YP, Nurika I (2019) Estimation of methane and electricity potential from canteen food waste. IOP Conf Ser Earth Environ Sci 230: 012075.
- Talaro PK (2009) Foundation in Microbiology, San Francisco: Pearson Benzamin.
- Tender L, Gray S, Groveman E, Lowy D, Kauffma P, et al. (2008) The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J Power Source 179: 571–575.
- Thi NBD, Kumar G, Lin CY (2016) Electricity generation comparison of food waste-based bioenergy with wind and solar powers: A mini review. Sustainable Environment Research 26: 197-202.
- Thi NBD, Kumar G, Lin CY (2015) An overview of food waste management in developing countries: current status and future perspective. J Environ Manag 157: 220-229.
- Tobiasen L, Kahle K, Hindsgaul C (2014) Waste to Energy – Energy Recovery of Green Bin Waste: Incineration/Biogas Comparison. Curr Sustainable Renewable Energy Rep 1: 136-149.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Mbugua JK, Ajuliu PK, Mbui DN, Mwaniki JM, Waswa AG (2022) Comparison of Microbial Fuel Cells and Anaerobic Digestion Technology in Nairobi Market and Slaughterhouse Waste Management. Environ Pollut Climate Change 6: 303. DOI: 10.4172/2573-458X.1000303
Copyright: © 2022 Mbugua JK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2963
- [From(publication date): 0-2022 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 2508
- PDF downloads: 455