Comparison of Lithium and Valproate Concentration in Serum During Three Different Patients Treated by Lithium Carbonate, Sodium Valproate and Their Combination: A Preliminary Study
Received: 25-Jan-2018 / Accepted Date: 05-May-2018 / Published Date: 15-May-2018
Abstract
Objective: To investigate the mutual influence of drug concentration in serum between lithium carbonate and sodium valproate.
Methods: A total of 24 patients with bipolar mania, schizoaffective disorder or schizophrenia accompanied by excited agitation were randomly divided into 3 groups (n=8 per group). Apart from antipsychotics, these patients received lithium carbonate, sustained release sodium valproate and lithium carbonate plus sustained release sodium valproate at a constant dose for 1 week, respectively. The drug concentrations of lithium and sodium valproate in serum were determined for 3 times.
Results: The drug concentration of lithium was 0.44 ± 0.20 mmol/L, 0.48 ± 0.20 mmol/L and 0.46 ± 0.10 mmol/L in the lithium carbonate group, and 0.54 ± 0.22 mmol/L, 0.57 ± 0.29 mmol/L, 0.58 ± 0.28 mmol/L in the lithium carbonate plus sodium valproate group, suggesting no significant difference. The drug concentration of sodium valproate was 91.9 ± 20.5 mg/L, 83.8 ± 26.2 mg/L and 74.5 ± 22.7 mg/L in the sodium valproate group, and 92.8 ± 23.5 mg/L, 93.3 ± 20.9 mg/L and 96.3 ± 31.4 mg/L in the lithium carbonate plus sodium valproate group suggesting no significant difference.
Conclusion: The mutual influence of lithium carbonate and sodium valproate maybe not significant.
Keywords: Lithium carbonate; Sodium valproate; Drug concentration; Mutual influence; Bipolar disorder
Introduction
Lithium carbonate and sodium valproate are the classic mood stabilizers and frequently applied in the treatment of bipolar affective disorder. However, favorable efficacy has not been achieved in some patients undergoing treatment with one mood stabilizer or even in combination with antipsychotics [1]. Therefore, combined application of mood stabilizers, especially for bipolar affective disorder patients with type I manic episode, mixed episode or rapid cycling is necessary [2]. In our previous studies, we found the efficacy of combined application of mood stabilizers were superior to that of one mood stabilizer alone [3,4]. Recently, increasing studies have confirmed that, in not only the treatment of acute bipolar affective disorder but the prophylactic management of the bipolar affective disorder, the lithium carbonate together with sodium valproate has definite effectiveness and preventive effects against lithium events [4,5]. To date, no evidence confirmed the mutual influence of lithium carbonate and sodium valproate. One study showed lithium carbonate and sodium valproate could not interfere with each other, sodium valproate rarely affected the pharmacokinetics of lithium carbonate, and the AUC, CMAX and CMIN were only slightly increased [6]. Although the combination therapy with lithium carbonate and sodium valproate can improve the efficacy and is suitable for rapid cycling bipolar disorder [7]. Sometimes this therapy should be performed with other antipsychotics [8]. In our previous study, lithium carbonate and sodium valproate were used in a rat model and results did not reveal that sodium valproate could increase the risk for lithium carbonate poisoning [9]. To date, there is no consensus on the effects of antipsychotics on the risk for lithium carbonate poisoning. In the present study, patients undergoing antipsychotics treatment were employed and the mutual influence of lithium carbonate and sodium valproate was investigated.
Patients and Methods
Patients
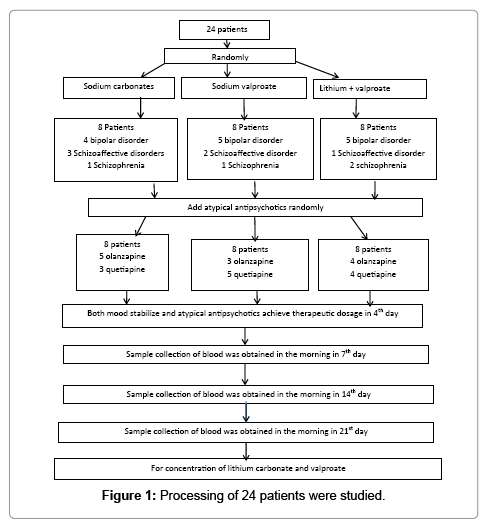
A total of 24 patients with bipolar affective disorder patients with manic episode, schizoaffective disorder or schizophrenia accompanied by obvious psychomotor excitement were selected. The diagnosis was made based on the DSM-IV bipolar disorder, schizoaffective disorder or schizophrenia criteria and their PANSS excitement factor score >14. Among these patients, the exclusion criteria are as following: (1) The patients age were <18 and >65 years old. (2) The patients have important organ disorder and endocrine disease. (3) The female patients were pregnant. (4) The patients had substance dependence. (5) The patients were not willing to join this study. 14 had bipolar affective disorder accompanied by manic episode (the number of episodes: 2~5; mean: 2.2 ± 2.5), 6 had schizoaffective disorder (the number of episodes: 1~4; mean: 1.6 ± 1.5) and 4 had schizophrenia (the number of episodes: 2~6; mean: 2.5 ± 2.8). There were 16 males and 8 females with a mean age of 37 ± 13 years (range: 19~60 years). All the patients were randomly assigned into 3 groups (n=8 per group) according to a table of random numbers and received randomly treatment with lithium carbonate, sodium valproate and lithium carbonate plus sodium valproate and their atypical antipsychotics.
Methods
Dosage and administration: The lithium carbonate was purchased from Hunan Qianjin Xiangjiang Pharmaceutical Co Ltd (Lot number: 050702) and sustained release sodium valproate (Depakine) from Sanofi Pharmaceutical Co Ltd (Lot number: H20010259). Patients were treated with lithium carbonate (1 g/d), sodium valproate (1 g/d) and lithium carbonate plus sodium valproate (1 g/d for each drug). The therapeutic doses of both drugs were achieved at day 4. In addition, atypical antipsychotics were also applied and their therapeutic doses were achieved within 1 week. The blood lithium and sodium valproate levels were determined when the both mood stabilizers and antipsychotics were applied at constant doses for 1st week. The antipsychotics included quetiapine (400~800 mg/d) and olanzapine (10~25 mg/d), and the doses of these antipsychotics increased according to the disease condition. In the lithium carbonate group, 4 patients suffered from bipolar disorder, 3 suffered from schizoaffective and 1 suffered from schizophrenia. In the sodium valproate group, 5 suffered from bipolar disorder, 2 suffered from schizoaffective and 1 suffered from schizophrenia. In the lithium carbonate plus sodium valproate group, 5 suffered from bipolar disorder, 1 suffered from schizoaffective and 2 suffered from schizophrenia. In respect of the antipsychotics, the mean dose of quetiapine was 660 ± 160 mg/d (range: 600~1000 mg/d) and that of olanzapine was 16.8 ± 5.0 mg/d (range: 15~20 mg/d) in the lithium carbonate group. In the sodium valproate group, the mean dose of quetiapine was 640 ± 150 mg/d (range: 600~800 mg/d) and that of olanzapine was 13.5 ± 9.0 mg/d (range: 10~25 mg/d). In the lithium carbonate plus sodium valproate group, the mean dose of quetiapine was 540 ± 180 mg/d (range: 400~800 mg/d) and that of olanzapine was 11.1 ± 4.5 mg/d (range: 10~20 mg/d). Significant difference was found in the dose of quetiapine, but not in that of olanzapine. Our study was passed though by Zhejiang Tongde Hospital Ethics Committee and very patients were informed and consent for our study. The knowing approval document were signed by their guardian. Demographics of all patients in different group were as following (Table 1).
| Disease | lithium carbonate (n=8) | combination therapy (n=8) | sodium valproate (n=8) | P |
|---|---|---|---|---|
| Bipolar disorder | 4 | 5 | 5 | ns |
| Schizoaffective disorder | 3 | 2 | 1 | ns |
| Schizophrenia | 1 | 1 | 2 | ns |
| GENDER | ||||
| Male | 5 | 6 | 5 | ns |
| Female | 3 | 2 | 3 | ns |
| AGE | 35±11 | 37±12 | 38±15 | ns |
| ANTI-PSYCHOTICS | ||||
| Quetiapine | 660±160 | 540±180 | 640±150 | P<0.05 |
| Olanzapine | 16.8±5.0 | 11.1±4.5 | 13.5±9.0 | ns |
| DOSES | ||||
| Lithium carbonate (g/d) | 1 | 1 | 1 | ns |
| Lithium carbonate (g/d) | 1 | 1 | 1 | ns |
Table 1: Demographics of patients in different groups.
Sample collection: The fasting blood was obtained in the morning in 7th day (2 ml for lithium carbonate group and sodium valproate; 4 ml for combination therapy group) and the lithium carbonate and sodium valproate levels in the blood were determined, which was repeated 14 days and 21 days later.
Detection of drug concentration: The blood lithium carbonate level was measured with a DSI-909 sodium potassium and lithium analyzer (Shanghai Xun-Da Medical Instrument Corporation Ltd.); the blood sodium valproate level was measured using an Abbott AXSYM System.
Study process: Processing of 24 patients were studied (Figure 1).
Statistics: Statistical analysis was performed with SPSS 10.0 and comparisons were conducted with t test.
Results
All the patients completed the study and no patients withdrew from the study. No significant differences in the blood lithium carbonate and sodium valproate levels were observed between these three groups (Table 2).
| lithium carbonate (n=8) | combination therapy (n=8) | sodium valproate (n=8) | t | P | |
|---|---|---|---|---|---|
| Lithium carbonate (mmol/L) | |||||
| First | 0.44±0.20 | 0.54±0.22 | -0.947 | 0.36 | |
| Second | 0.48±0.20 | 0.57±0.29 | -0.722 | 0.482 | |
| Third | 0.46±0.10 | 0.58±0.28 | -1.113 | 0.276 | |
| Sodium valproate (mg/L) | |||||
| First | 91.9±20.5 | 92.8±23.5 | -0.081 | 0.937 | |
| Second | 83.8±26.2 | 93.3±20.9 | -0.803 | 0.435 | |
| Third | 74.5±22.7 | 96.3±31.4 | -1.588 | 0.135 |
Table 2: Blood lithium carbonate and sodium valproate levels in different groups.
Discussion and Conclusion
Lithium carbonate and sodium valproate have been approved by FDA to apply in the treatment of acute mania. In a previous study, the drug concentrations were determined in type I bipolar disorder patients receiving lithium carbonate plus sodium valproate and lithium carbonate plus placebo. The results showed the blood lithium carbonate level ranged from 0.8 mmol/L to 1.0 mmol/L and that of sodium valproate was 50~125 mg/ml. Clinical observation revealed recurrence or ineffectiveness in 5 of 7 patients receiving lithium carbonate plus placebo. However, in the patients treated with lithium carbonate plus sodium valproate, recurrence was not found suggesting effectiveness (X2=5.61, df=1, P=0.014). Among the 5 patients, 3 had at least one moderate to severe side effect which last for 4 weeks or longer, which significantly different from patients treated with lithium carbonate plus sodium valproate (X2=3.93, df=1, P=0.041) [10]. Recently, increasing researches focus on the interaction or mutual influence in drug concentration between lithium carbonate plus sodium valproate [3,9]. In our previous study, the results showed sodium valproate did not increase the serum lithium carbonate level in a rat model [8] and clinically lithium poisoning is not found. Especially, combination therapy with lithium carbonate plus sodium valproate not only has no risk for lithium poisoning, but improves the efficacy [11]. For this reason, we conducted this study. In the present study, a total of 24 patients were randomly assigned to lithium carbonate group, sodium valproate group and combination therapy group (n=8 per group). When the drug concentration reached a stable level, the serum lithium carbonate and sodium valproate levels were measured. Our results revealed there were no remarkable differences in the serum lithium carbonate and sodium valproate levels at different time points. This finding suggested the mutual influence of blood lithium carbonate and sodium valproate was minor and therefore, the drug concentration was not interfered with each other when combination therapy was performed. This may be explained by almost no interaction between lithium carbonate and sodium valproate and sodium valproate cannot affect the pharmacokinetics of lithium carbonate. Moreover, the AUC, CMAX and CMIN of sodium valproate were only slightly increased in patients received combination therapy [6]. In the present study, these patients were treated with not only atypical antipsychotics but lithium carbonate in combination with sodium valproate. Therefore, we could draw a conclusion that under combination therapy with multiple drugs, there was no interaction between lithium carbonate and sodium valproate. Our results were consistent with one study in which patients received not only chlorpromazine but lithium carbonate and sodium valproate and results revealed no interaction between lithium carbonate and sodium valproate [8]. Previously, we conducted an evidence-based survey which investigated the application of lithium carbonate and sodium valproate by psychiatrists with senior positions. They have agreement on whether sodium valproate can increase blood lithium level or the risk for lithium poisoning. Combination therapy cannot increase the risk for lithium poisoning (88% of psychiatrists), and the lithium poisoning is unrelated to sodium valproate (85%) but caused by lithium carbonate itself (60%). A majority of psychiatrists (94%) do not clinically encounter lithium poisoning and the experience on the clinical application of lithium carbonate and sodium valproate derives from clinical practice (60%) [12]. Our study further confirmed that there was no interaction between lithium carbonate and sodium valproate, two common mood stabilizers, which may be closely associated with the metabolism of both drugs [12]. There is no obvious interaction between lithium carbonate and sodium valproate, but combination therapy with lithium carbonate and sodium valproate increases the efficacy, which may be related to the synergistic effects of both drugs. In recent years, in the treatment of affective disorder, more attention has been paid to the regulation of signal transmission and the gene expression in neural pathway. Among these genes, the expression of transcription factor polyoma enhancer binding protein (PEBP2β) is increased by treatment. Study showed lithium carbonate and sodium valproate could enhance the functions of PEBP2β in the prefrontal cortex, one of which was to regulate the expression of Bcl-2, an important neuroprotective protein. Further study also indicated not only lithium carbonate but sodium valproate could increase the number of cells expressing PEBP2β, which was not observed after treatment with benzodiazepine drugs (the number of cells positive for Bcl-2 was not changed in the layer II/III of the prefrontal cortex of mice). These findings implied the synergistic effects of both drugs [13]. The neuroprotective effects are the new knowledge on the therapeutic effects of mood stabilizers. Lithium carbonate can combat with the nerve damage caused by numerous toxic substances exerting neuroprotective effects. For example, lithium carbonate can combat with neurotoxicity of glutamic acid, cytotoxicity of calcium overload and apoptosis of all cause, etc. Recently, studies also found similar neuroprotective effects of sodium valproate which conferred neurotrophic effects on neurons in vitro [14]. Our results suggested that the mutual influence of lithium carbonate and sodium valproate maybe not significant. But drug concentration is a only index, not completely reflecting mutual influence of lithium carbonate and sodium valproate. So next study should be stressed on their pharmacokinetics.
Acknowledgement
We thank Adikon biological Measurement Company to help to test drug concentration. We thank Maria Uscinska made the final amendments of the manuscript.
Conflict of Interests
All authors declare no conflicts of interest with any commercial or other association in connection with submitted article
References
- Solomon DA, Keitner GI, Ryan C, Miller IW (1998) Lithium plus valpoate as maintenance polypharmacy for patients with bipolar I disorder: A review. J Clin Psychopharmacol 18: 38-49.
- Mitchell P, Withers K, Jacobs G (1994) Combining lithium and sodium valproate for bipolar disorder. Aust N Z J psychiatry 28: 141-143.
- Jin WD, Chen Z, Chen J (2007)Â Advances in the pharmacology and combination application of lithium carbonate and sodium valproate. Chin J Physiol 40: 120-122
- Wang HQ, Wang NX, Jin WD (2008) The efficacy of lithium carbonate plus sodium valproate and lithium carbonate alone in the treatment of manic episode. Chinese Journal of Nervous and Mental Diseases 34: 98-100.
- Geddes JR, Goodwin GM, Rendell J, Azorin JM (2010) Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet 375: 385-395.
- Granneman GR,Schneck DW, Cavanaugh JH, Witt GF (1996) Pharmacokinetic interaction and side effects resulting from concomitant administration of lithium and divalproex sodium. J Clin Psychiatry 5: 204-206.
- Sharma V, Persad M, MaZanian D, Karunaratne K (1993) Treatmnet of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry 38: 137-139.
- Reischies FM, Hartikainen J, Berghofer AM (2002) Initial triple therapy od acute mania, adding lithium and valpoate to neuroleptics. Pharmaco psychiatry 35: 344-246
- Jin WD, Wang HQ, Zhang JT (2007) Study of effects of sodium valproate on serum lithium levels in rats. Chinese Journal of Clinical Pharmacy 16: 116-118.
- Solomon DA, Ryan CE, Keitner GI, Miller IW, Shea MT, et al. (1997) A pilot study of lithium carbonate plus divalproex sodium for the continuation and maintenance treatment of patients with bipolar I disorder. J Clin Psychiatry 58: 95-99.
- Keck PE, McElroy SL, Strakowski SM, Bourne ML, West SA, et al. (1997) Compliance with combination with maintenance treatment in bipolar disorder. Psychopharmacol Bull 33: 87-91.
- Jin WD, Chen J, Tang XX (2007)Is the combination therapy with two mood stabilizers feasible--- a survey in psychiatrists. J Clin Psychol Med 17: 63-64
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, et al. (1999) The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in CNS. J Neurochem 72: 879-882
- Lagace DC, Eisch AJ (2005) Mood-stabilizing drugs: Are their neuroprotective aspects clinically relevant? Psychiatr Clin North Am 28: 399-414.
Citation: Zhu JF, Jin WD, Jin R, Ma YC, Ren ZB (2018) Comparison of Lithium and Valproate Concentration in Serum During Three Different Patients Treated by Lithium Carbonate, Sodium Valproate and Their Combination: A Preliminary Study. Neurosci Psychiatry 1: 102.
Copyright: © 2018 Zhu JF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 5194
- [From(publication date): 0-2018 - Jan 23, 2025]
- Breakdown by view type
- HTML page views: 4457
- PDF downloads: 737