Research Article Open Access
Comparison of 23-Valent Pneumococcal IgG ELISA with Multiplex 13-Valent Serotype-Specific Antibody Assay as Diagnostic Tools in Subjects with Suspected Antibody Deficiency
Magdalena Dziadzio1,2*, Gabriela Barcenas Morales G4, Jennifer Harvey1, Roy Smith1, Joanna Lukawska2, Fariba Tahami1, Lourdes Ceron-Gutierrez3, Rainer Doffinger3, Suranjith Seneviratne1, Ronnie Chee1 and Helen Baxendale31Department of Immunology, Royal Free Hospital, London
2Department of Specialist Allergy and Clinical Immunology, University College London Hospitals
3Department of Immunology Addenbrooke’s and Papworth Hospitals, Cambridge
4Laboratorio de Immunologia, UNAM, FES-Cuautitlan, Mexico
- *Corresponding Author:
- Magdalena Dziadzio
Department of Specialist Allergy and Clinical Immunology
University College London Hospitals, Royal Free Hospital, London
Tel: 0203 456 5242
E-mail: magdalena.dziadzio@nhs.net
Received date: February 02, 2017; Accepted date: March 02, 2017; Published date: March 06, 2017
Citation: Dziadzio M, Morales GB, Harvey J, Smith R, Lukawska J, et al. (2017) Comparison of 23-Valent Pneumococcal IgG ELISA with Multiplex 13-Valent Serotype-Specific Antibody Assay as Diagnostic Tools in Subjects with Suspected Antibody Deficiency. J Mol Immunol 2:108.
Copyright: © 2017 Dziadzio M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Immunology
Abstract
The aim of this study was to compare the method agreement between the Pneumococcal 23vELISA (VaccZyme™, the Binding Site) and 13-valent serotype-specific multiplex assay in evaluating vaccination responses to Pneumovax® in patients with suspected antibody deficiency.
Diagnostic accuracy in antibody deficiencies is important as potential therapies are costly and lifelong. The literature regarding interpretation of results shows no consensus; this study was needed to guide our practice and to see if the multiplex assay is superior to 23vELISA or whether the two assays could be used together.
Pneumococcal antibody levels from a sample cohort of 64 patients referred to our Immunology Clinic for investigations of suspected immunodeficiency over the period of 6 month were analysed before and after testvaccination with Pneumovax® using both assays. There was good method agreement between ELISA and multiplex for serotype-specific individual IgG thresholds of 0.35 mg/l and 0.5 mg/l but not for 1.3 mg/l.
Thirteen patients had discordant results by the two methods. We propose that, the two assays studied could be used together to complement each other but prospective larger studies are needed to validate this. The vaccination results have to be interpreted with critical clinical judgement as meeting the “protective” antibody threshold is not a surrogate of function, especially in the context of immunodeficiency. We propose an in-house diagnostic flowchart for the assessment of vaccination responses.
Keywords
Primary immunodeficiency; Vaccine response; Pneumococcal assays; Method agreement; Diagnosis
Background
In patients with suspected immunodeficiency, the evaluation of vaccination responses to polysaccharides guides diagnosis and therapeutic interventions [1,2]. There is, however, controversy about the definitions for the interpretation of test results and the robustness of Pneumococcal serology in diagnosing clinically relevant antibody deficiency.
There is currently no international consensus regarding the “gold standard” assay, nor what constitutes an abnormal vaccine response [3-5]. A WHO recommendation for IgG titres protecting against invasive pneumococcal disease assumes a minimum antibody concentration of 0.35 mg/l for all serotypes in a test setting without pre-absorption [6].
In the past, serological assessment of vaccine responses to unconjugated Pneumococcal vaccine (Pneumovax®) has mainly relied on Pneumococcal 23vELISA which measures a total IgG Pneumococcal response. Serotype-specific assays have been developed alongside the new conjugated Prevenar™ (or Prevnar 13 in USA) vaccines and are now replacing 23vELISA IgG. Serotype-specific assays can be based on single serotype ELISAs, flow cytometry or multiplex technology.
There are a few studies looking specifically at diagnostic method agreement between 23vELISA and serotype-specific assays, usually measured by individual ELISAs in assessing vaccine responses in the context of suspected immunodeficiency. The ones published evaluated small numbers of individual Pneumococcal serotypes.
In one study, vaccine responses to Pneumovax® were analysed in healthy children and adults and in patients referred with recurrent infections and/or a diagnosis of specific antibody deficiency (SPAD). The post-vaccination results generated using single-serotype ELISA to 5 serotypes, 23vELISA and 14-valent xMAP Pneumo multiplex assays [7] showed good correlation (correlation coefficient 0.6-0.76) between the total Pneumococcal IgG levels on 23vELISA and the number of serotypes to which the response was inadequate on xMAP.
Schütz et al. evaluated the correlation between IgA and IgM 23vELISA and four individual serotypes in 20 healthy individuals [8]. They found that serotype-specific IgA but not IgM correlated significantly with 23vELISA post-vaccination. Cavagliere et al. studied vaccination responses in CVID patients on IVIG replacement therapy with IgM and IgA ELISAs and found poor linear correlation between pre- and post-vaccination single serotype IgM and IgA concentrations as well as by IgA and IgM 23vELISAs [9].
Balmer et al. [10] compared the results from 23vELISA (without preadsorbtion stage) to those obtained by a capsular polysaccharide serotype-specific IgG ELISA assay to 9 common serotypes. Discrepancies in 21/47 (45%) of the results were observed between the two assays and were considered to be due to lack of specificity and the presence of C-polysaccharide non-capsular IgG. Nowadays, 23vELISA assay incorporates an adsorption stage to eliminate non-capsular Cpolysaccharide antibodies.
The aim of this study was to compare the method agreement between the Pneumococcal 23vELISA (VaccZyme™, the Binding Site) and 13-valent multiplex serotype-specific multiplex assay [11] in evaluating responses to Pneumovax® in patients with suspected antibody deficiency.
Methods
Sixty-four consecutive patients referred to the immunology clinic at the Royal Free Hospital for investigations of immunodeficiency received test-vaccination with Pneumovax®. Pneumococcal IgG levels at baseline and 4 weeks post-vaccination were measured using both assays. 23vELISA assay (VaccZyme™, the Binding Site) was carried out at the Royal Free Hospital while 13-valent multiplex testing was done at Addenbrooke’s Hospital with the validated method developed as described by Biagini et al. in [11]. At the time of the study both laboratories were part of the NHS and accredited.
Adequate 23vELISA response was defined as a ≥ 4-fold increase in Pneumococcal IgG levels from baseline. Adequate multiplex response was defined as serotype-specific IgG concentration of ≥ 0.35 mg/l (“protective”) and ≥ 0.5 mg/l (“optimal”, as per Addenbrooke’s interpretative comment) for at least 9/13 (≥ 70%) of serotypes tested [6]. IgG threshold of ≥ 1.3 mg/l, theoretically defined as protective in the American literature [4] was also analysed. Methods agreement was analysed using Cohen’s Kappa and McNemar’s tests.
Patients with anti-Haemophilus and/or tetanus IgG below the protective levels (<0.15 mg/L and <0.1 IU/L, respectively) were also vaccinated with Menitorix.
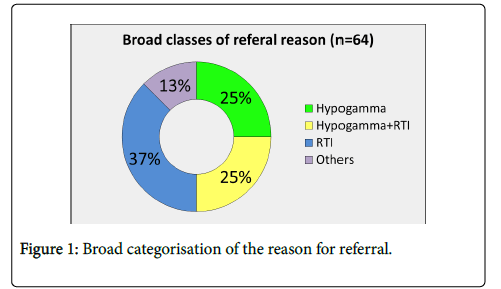
Of the sixty-four patients (median age 46 years, range 18–81 years, F=38, M=26), twenty-seven patients (37%) were referred with recurrent ( ≥ 4/year) respiratory tract infections (RTI) and no previous serum immunoglobulin result, 25% with RTI and known hypogammaglobulinaemia (one or more Ig below the lower limit of normal range: IgG<7 g/L, IgA<0.7 g/L, IgM<2.4 g/l), 25% with incidental hypogammaglobulinaemia and 13% miscellaneous (recurrent infections not RTI, drugs causing hypogammaglobulinaemia) (Figure 1).
Results
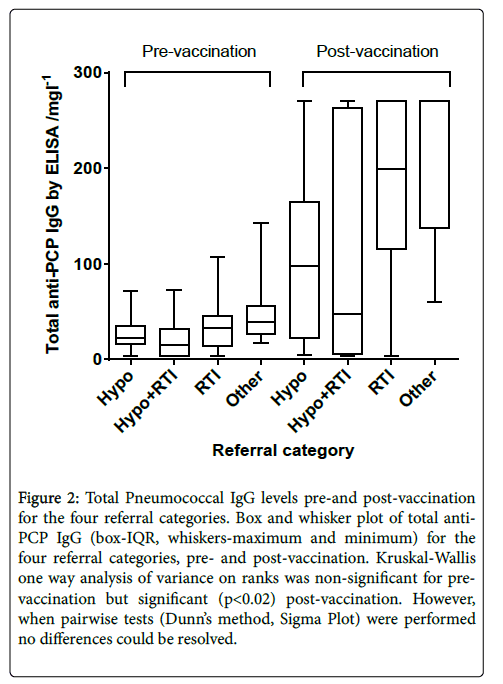
Figure 2 shows total Pneumococcal IgG pre-and post-vaccination , divided by the patient referral categories. A large range of responses is seen, in particular in the group of patients referred with RTI and hypogammalobulinaemia.
Figure 2: Total Pneumococcal IgG levels pre-and post-vaccination for the four referral categories. Box and whisker plot of total anti- PCP IgG (box-IQR, whiskers-maximum and minimum) for the four referral categories, pre- and post-vaccination. Kruskal-Wallis one way analysis of variance on ranks was non-significant for pre-vaccination but significant (p<0.02) post-vaccination. However, when pairwise tests (Dunn’s method, Sigma Plot) were performed no differences could be resolved.
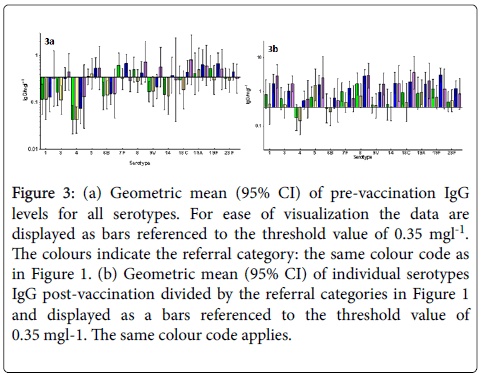
Figures 3a and 3b show geometric mean (95% CI) of individual serotypes IgG pre (a) and post-vaccination (b) divided by the referral categories in Figure 1 and displayed as bars referenced to the threshold value of 0.35 mg/l.
Figure 3: (a) Geometric mean (95% CI) of pre-vaccination IgG levels for all serotypes. For ease of visualization the data are displayed as bars referenced to the threshold value of 0.35 mgl-1. The colours indicate the referral category: the same colour code as in Figure 1. (b) Geometric mean (95% CI) of individual serotypes IgG post-vaccination divided by the referral categories in Figure 1 and displayed as a bars referenced to the threshold value of 0.35 mgl-1. The same colour code applies.
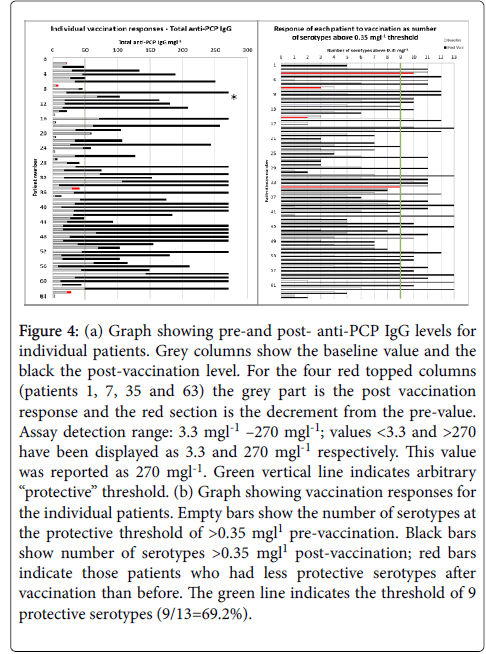
Again, large variability in pre- and post-vaccination results for the individual serotypes is observed, and the post-vaccination values are generally lower for most serotypes for hypogamma and hypogamma with RTI groups. Individual patient vaccination responses for 23vELISA and for serotype IgG are shown in Figures 4a and 4b, respectively.
Figure 4: (a) Graph showing pre-and post- anti-PCP IgG levels for individual patients. Grey columns show the baseline value and the black the post-vaccination level. For the four red topped columns (patients 1, 7, 35 and 63) the grey part is the post vaccination response and the red section is the decrement from the pre-value. Assay detection range: 3.3 mgl-1 –270 mgl-1; values <3.3 and >270 have been displayed as 3.3 and 270 mgl-1 respectively. This value was reported as 270 mgl-1. Green vertical line indicates arbitrary “protective” threshold. (b) Graph showing vaccination responses for the individual patients. Empty bars show the number of serotypes at the protective threshold of >0.35 mgl1 pre-vaccination. Black bars show number of serotypes >0.35 mgl1 post-vaccination; red bars indicate those patients who had less protective serotypes after vaccination than before. The green line indicates the threshold of 9 protective serotypes (9/13=69.2%).
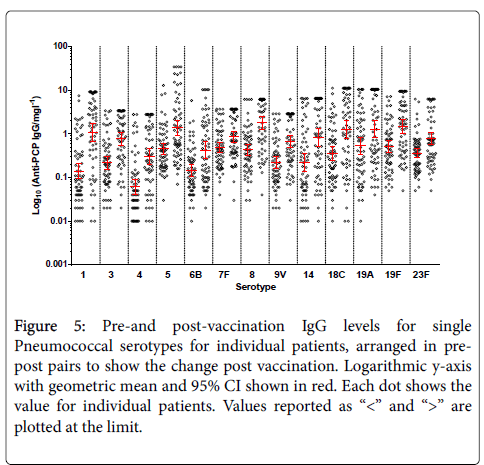
It confirms large variability between the patients for total Pneumococcal IgG. When the number of serotypes >0.35 mg/l is analysed, 19% of patients have 9/13 serotypes within the level of “protection” at baseline. Figure 5 shows pre-and post-vaccination IgG levels for single Pneumococcal serotypes for individual patients, arranged in pre-post pairs to show the change post vaccination. Again, on this logarithmic scale, large variability in concentrations between the serotypes is evident.
Figure 5: Pre-and post-vaccination IgG levels for single Pneumococcal serotypes for individual patients, arranged in prepost pairs to show the change post vaccination. Logarithmic y-axis with geometric mean and 95% CI shown in red. Each dot shows the value for individual patients. Values reported as “<” and “>” are plotted at the limit.
Twenty-eight patients (43.7%) had abnormal vaccine response as measured by 23vELISA, 21 (32.8%) by multiplex at ≥ 0.35 mg/l, 29 (43.7%) at ≥ 0.5 mg/l and 48 (75%) at ≥ 1.3 mg/l threshold. In patients with abnormal vaccine responses on 23vELISA, the most common diagnoses were: CVID (n=8, 12.5%) using ESID criteria [12] and possible CVID (n=10) (Table 1) [12].
| Diagnosis | Referral | ||||
|---|---|---|---|---|---|
| Hypogamma | Hypogamma +RTI |
RTI | Other | Total | |
| No antibody deficiency | 2 | 1 | 14 | 7 | 24 |
| CVID | 3 | 4 | 1 | 8 | |
| Possible CVID | 1 | 1 | 2 | ||
| Hypogamma with SPAD-Ps | 3 | 1 | 1 | 1 | 6 |
| Hypogamma without SPAD | 2 | 6 | 2 | 10 | |
| Secondary hypogamma with SPAD-Ps | 3 | 2 | 1 | 6 | |
| IgA deficiency with SPAD | 1 | 1 | |||
| IgA deficiency without SPAD | 1 | 1 | |||
| SPAD-Ps | 3 | 3 | |||
| SPAD-Ps (MenC) | 1 | 1 | |||
| Subclass deficiency (IgG2) without SPAD | 1 | 1 | |||
| Good's with SPAD-Ps and Pr | 1 | 1 | |||
| Grand Total | 16 | 16 | 24 | 8 | 64 |
Table 1: Cross tab between referral category and diagnosis after vaccination response considered. The number of patients is shown in each cell along with marginal totals.
Four patients were diagnosed with SPAD. Twenty-four patients (37.5%) had no antibody deficiency (normal serum immunoglobulin levels and normal vaccine responses) by ELISA, twenty-three (36%) at 0.35 mg/l and thirteen (20%) at 1.3 mg/l by multiplex. Agreement between the assays was good for ≥ 0.35 mg/l threshold (agreement of 79.6%, Cohen's Kappa=0.57, CI 0.370-0.782), and for 0.5 mg/l (79.7%, Cohen’s Kappa=0.59, CI 0.589 (0.390-0.788) but poor for 1.3 mg/l threshold (McNemar p<0.00002; Cohen's Kappa=0.41, CI 0.198-0.626).
Of 28 patients who showed an abnormal response to Pneumovax by 23v-ELISA, 27 underwent test-vaccination with Menitorix. Normal responses to both Tetanus and Haemophilus were seen in 18/27 patients and abnormal responses in 9 patients: tetanus only or Haemophilus only in 3 patients each and to both in 3 other patients.
Discordant outcomes of vaccine responses evaluated by the two assay were seen in 7/64 (11%) of patients (ELISA vs. ≥ 0.35 mg/l threshold). Three patients had a normal response on 23vELISA but abnormal response on multiplex; two of those patients had excellent Pneumococcal IgG titres on 23vELISA (>270 mg/l) but post-vaccination response to 7/13 and 8/13 serotypes respectively; the third patient had a 4-fold total Pneumococcal IgG increase but post-vaccination protective levels to only 2/13 serotypes. Lack of response was not specific for any given serotype, hence it could not account for the discrepancy between the assays. Four patients with abnormal 23v ELISA result post-vaccination had normal response by multiplex assay at 0.35 mg/l threshold. All four patients had abnormal response at 1.3 mg/l threshold. This group was heterogeneous in terms of clinical presentation and baseline serum immunoglobulin levels. One of these patients also failed to respond to Menitorix and was diagnosed with CVID following ESID criteria. No patients with undetectable (<3.3 mg/l) Pneumococcal IgG by 23vELISA pre-immunisation had normal vaccine responses by either ELISA or multiplex.
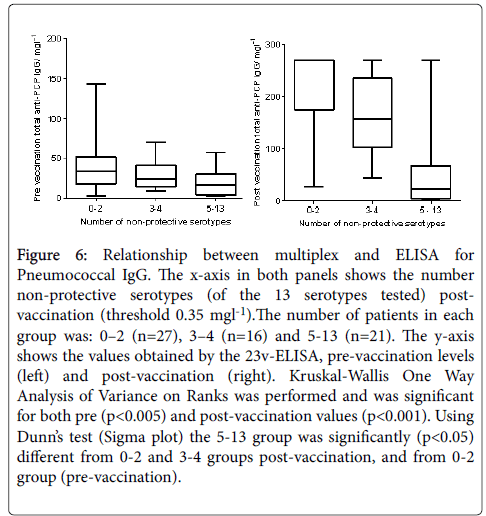
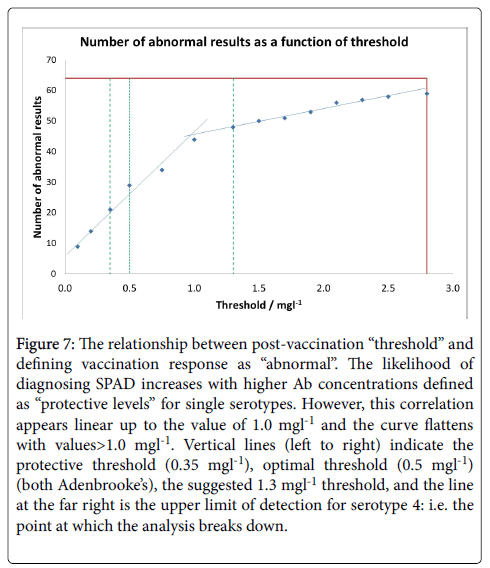
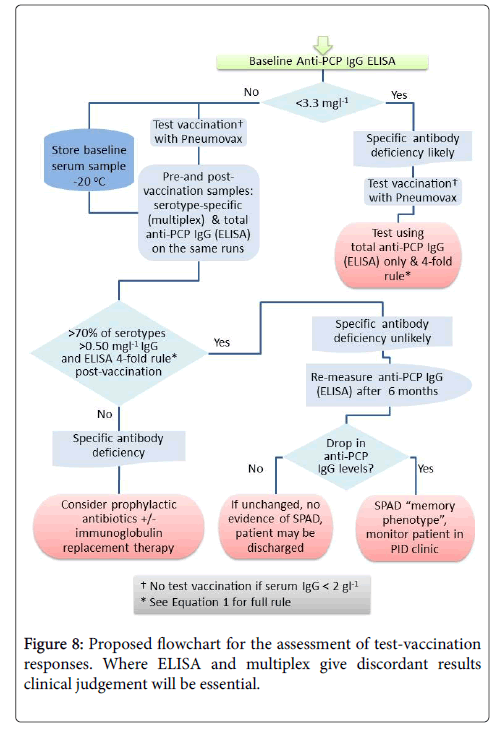
Pneumococcal IgG levels measured by 23vELISA positively correlated with the number of Pneumococcal serotypes at or above protective threshold, in particular for the post-vaccination values (Figure 6). The likelihood of diagnosing SPAD increased with higher antibody concentrations defined as “protective levels” for single serotypes (Figure 7). An in-house diagnostic protocol for Pneumococcal responses has been designed (Figure 8). As 0.5 mg/l showed “mathematically” the best correlation with 23vELISA and was defined as “optimal” by Addenbrooke’s lab, this was chosen for the flow chart.
Figure 6: Relationship between multiplex and ELISA for Pneumococcal IgG. The x-axis in both panels shows the number non-protective serotypes (of the 13 serotypes tested) postvaccination (threshold 0.35 mgl-1).The number of patients in each group was: 0–2 (n=27), 3–4 (n=16) and 5-13 (n=21). The y-axis shows the values obtained by the 23v-ELISA, pre-vaccination levels (left) and post-vaccination (right). Kruskal-Wallis One Way Analysis of Variance on Ranks was performed and was significant for both pre (p<0.005) and post-vaccination values (p<0.001). Using Dunn’s test (Sigma plot) the 5-13 group was significantly (p<0.05) different from 0-2 and 3-4 groups post-vaccination, and from 0-2 group (pre-vaccination).
Figure 7: The relationship between post-vaccination “threshold” and defining vaccination response as “abnormal”. The likelihood of diagnosing SPAD increases with higher Ab concentrations defined as “protective levels” for single serotypes. However, this correlation appears linear up to the value of 1.0 mgl-1 and the curve flattens with values>1.0 mgl-1. Vertical lines (left to right) indicate the protective threshold (0.35 mgl-1), optimal threshold (0.5 mgl-1) (both Adenbrooke’s), the suggested 1.3 mgl-1 threshold, and the line at the far right is the upper limit of detection for serotype 4: i.e. the point at which the analysis breaks down.
Discussion
Individual Pneumococcal IgG levels depend on vaccination history, Pneumococcal exposure, immune status and age. For example, unconjugated Pneumovax showed low to moderate effectiveness against pneumococcal pneumonia serotypes covered by the vaccine in people aged 65 years or older [13].
Protective antibody thresholds differ between individual Pneumococcal serotypes [3,14]. It is not clear what antibody level can be defined as protective in the context of suspected immunodeficiency. WHO recommends a cut-off of ≥ 0.35 mg/l as a “protective” singleserotype IgG concentration in children vaccinated with conjugate Pneumococcal vaccine [6]. The threshold of protection in adults has been debatable; the recent American Academy of Allergy, Asthma & Immunology (AAAAI) guidelines have defined a “protective” (normal or adequate) response to each pneumococcal serotype as an antibody titre of ≥ 1.3 mg/l. Normal reference ranges for individual Pneumococcal serotypes vary and hence evaluation of vaccine responses using fold increase may be difficult [3,7]. Borgers et al. [3] showed that, depending on the serotype, only 39–90% of healthy controls had a post-vaccination IgG concentration ≥ 1.3 mg/l. Values <5th percentile of controls (using individual serotype-specific cut-offs) for <70% serotypes response were observed in 4% of healthy controls (n=75) and in 11/99 (11%) of patients with RTI [7].
In our cohort, abnormal vaccine response was observed in 32.8% of patients on multiplex for 0.35 mg/l, 45.3% for 0.5 mg/l and 43.7% on 23vELISA; vaccine responses were abnormal in 75% of patients with ≥ 1.3 mg/l multiplex threshold. Our results also demonstrate that the comparison of outcomes between 23vELISA and multiplex using threshold of ≥ 1.3 mg/l showed poor method agreement. 23vELISA with a criterion of 4-fold rise in antibody titre classifies fewer patients as abnormal in comparison to the 1.3 mg/l type-specific multiplex. Therefore, defining the thresholds fundamental as it has diagnostic and therapeutic implication (Figure 7), especially as treatment with antibody replacement is very costly.
Pneumococcal assays may not be sufficiently robust to detect abnormal vaccine response if used alone. We therefore suggest that both assays could be used together for each patient, except for the individuals with undetectable levels of anti-PCP IgG at baseline where 23v-ELISA assay should be sufficient (Figure 8).
As other groups, we have observed large variation in IgG levels for individual serotypes both at baseline and post-vaccination. Both total 23v-ELISA and Luminex are characterised by rather large inter-assay variability (CV for ELISA 5-20%, Luminex up to 50% [15]. Evaluation of a fold increase in individual serotype concentration is difficult when assessing vaccine response because of the large variability amongst the serotypes, in particular different upper and lower limits of detection [3,7]. Nasal carriage of Pneumococcus occurs in about 30% of healthy individuals and may lead to higher baseline IgG for most serotypes, in particular for serotypes 1, 5, 18C, 19A, 19F [15], although this was not confirmed in our study (Figure 2).
The main limitation of 23vELISA assay is its inability to distinguish between high responses to a few individual serotypes and a good overall response, as shown by our study. Multiplex assays, on the other hand, do not test responses to all serotypes in Pneumovax. Neither 23vELISA nor 13-multiplex assay adequately take into account the problem of priming with Prevenar 13 prior to measuring response to Pneumovax. For this reason, the best but costly approach would be to measure a 23 serotype panel by multiplex and to focus on serotypes the individual has not been exposed to previously in the form of Prevenar.
In summary, in our hands, undetectable baseline total Pneumococcal IgG on ELISA predicted lack of Pneumococcal vaccine responses as determined by both assays. Pneumococcal 23vELISA and multiplex show good (approximately 80%) diagnostic agreement for abnormal test-vaccination response using the criteria stipulated with a serotype-specific IgG threshold of ≥ 0.35 mg/l and/or of 0.5 mg/l for ≥ 9/13 ( ≥ %70) serotypes and total Pneumococcal IgG threshold of 4- fold rise. When a threshold of ≥ 1.3 mg/l is used, agreement between the assays is poor. The serotype-specific IgG protective threshold of ≥ 1.3 mg/l is not transferrable to our cohort. Larger prospective study would be required to see if the 0.5 mg/l threshold is superior to 0.35 mg/l. Whilst great efforts have been made to generate guidelines to support diagnosis of poor vaccine response, in the absence of standardised assays and the establishment of normal range responses through larger cohort analysis, current pneumococcal assays may guide but not lead the clinical management of patients with immunodeficiency. Vaccine responses should be carefully evaluated by clinicians when they formulate a diagnosis and to justify therapeutic interventions with prophylactic antibiotics and/or expensive IVIG or ScIg. Utilization of identical laboratory methods and definitions is a fundamental component for comparability across centres.
Acknowledgement
We would like to thank Professor Bodo Grimbacher for his constructive comments and suggestions. We also thank Annabel Mai for her help with the samples. Dr. Gabriela Barcenas Morales was supported by a sabbatical grant of PASPA of Universidad National Autonoma de Mexico.
References
- Parvaneh N, Casanova JL, Notarangelo L, Conley ME (2013) Primary immunodeficiencies: a rapidly evolving story. J Allergy Clin Immunol 131:314-323.
- Eibl ME, Wolf HM (2015) Vaccination in patients with primary immune deficiency, secondary immune deficiency and autoimmunity with immune regulatory abnormalities. Immunotherapy 7:1273-1292.
- Borgers H, Meyts I, De Boeck K, Raes M, Sauer K, et al. (2012) Fold-increase in antibody titer upon vaccination with pneumococcal unconjugated polysaccharide vaccine. Clin Immunol 145:136-138.
- Orange JS, Ballow M, Stiehm RE, Ballas ZK, Chinen J, et al. (2012) Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 130:S1-24.
- Bossuyt X, Borgers H, Moens L, Verbinnen B, Meyts I (2011) Age-and serotypedependent antibody response to pneumococcal polysaccharides. J Allergy Clin Immunol 127: 1079-1080.
- WHO (2012) Weekly epidemiological record. Pneumococcal vaccines WHO position paper 87: 129-144.
- Borgers H, Moens L, Picard C, Jeurissen A, Raes M, et al. (2010) Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol 134:198-205.
- Schütz K, Hughes RG, Parker A, Quinti I, Thon V, et al. (2013) Kinetics of IgM and IgA antibody response to 23-valent pneumococcal polysaccharide vaccination in healthy subjects. J Clin Immunol 33:288-296.
- Cavaliere FM, Milito C, Martini H, Schlesier M, Dräger R, et al. (2013) Quantification of IgM and IgA anti-pneumococcal capsular polysaccharides by a new ELISA assay: a valuable diagnostic and prognostic tool for common variable immunodeficiency. J Clin Immunol 33:838-846.
- Balmer P, North J, Baxter D, Stanford E, Melegaro A, et al. (2003) Measurement and interpretation of pneumococcal IgG levels for clinical management. Clin Exp Immunology 133: 364-369.
- Biagini RE, Schlottman SA, Sammons DL, Smith JP, Snawder JC, et al. (2003) Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin Diagn Lab Immunol 10:744-750.
- https://esid.org/Working-Parties/Clinical/Resources/Diagnostic-criteria-for-PID2
- Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, et al. (2017) Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study (APSG-J). Lancet Infect Dis 17: 313–321.
- Balloch A, Licciardi PV, Tang MLK (2013) Serotype-specific anti-Pneumococcal IgG and immune competence: critical differences in interpretation criteria when different methods are used. J Clin Immunol 33:335-341.
- Whitelegg AME, Birtwistle J, Richter A, Campbell JP, Turner JE, et al. (2012) Measurement of antibodies to pneumococcal, meningococcal and haemophilus polysaccharides, and tetanus and diphtheria toxoids using a 19-plexed assay. J Immunol Methods 377:37-46.
Relevant Topics
- Bacteriostatic antibiotics
- Cell signaling and activation
- Chemokines
- Class I MHC molecules
- Class II MHC molecule
- Colitis Antibiotics
- Immune response
- Immunochemistry
- Immunogenicity of biopharmaceuticals
- Immunogenomics
- Immunoglobulins
- Immunoglycomics
- Immunomodulatory xenobiotics
- Immunopharmacology
- Immunoproteomics
- Immunosenescence
- Immunotolerance
- Molecular Immunology
- Non classical MHC class I molecules
Recommended Journals
Article Tools
Article Usage
- Total views: 4266
- [From(publication date):
June-2017 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 3268
- PDF downloads : 998