Comparison between the Apoptotic Effect of Natural Milk Thistle Tincture and Legalon on Hepatocellular Carcinoma Cell Line (Hepg2): An In Vitro Study
Received: 29-Sep-2022 / Manuscript No. jbcb-22-76221 / Editor assigned: 03-Oct-2022 / PreQC No. jbcb-22-76221 / Reviewed: 15-Oct-2022 / QC No. jbcb-22-76221 / Revised: 23-Oct-2022 / Manuscript No. jbcb-22-76221 / Published Date: 31-Oct-2022
Abstract
Purpose: The purpose of this study was to compare the apoptotic effects of the natural milk thistle seeds as a tincture and the commercial silymarin drug (Legalon) on HepG2 cell line.
Methods: The inhibitory concentration (IC50) was determined via the MTT assay for the natural milk thistle seeds tincture and the commercial silymarin drug. The cell cycle distribution assay was determined via flow cytometry. Apoptosis was confirmed by Annexin V -FITC/PI double staining and the percentages of change in the mean levels of P53, Bcl2, Bax, cytochrome c, and caspase 3 were evaluated via ELISA kits assay.
Results: Milk Thistle tincture and silymarin treated HepG2 cells showed cell cycle arrest at G2M. Both substances showed significant apoptotic effects on HepG2 where the percentage of early apoptotic, late apoptotic, and necrotic cells were higher in the HepG2 cells treated with milk thistle tincture than in those treated with silymarin. The mean levels of P53, Bax, cytochrome c, and caspase 3 increased significantly in the milk thistle tincture treated HepG2 cells compared to silymarin treated ones.
Conclusion: The natural milk thistle seed tincture and the commercial silymarin drug (Legalon) induce the hepatocellular apoptosis by activating the mitochondrion-dependent pathway and cell cycle arrest at G2/M. Milk thistle seeds tincture showed a more significant apoptotic effect on HepG2 cells than the silymarin drug (Legalon) as milk thistle seeds contain other constituents in addition to silymarin such as taxifolin, kaempferol, apigenin, quercetin oleic, and palmitic acid.
Keywords
Milk thistle seeds; Silymarin; HepG2; Legalon; Apoptosis
Introduction
Nature is continuously proving that it has a great wealth of natural molecules endowed with cytotoxic activity towards a large panel of tumor cells. Some of these molecules are used in chemotherapy, and others have shown great anti-tumor and anti-metastatic potential in preclinical trials. Silymarin is a flavonolignan called silybin which represents 1.5–3% of the fruit’s dry weight. Milk thistle seeds are seeds containing other flavonoids besides flavonolignans such as taxifolin, quercetin, dihydrokaempferol, kaempferol, apigenin, naringin, eriodyctiol, and chrysoeriol. In addition, they contain fixed oil (35- 55%) (linoleic acid 24-30%, oleic acid 8-12% and palmitic acid), tocopherol, sterols, and proteins.
The production of silymarin from the fruits of S.marianum requires an initial defatting step. The absorption of silymarin by the gastrointestinal tract is only between 20–50% due to its poor water solubility. The poor water solubility and bioavailability of silymarin led to the development of enhanced formulations. Silipide (trade name Siliphos) a drug that is composed of silymarin and phosphatidylcholine (lecithin), is about 10 times more bioavailable than silymarin [1]. Complexation with phosphatidylcholine greatly increases the bioavailability of silymarin, probably by facilitating its passage across the gastrointestinal mucosa [2]. Phosphatidylcholines are the most abundant phospholipids in animal tissues and typically contain saturated fatty acids (palmitic, stearic), monounsaturated (oleic acid), linoleic, or arachidonic acid [3]. The chemical composition of milk thistle fruit besides silymarin also include fixed oil (60% linoleic acid; 30%, oleic acid; 9% palmitic acid) [4], Accordingly we studied the effect of silymarin in its commercial form (Legalon) drug and the effect of the natural crude extract of milk thistle seed (fruit) (Milk thistle mother tincture) (hydroethanolic liquid extract) we prepared Milk thistle mother tincture with extraction Ratio (1: 1) homeopathic preparation containing the natural oils linoleic acid, oleic acid and palmitic acid that present naturally together with silymarin in addition to flavonoids, sterols, sugars and proteins that may provide stronger effect on hepatocellular carcinoma cell line than silymarin .
Methodology
Preparation of milk thistle mother tincture (hydroethanolic liquid extract): It was prepared with extraction ratio (1: 1) homeopathic preparation in which the seeds were separated and washed using cold water to be cleaned from dust. Then, they were dried at room temperature for five days. Next, 50 g of powdered milk thistle seeds were added to 50 ml ethanol (70 per cent V/V) and purified water. Maceration time: 3 weeks (in a dark sealed bottle & away from light).
Preparation of silymarin: It was prepared with concentration 140 mg/ ml. Silymarin drug (Legalon 140 mg) was purchased from (CID)- Egypt under the license of MADAUS GmbH- Germany.
The human Hepatocellular carcinoma cell line (HepG2) with a high metastatic potential was obtained from VACSERA – Egypt. These cells were cultured in RPMI1640 medium supplemented with 10% heatinactivated FBS at 37˚C in a humidified incubator containing 5% CO2.
MTT Assay
The assay is based on the cleavage of the yellow tetrazolium salt MTT into purple formazan by metabolically active cells, which can be photometrically quantified. The increase in the number of living cells increases the total metabolic activity, leading to stronger color formation [5]. For the assay, HepG2 cells (2×105 cells/well in 100μL of complete RPMI1640) were placed in each well of a 96-well-flat-bottom plate. The cells were allowed to adhere for 24h. Then they were treated with 300mg/ml amygdalin, 300mg/ml amygdalin with 20μM of zinc and 300mg/ml amygdalin with 800μM of zinc at which 100μL of each was added separately to the cells. The blank wells contained the above concentrations of samples in growth medium but with no cells. After 48h of incubation, the media were replaced with 100μL of serum-free RPMI1640. 50μL of MTT solution (5mg/mL) was added to each well. After 4h of post-incubation, the medium was removed and 50μL of DMSO was added to each well to solubilize the formazan.
The plate was read at a wavelength of 570nm. The viability percentage was calculated by the formula OD of treated cells/OD of control cells ×100%. The data obtained were analyzed using Master Plex Reader Fit program to determine the half-maximal inhibitory concentration (IC50) for the samples.
Flow cytometric analysis of cell cycle profile
The percentages of the cells distributed in different phases of the cell cycle were determined with flow cytometry using Propidium Iodide (PI) staining [6]. Briefly, the HepG2 treated cells were harvested, washed with PBS, and centrifuged at 3,000 rpm for 3min. Pellets were fixed in 70% cold ethanol at 4°C for 1 hour. After fixation, the cells were washed with PBS twice and incubated with 0.5mg/mL RNase (RNase type I-A; Sigma) for 1 hour to avoid double-stranded RNA staining. Lastly, nuclear DNA staining was carried out using propidium iodide (50μg/mL) in PBS solution for 40min at room temperature. The DNA histograms reflecting cell cycle distribution were determined using BD FAC II Caliber flow cytometer (Becton-Dickinson, Oxford, UK).
Annexin V-FITC/PI double staining
The induction of apoptosis by amygdalin was confirmed by double staining of annexin V-FITC and Propidium Iodide (PI) by Annexin V-FITC Apoptosis Detection Kit; Key GEN, Nanking, China [7]. Briefly, 5×105 of the treated HepG2 cells were resuspended in a binding buffer (10mM HEPES, pH 7.4, 140mM NaCl, 1mM MgCl2, 5mM KCl, 2.5mM CaCl2), stained with 5μl of annexin V-FITC for 10min, and then stained with 5μl of PI for another 10min. The cells were then analyzed with a flow cytometer (FACScan; BD Biosciences, Milano, Italy).
P53, Bcl2, Bax, cytochrome c, and caspase 3 ELISA kit assay
Determination of Human p53
Detection of human p53 concentration in cell culture media was done by ELISA kit (Cat. No.CS0070) from Sigma-Aldrich, Inc, p53 ELISA [8]. Briefly, according to the manufacturer's instructions, 100μL of standards, samples and controls were added and the plate was covered and incubated for 2 hours at room temperature, washed 4 times, and then allowed to dry. 100μL of anti-p53 detection antibody was added, covered, and incubated for 1 hour, washed 4 times, and then allowed to dry, 100μL of anti-Rabbit IgG-HRP working solution was added, covered, and incubated for 30 minutes at room temperature, then washed 4 times and allowed to dry, 100μL of stabilized chromogen was added and incubated for 30 minutes at room temperature in the dark, 100μL of stop solution was added and finally, reading was done on a spectrophotometer at 450nm.
Determination of Bcl-2
Quantitative detection of human Bcl-2 concentration in cell culture media was done using ELISA kit (Cat. No. 99-0042) from Zymed® laboratories - Invitrogen immunodetection [9]. Briefly, 100μL of sample diluent was added in duplicate to all standard and blank wells. 80μL of sample diluent was added in duplicate to the sample wells. 20μL of each sample was added in duplicate to the designated wells. 100μL of diluted biotin-conjugate was added to all wells, and then incubated for 2 hours and washed. 100μL of diluted streptavidin-HRP was added, then incubated for 1 hour and washed. Furthermore, 100μl of the mixed TMB substrate solution was added. The enzyme reaction was stopped by quickly pipetting 100μL of stop solution .Final reading was done on a spectrophomter at 450 nm
Determination of Bax
Quantitative determination of Bax concentration in cell culture media was done using the ELISA kit (Cat. No. EIA-4487) from DRG International Inc., USA [10]. Briefly, 100μL of assay buffer, 100μL of standard, and 100μL of the samples were pipetted into the appropriate wells. The plate was incubated after sealing for 1 hour at room temperature on a plate shaker at ~500 rpm. The content was discarded and washed 4 more times. In addition, 100μL of yellow antibody was pipetted into each well except for the blank ones; it was incubated, discarded, and washed 4 more times. 100μL of the blue conjugate was added to each well except the blank one. The plate was incubated, discarded, and washed. 100μL of substrate solution was pipetted into each well and incubated for 30 minutes at room temperature on a plate shaker at ~500 rpm. Finally, 100μL of stop solution was pipetted to each well. The plate reader was blanked.
Determination of Mitochondrial Cytochrome C
Cytochrome c in vitro ELISA (Enzyme-Linked Immunosorbent Assay) kit (Cat. No. ab119521) from Abcam is designed for accurate quantitative measurement of human cytochrome c concentrations in the cell lysate [11]. Briefly, 100μL of the prepared standards, 100μL of samples, and 50μL of 1X biotin-conjugated antibody were added. The plate was covered and incubated for 2 hours at room temperature. Furthermore, 100μL of 1X streptavidin-HRP was added and the plate was covered and incubated at room temperature for 1 hour. 100μL of TMB substrate solution was added and the plate was incubated at room temperature for 10 minutes. The enzyme reaction was stopped by adding 100μL of stop solution. Finally, reading was done on a spectrophotometer at 450nm 2.4.5 Experimental Manipulations or Interventions
Detrmination of Human Caspase-3 (active):
Detection of the human active caspase-3 concentration in cell culture media was measured by the Invitrogen Caspase-3 (active) Human ELISA kit (Cat. No.KHO1091) [12]. Briefly, 100μl of the standard diluent buffer was added to the zero standard wells. 100μl of standards, controls, and diluted samples were added to appropriate wells, the plate was covered, then incubated and washed. Furthermore, 100μl of caspase-3 (active) detection antibody solution was pipetted into each well except for the blank one. The plate was covered, and then incubated for 1 hour at room temperature. Moreover 100μl of antirabbit IgG HRP working solution was added to each well except for the chromogen blank one. The plate was covered then incubated for 30 minutes and washed. 100μl of stabilized chromogen was added to each well, and then 100μl of stop solution was added. Final reading was done on a spectrophotometer at 450nm.
Results
MTT assay
The inhibitory concentrations (IC50) of milk thistle extract treated HepG2 cells and silymarin (Legalon) treated HepG2 cells were 165 mg/ ml and 51.47 mg/ml, respectively.
Flow cytometric analysis of cell cycle profile
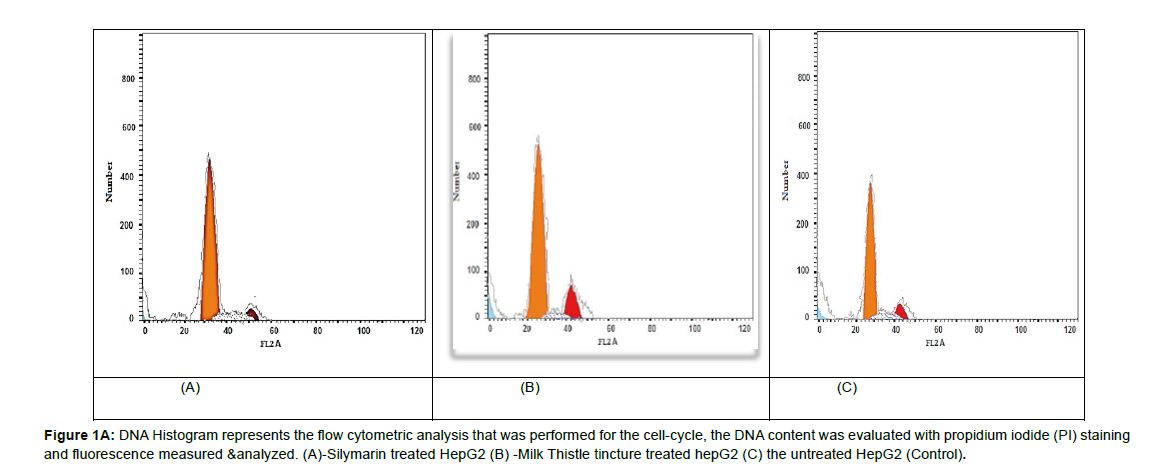
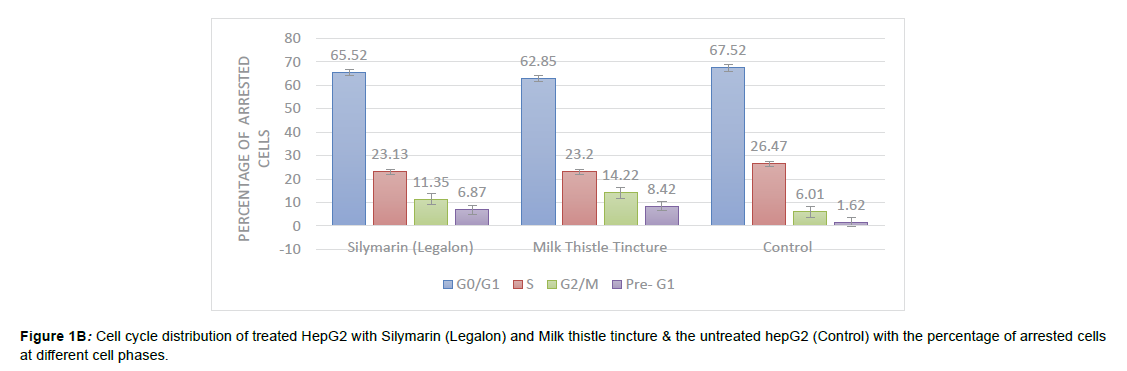
There was a significant accumulation of cellular DNA arrest throughout G2/M phase in all treated HepG2 cells compared to controls (P= 0.000**).The milk thistle tincture significantly influenced the cell cycle arrest at G2/M more than silymarin (P= 0.000 **) as shown in (Figures 1( A) & Figure 1( B)).
Figure 1A: DNA Histogram represents the flow cytometric analysis that was performed for the cell-cycle, the DNA content was evaluated with propidium iodide (PI) staining and fluorescence measured & analyzed. (A)-Silymarin treated HepG2 (B) -Milk Thistle tincture treated hepG2 (C) the untreated HepG2 (Control).
Figure 1A: DNA Histogram represents the flow cytometric analysis that was performed for the cell-cycle, the DNA content was evaluated with propidium iodide (PI) staining and fluorescence measured & analyzed. (A)-Silymarin treated HepG2 (B) -Milk Thistle tincture treated hepG2 (C) the untreated HepG2 (Control).
Annexin V-FITC/PI double staining
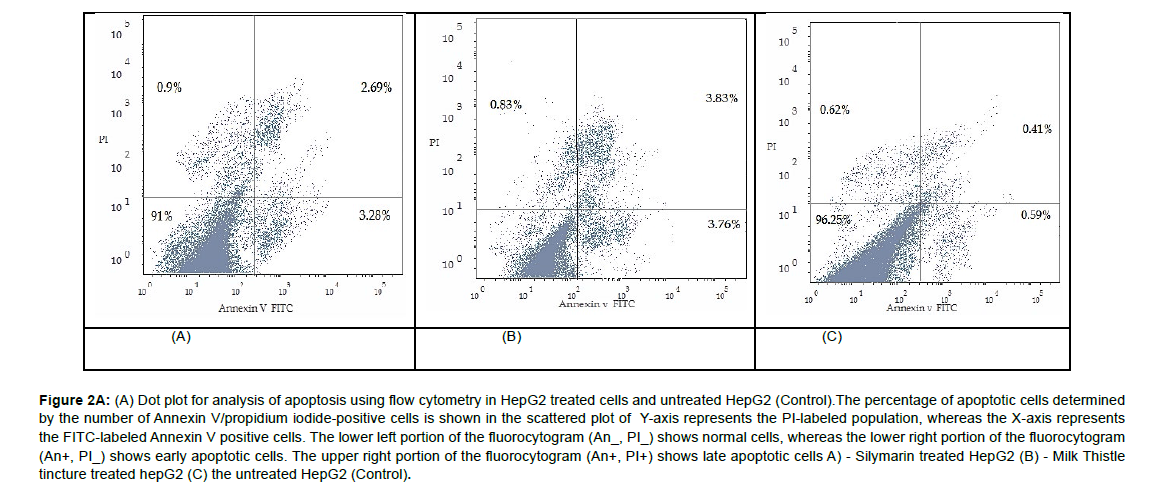
The percentages of early apoptotic, late apoptotic, and necrotic cells were higher in the milk thistle tincture treated HepG2 cells (3.76%,3.83%, and 0.83%, respectively) than in the silymarin treated ones (3.28%, 2.69%, and 0.9%, respectively) (Figure 2(A) & Figure 2(B)).
Figure 2A: (A) Dot plot for analysis of apoptosis using flow cytometry in HepG2 treated cells and untreated HepG2 (Control).The percentage of apoptotic cells determined by the number of Annexin V/propidium iodide‑positive cells is shown in the scattered plot of Y-axis represents the PI-labeled population, whereas the X-axis represents the FITC-labeled Annexin V positive cells. The lower left portion of the fluorocytogram (An_, PI_) shows normal cells, whereas the lower right portion of the fluorocytogram (An+, PI_) shows early apoptotic cells. The upper right portion of the fluorocytogram (An+, PI+) shows late apoptotic cells A) - Silymarin treated HepG2 (B) - Milk Thistle tincture treated hepG2 (C) the untreated HepG2 (Control).
P53, Bcl2, Bax, cytochrome c, and caspase 3 ELISA kit assay
Statistical analysis using one-way ANOVA revealed a highly significant increase in the mean levels of P53, cytochrome c, Bax, and caspase-3 in the treated HepG2 cells compared to their mean levels in the untreated HepG2 cells (controls) (P= 0.000**). However, there was a concomitant decrease in the mean level of Bcl2 in all treated HepG2 cells compared to the untreated ones (P =0.000**).
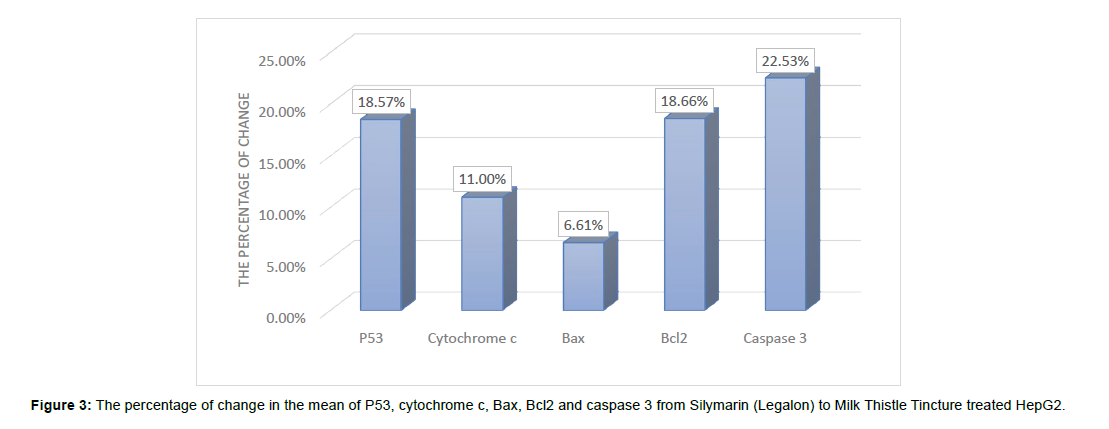
The percentages of change in the mean levels of P53, Bax, cytochrome c, and caspase 3 increased significantly in the milk thistle tincture treated HepG2 cells compared to those in the silymarin treated cells by 18.57%, 6.60 %, and 22.53%, respectively (Figure 3).
Discussion
In the present work, we studied the apoptotic effects of the natural milk thistle seeds as a tincture and the commercial silymarin drug (Legalon) on HepG2 cell line. The inhibitory concentration (IC50) was determined via the MTT assay for the natural milk thistle seeds tincture and the commercial silymarin drug. The cell cycle distribution assay was determined via flow cytometry. Apoptosis was confirmed by Annexin V -FITC/PI double staining and the percentages of change in the mean levels of P53, Bcl2, Bax, cytochrome c, and caspase 3 were evaluated via ELISA.
The distribution of DNA in the cell cycle was studied by flow cytometer. There was a significant accumulation of cellular DNA arrest throughout the G2/M phase in the natural milk thistle Tincture and the commercial silymarin drug (Legalon) treated HepG2 cell line with a significant pre-G1 phase apoptotic incidence. Silymarin inhibits cell cycle progression by induction of cyclin-dependent kinase inhibitors such as p21 and p27 and enhancement of apoptotic signals through regulation of the Akt or p38/JNK mitogen-activated protein kinase (MAPK) signaling pathway [13]. Moreover, silymarin can modulate the Chk2-Cdc25C-Cdc2/ cyclin B1 pathway for G2-M arrest [14]. On the other hand, Milk thistle seeds in addition to Silymarin it contains Taxifolin, kaempferol Palmitic acid and Apigenin. Taxifolin (TAX) can induce cell growth arrest, variation in molecules controlling cell cycle operative in the G2 phase of the cell cycle and apoptosis in a concentration dependent approach [15]. Also, a milk thistle seed contains Apigenin that led to cell cycle arrest at G2M phase via augmenting levels of ROS, reducing glutathione, and inducing TNF-R and TRAIL-R [16]. Moreover, Kaempferol can induce G2/M cell cycle arrest via the Chk2/Cdc25C/Cdc2 pathway and Chk2/p21/Cdc2 pathway [17]. Furthermore, Palmitic acid induces G2/M arrest [18].
We further tested apoptotic cell death in the HepG2 treated cells by staining phosphatidylserine with annexin V conjugated with fluorescein isothiocyanate (FITC) and DNA with propidium iodide (PI). There were significantly elevated percentages of early apoptotic, late apoptotic, and necrotic cells in the group of HepG2 cells treated with the natural milk thistle Tincture and the commercial silymarin drug (Legalon) in comparison with the control group (P<0.05). Moreover, the percentages of early apoptotic, late apoptotic, and necrotic cells were higher in the group of cells treated with the natural milk thistle Tincture than those in the group treated with the commercial silymarin drug (Legalon).
In our study, the statistical analysis revealed a highly significant increase in the mean level of p53 in the HepG2 cells treated with the natural milk thistle Tincture and the commercial silymarin drug (Legalon)compared to the control group (P<0.001). The basal level of p53 and the level of phosphorylation of p53 were increased after silymarin treatment which suggested that the induction of apoptosis by silymarin is mediated via p53 protein expression .Also phosphorylation of p53 increases its half-life and thus increases its accumulation and functional activation of p53 in response to DNA damage [19]. By P53 activation APAF-1 will be activated, APAF-1 binds to cytochrome c and procaspase-9 in the presence of ATP to form the apoptosome (Multiprotein complex) this results in activation of procaspase-9 through autocatalytic cleavage, initiating a cascade of downstream effector caspases, especially caspase-3 which cleave several cell proteins and eventually leading to apoptosis [20].
On the other hand, Our study showed a highly significant increase in the mean level of p53 in Milk Thistle seed Tincture treated HepG2 cells compared to the mean level of p53 in Silymarin treated HepG2 cells with percentage of increase by 18.57% that could be explained by presence of Oleic acid and kaempferol in addition to silymarin in the milk thistle seeds. Oleic acid provides valuable anticancer effects in vitro and in vivo through induction of cell death via autophagy and apoptosis [21]. Milk Thistle seeds also contains kaempferol that can induce DNA fragmentation and upregulation of p53 expression and phosphorylation leading to disrupting cell proliferative signaling, hence apoptosis [16].
In our study, the statistical analysis revealed a highly significant increase in the mean level of Cytochrome C in the HepG2 cells treated with the natural milk thistle Tincture and the commercial silymarin drug (Legalon)compared to the control group (P<0.001). Silymarin treatment can significantly decrease the mitochondrial membrane potential of HepG2 cells leading to opening of mitochondrial permeability transition pores and accordingly, release of cytochrome c from the intermembrane space into the cytosol [22]. In harmony with this result, a previous study demonstrated that, silymarin treatment resulted in a dose-dependent increase in cytochrome c release from mitochondria [19].
On the other hand our study showed a highly significant increase in the mean level of Cytochrome C in Milk Thistle seed Tincture treated HepG2 cells Compared to the mean level of Cytochrome c in Silymarin (Legalon) treated HepG2 cells with percentage of increase by 11% that could be explained by that Milk Thistle seeds contains Apigenin, linoleic acid in addition to presence of Silymarin. Apigenin shows anticancer effect by hyperacetylation of H3 on the p21/waf1 promoter [4] Correspondingly, Bax overexpression as well as the release of cytochrome c [16]. Furthermore Milk thistle seeds contain linoleic acid that promotes cell apoptosis through the release of cytochrome C [23].
Regarding the Bcl2 family (the regulators of apoptosis Bax & Bcl- 2), p53 is a positive transcriptional activator for Bax and a negative transcriptional activator for Bcl-2. Also Bcl-2 can prolong cell survival by suppressing apoptosis, and Bax can enhance apoptosis. Bcl-2 can block mitochondrial permeability transition pores opening, thereby preventing release of caspase activators from mitochondria [22].
In our study, the statistical analysis revealed a highly significant increase in the mean level of Bax and a significant decrease in the mean of Bcl2 in the HepG2 cells treated with the natural milk thistle Tincture and the commercial silymarin drug (Legalon) compared to the control group. Silymarin activates the p53 pathway leading to downregulation of Bcl-2 and up-regulation of Bax [22]. In harmony with this result, a previous study demonstrated that silymarin treatment significantly induced apoptosis by increasing Bax and decreasing Bcl-2 protein expression [24]. On the other hand, there was a highly significant increase in the mean level of Bax in Milk Thistle seed tincture treated HepG2 cell line Compared to the mean of Bax level in Silymarin (Legalon) treated HepG2 cell line. With percentage of increase by 6.60%. Moreover, there was a highly significant decrease in the mean level of Bcl2 in Milk Thistle seed tincture treated HepG2 cell line Compared to the mean of Bcl2 level in Silymarin (Legalon )treated HepG2 cell line with percentage of change by 18.66%.
That could be explained by the presence of Quercetin, Taxifolin, Tocopherol and Silymarin in the Milk Thistle seeds. Quercetin downregulates Bcl2, upregulates BAX, P53, release of Cytochrome C as well as cleavage of Caspase 3 and Caspase 9 that provide involvement of intrinsic mitochondrial pathway [25]. Quercetin also increases Bax translocation from the cytosol to the mitochondrial membrane, an event that promotes apoptotic death [26]. Taxifolin significantly enhance the expression of Bax which was concomitant with decline in the expression of Bcl-2 [27]. Moreover, a Milk Thistle seed contains Tocopherol that enhances Bax mitochondrial relocalization in cancer cells [28]. Furthermore, tocopherol induces apoptosis by also binding to the BH3 domain of antiapoptotic proteins Bcl-2 and thereby blocking its activity [29].
In our study, the statistical analysis revealed a highly significant increase in the mean level of Caspase-3 in the HepG2 cells treated with the natural milk thistle Tincture and the commercial silymarin drug (Legalon)compared to the control group (P<0.001). The treatment with silymarin inducing expression of APAF-1 that interacts with the released cytochrome c-forming apoptosome that activates the caspase-3 in the hepatocellular carcinoma cells. Moreover, the significant increase in P53 may induce apoptosis by activating many pro-apoptotic genes, which ultimately activate APAF-1 [22].
On the other hand, our study showed a highly significant increase in the mean level of Caspase-3 in Milk Thistle seed Tincture treated HepG2 cells Compared to the mean level of Caspase-3 in Silymarin (Legalon) treated HepG2 cells with percentage of increase by 22.53%.
That could be explained by the presence of palmitic acid, linoleic acid, kaempferol, taxifolin and Quercetin in addition to silymarin in the Milk Thistle. A Milk thistle seeds contain palmitic acid and linoleic acid which increase the activity of caspase-3 which is a key component of the pro-apoptotic machinery of cells [30&31]. Also, Milk thistle extract contains kaempferol which triggers expression of cleaved caspase-3 .kaempferol induces apoptosis in HepG2 cells in a dose-dependent manner [32]. Milk thistle extract contains taxifolin that significantly enhance the expression of Bax and cleaved caspase-3 which was concomitant with decline in the expression of Bcl-2 [27]. Milk thistle extract contains Quercetin that activates caspase-3 and caspase-9 and releases cytochrome c [26].
So, the milk thistle extract and pure silymarin may induce hepatocellular apoptosis by activating the mitochondrion-dependent pathway. Milk thistle extracts showed higher apoptosis effect on HepG2 than Silymarin drug.
References
- Morazzoni P, Montalbetti A, Malandrino S, Pifferi G ( 1993) Comparative pharmacokinetics of silipide and silymarin in rats. Eur J Drug Metab Pharmo 18: 289-297.
- Nitin Dixit, Sanjula Baboota, Kanchan Kohli, S Ahmad, Javed Ali (2007) Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Pharmco Bio 39: 172-179.
- https://www.elsevier.com/books/essentials-of-medical-biochemistry/ha/978-0-12-095461-2
- Bijak M (2017) Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry,Bioavailability,andMetabolism. Molecules 22: 1 -11.
- Sylvester PW (2011) Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol 716: 157-168.
- https://pubmed.ncbi.nlm.nih.gov/15220539/
- Zhao G, Han X, Cheng W, Ni J, Zhang Y, et al. (2017) Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol Rep 37: 2277-2285.
- Balmer MT, Katz RD, Liao S, Goodwine JS, Gal S (2014) Doxorubicin and 5-fluorouracil induced accumulation and tran-scriptional activity of p53 are independent of the phosphorylation at serine 15 in MCF-7 breast cancer cells. Cancer Biol Ther 15: 1000-1012.
- Adhami VM, Aziz MH, Mukhtar H, Ahmad N (2003) Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis path-way by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res 9: 3176-3182.
- Oguchi T, Ono R, Tsuji M, Shozawa H, Somei M, et al. (2017) Cilostazol sup-presses Aβ-induced neurotoxicity in SH-SY5Y cells through inhi- bition of oxidative stress and MAPK signaling pathway. Front. Aging Neurosci 9: 337-348.
- Manickam P, Kaushik A, Karunakaran C, Bhansali S (2017) Recent advances in cytochrome c biosensing technologies. Biosens Bioelectron 87: 654-668.
- Schroeter H, Spencer JP, Rice-Evans C, Williams RJ (2001) Flavonoids protect neurons from oxidized low-density-lipoprotein- induced apoptosis involving c-Jun N-terminal Kinase (JNK), c-Jun and caspase-3. Biochem J 358: 547-557.
- Won DH, Kim LH, Jang B, Yang IH, Kwon HJ, et al. (2018) In vitro and in vivo anti-cancer activity of silymarin on oral cancer. Tumor Biology 40.
- Ramasamy K, Agarwal R (2008) Multitargeted therapy of cancer by silymarin. In Cancer Lett 269: 352-362.
- Razak S, Afsar T, Ullah A, Almajwal A, Alkholief M, et al. (2018) Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/ β -catenin signaling pathway. BMC Cancer 18: 1043-1056.
- Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, et al. (2018) Flavonoids in Cancer and Apoptosis. Cancers11: 28-36.
- Gao Y, Yin J, Rankin GO, Chen YC (2018) Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells. Molecules 23: 1095-1110.
- Yung Hsuan Hsiao, Ching I Lin , Hsiang Liao , Yue Hua Chen, Shyh-Hsiang Lin (2014) Palmitic Acid-Induced Neuron Cell Cycle G2/M Arrest and Endoplasmic Reticular Stress through Protein Palmitoylation in SH-SY5Y Human Neuroblastoma Cells. Int J Mol Sci 15: 20876-20899.
- Katiyar S K, Roy AM, Baliga MS (2005) Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol Can Therap 4: 207 -216.
- Würstle ML, Rehm M (2014) A systems biology analysis of apoptosome formation and apoptosis execution supports allosteric procaspase-9 activation. J Biol Chem 289: 26277 -26289.
- Jiang L, Wang W, He Q, Wu Y, Lu Z, et al. (2017) Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci Rep 7: 11277-11288.
- Ramakrishnan G, Lo Muzio L, ElinosBáez CM, Jagan S, Augustine TA, et al. (2009) Silymarin inhibited proliferation and induced apoptosis in hepatic cancer cells. Cell Proli 42: 229-240.
- Zhang Y, Xue R, Zhang Z, Yang X, Shi H (2012) Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis11: 1-9.
- Sekeroglu A, Sarica M, Demir E, Ulutas Z (2015) Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Asian J Chem 20: 4047 -4050.
- Shikha Srivastava, Ranganatha R Somasagara MH, Mayilaadumveettil Nishana, Satish KumarTadi, Mrinal Srivastava, et al. (2016) Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci Rep 12: 240-249.
- GranadoSerrano AB, Martín MA, Bravo L, Goya L, Ramos S (2006) Quercetin Induces Apoptosis via Caspase Activation, Regulation of Bcl-2, and Inhibition of PI-3-Kinase/Akt and ERK Pathways in a Human Hepatoma Cell Line (HepG2). J Nut 136: 2715 -2721.
- Guo L, Zhou W, Liu Z, Wang M, Chen D, et al. (2019) Taxifolin inhibits the development of scar cell carcinoma by inducing apoptosis, cell cycle arrest, and suppression of PI3K/ AKT/mTOR pathway. JBUON 24: 853 -858.
- Wang XF, Dong L, Zhao Y, Tomasetti M, Wu K (2006) Vitamin E analogues as anticancer agents: Lessons from studies with α-tocopheryl succinate. Mole Nutri Food Res 50: 675 -685.
- Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, et al. (2006) α-Tocopheryl Succinate Induces Apoptosis in Prostate Cancer Cells in Part through Inhibition of Bcl-xL/Bcl-2 Function. J Biol Chem 281: 11819 -11825.
- Ahn JH, Kim MH, Kwon HJ, Choi SY, Kwon HY (2013) Protective Effects of Oleic Acid Against Palmitic Acid-Induced Apoptosis in Pancreatic AR42J Cells and Its Mechanisms. Korean J Physio Pharmacol 17: 43 -50.
- Plötz T, Krümmel B, Laporte A, Pingitore A, Persaud S, et al. (2017) The monounsaturated fatty acid oleate is the major physiological toxic free fatty acid for human beta cells. Nutri Dia 7: 305.
- Guo H, Ren F, Zhang L, Zhang X, Yang R, et al. (2016) Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Mole Med Repo 13: 2791 -2800.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Nasraldin KM, Desouky MAE, Fahmi AA (2022) Comparison between the Apoptotic Effect of Natural Milk Thistle Tincture and Legalon on Hepatocellular Carcinoma Cell Line (Hepg2): an In Vitro Study. J Biochem Cell Biol, 5: 166.
Copyright: © 2022 Nasraldin KM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1850
- [From(publication date): 0-2022 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 1638
- PDF downloads: 212