Research Article Open Access
Comparison of Alkali-Tolerant Fungus Myrothecium Sp. IMER1 and White-Rot Fungi for Decolorization of Textile Dyes and Dye Effluents
| Juan Huang1, Yun Fu2 and Youxun Liu2* | |
| 1School of Basic Medical Sciences, Xinxiang Medical University, Jinsui Avenue, Xinxiang, Henan, China | |
| 2School of Life Science and Technology, Xinxiang Medical University, Jinsui Avenue, Xinxiang, Henan, China | |
| Corresponding Author : | Youxun Liu The Laboratory of biochemistry School of Basic Medical Sciences Xinxiang Medical University Jinsui Avenue, Xinxiang, Henan, China Tel: 86-373-3029127 Fax: 86-373-3831739 E-mail: liuyouxun@126.com |
| Received April 01, 2014; Accepted April 24, 2014; Published April 30, 2014 | |
| Citation: Huang J, Fu Y, Liu Y (2014) Comparison of Alkali-Tolerant Fungus Myrothecium Sp. IMER1 and White-Rot Fungi for Decolorization of Textile Dyes and Dye Effluents. J Bioremed Biodeg 5:221. doi:10.4172/2155-6199.1000221 | |
| Copyright: © 2014 Huang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
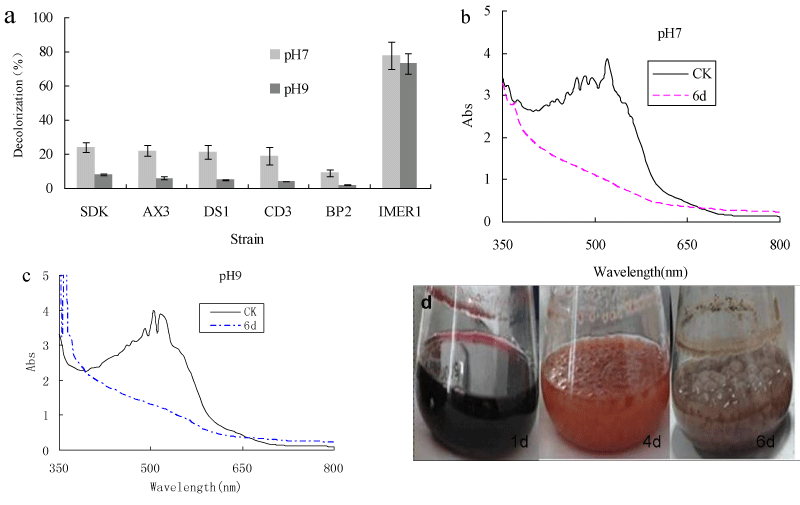
A new isolated nonligninolytic fungus, strain Myrotheciumsp. IMER1, was found to decolorize five different synthetic dyes when grown on dye-containing agar plates. The capability of Myrothecium sp. IMER1 for decolorization of Remazol Brilliant Blue R (RBBR) and dye effluents was compared with that of five white-rot fungi. More than 65% RBBR removal by Myrotheciumsp. IMER1was observed at various pHs (5-10). About 60-95% of decolorization was observed with these white-rot fungi in the acidic pH range of 5.0-6.0, whereas color removal rate was less than 30% in the basic pH range of 8.0-10.0. Myrothecium sp. IMER1 had a more efficient decolorization of the dye in a broad pH range than white-rot fungi tested. In comparison with color removal performance, Myrotheciumsp. IMER1 was approximately 2-5-fold better than white-rot fungi tested in the basic pH range. Additionally, the visual observation and Ultraviolet-Visible (UV-VIS) spectral analysis demonstrated that decolorization of dye by Myrothecium sp. IMER1 was due to biodegradation and biosorption. Biomass production was not affected by changes in the pH range of 7-10, indicated that Myrothecium sp. IMER1 is alkali-tolerant fungus. Decolorization of dye effluents by Myrothecium sp. IMER1 at pH 7 and 9 was 73 and 70%, respectively, while less than 25% of decolorization was observed in the case of white-rot fungi tested. Our results showed that Myrothecium sp. IMER1 exhibited efficient decolorization of dye effluents comparedtowhite-rot fungi, indicatingthat the alkali-tolerant strain Myrothecium sp. IMER1 will be potential candidates for wastewater treatment of dye effluents, especially alkaline dye effluents.
Due to the complexity of the biodegradation mechanism of ligninolytic system, requirements for some redox mediators and low pH for the optimum activity of the enzymes will be present in a wastewater, which are major disadvantages of bioremediation by white rot fungi [14,18]. On the other hand, studies of nonligninolytic fungi degrading dyes are few [19]. Such studies are very interesting not only from the standpoint of comparative biology but also with the expectation of finding better fungi for use in the treatment of dye effluents. Our previous studies showed that the nonligninolytic fungus, Myrothecium sp. IMER1, can decolorize dyes and dye effluents. Furthermore, unlike white rot fungi, this strain can grow at high pH [20-22].
However, decolorization efficiency of dye/dye effluents by Myrothecium sp. IMER1 and white-rot fungi were not systematically compared. Therefore, the major objective of this study is to investigate efficiencies of different white rot fungi strains and Myrothecium sp. IMER1 for decolorization of the dye (RBBR) and dye effluents at various pHs. The results would help to evaluate decolorizing performance of these strains tested and select the right strains for an effective decolorization process.
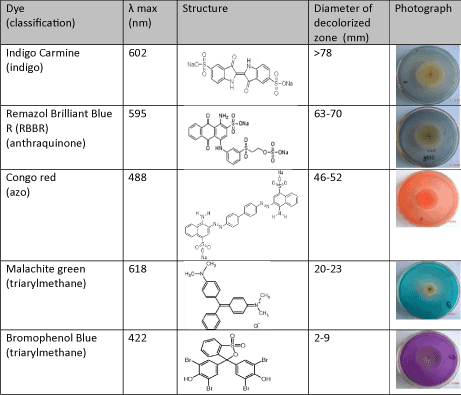
Thus it is seen that that dye decolorization by fungus was dependent on dye structures. Spadaro et al reported that aromatic rings with substituents such as hydroxyl, amino, acetamido, or nitro functions were mineralized to a greater extent than unsubstituted rings in dye decolorization by Phanerochaete chrysosporium[26]. Knapp et al reported that relatively small structural differences could markedly affect decolorization and this might be due to electron distribution and charge density as well as steric factors [27].
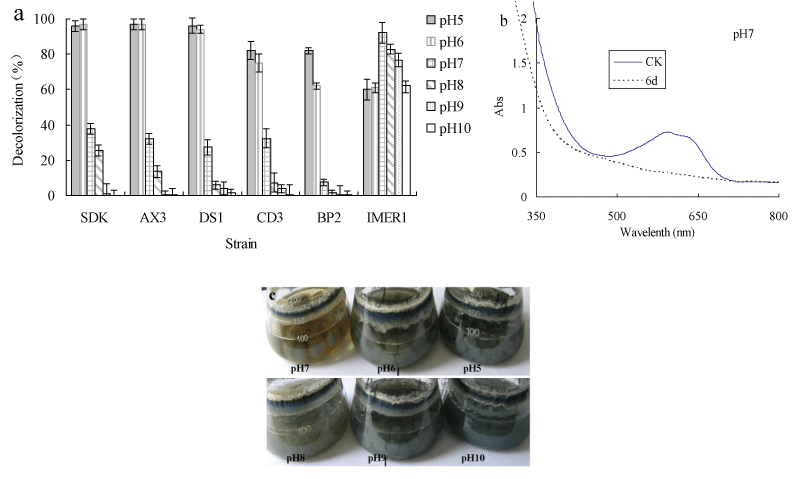
Decolorization of dyes by most fungi could be due to adsorption to microbial cells or to biodegradation [32,33]. If the dye removal were attributed to biodegradation, either the major UV-VIS light absorbance peak would completely disappear or a new peak would appear. In adsorption, cells of fungus become deeply colored because of adsorbing dyes [34,35]. The absorbance spectra of the dye were scanned before and after decolorization and changes in its absorption spectrum (350-800 nm) were recorded. The absorption peaks of RBBR in the visible region disappeared, suggesting that removal of dye by Myrothecium sp. IMER1 should be partly attributed to biodegradation (Figure 1b). In addition, in the decolorization of RBBR by Myrothecium sp. IMER1, the cells were stained deeply colored at pHs (5.0-10.0), indicating that the dye was adsorbed on the cell surface, which was partly due to biosorption (Figure 1c). It suggested that decolorization by Myrothecium sp. IMER1 involved biosorption and biodegradation. This result was in agreement with our previous studies [20,21].
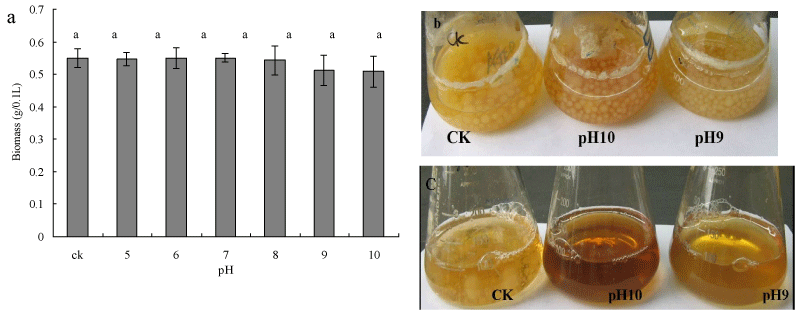
To investigate the effect of pH on growth of Myrothecium sp. IMER1, biomass production was evaluated by determining the dry mass of mycelia. No decrease or increase in biomass production was observed throughout the study in the pH range of 5.0-8.0 (Figure 2a). Interestingly, cells of Myrothecium sp. IMER1 grew almost as well as the control at pH 9.0 and 10.0 (Figure 2b). Although biomass production of Myrothecium sp. IMER1 decreased slightly with increase pH value (9.0-10.0), data statistically analyzed showed that there were no significantly difference in biomass production between control group and experimental group. In comparison with Myrothecium sp. IMER1, growth of white-rot fungi tested such as Pleurotus sp. BP2 was strongly inhibited and low biomass production was observed in the basic pH range of 9.0-10.0 (Figure 2c). The results indicated that biomass production of Myrothecium sp. IMER1 might not be affected by changes in the pH range of 7-10, which also confirmed that Myrothecium sp. IMER1 is alkali-tolerant fungus. Previously, Sulistyaningdyah et al. had reported that Myrothecium verrucaria 24G-4 is an alkali-tolerant fungus and can grow at pH10.0 [36].
It was reported that most white-rot fungi only exhibit good decolorization ability in the acidic pH range of 3-6, which due to low pH for the optimum activity of extracellular ligninolytic enzymes [39,40]. The decolorization capability of Myrothecium sp.IMER1 under alkaline conditions will be an advantage in the case of practical applications to dye effluents, because pulping, bleaching and dye-producing are mainly performed under highly alkaline conditions and their effluents generated are also alkaline.
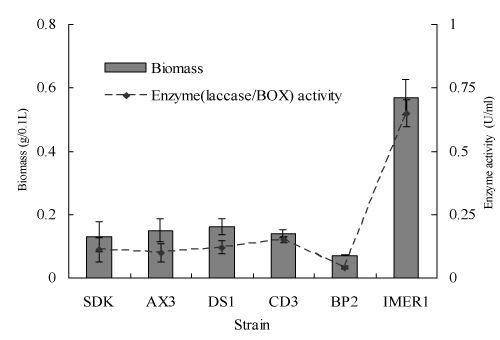
It has previously been reported that BOX as a main extracellular oxidoreductase of Myrothecium sp. IMER1 plays a major role in the dye decolorization, while in the case of many white-rot fungi; main decolorization enzyme is laccase [20-22]. Therefore, BOX/laccase activity and the biomass production of Myrothecium sp. IMER1/white-rot fungi tested were evaluated during decolorization. Both enzyme activity and biomass production of Myrothecium sp. IMER1 were higher than that of all white-rot fungi tested, which attributed to the fact that Myrothecium sp. IMER1 is alkali-tolerant fungus and BOX activity keeps good under neutral and alkaline pHs (Figure 4).
References
- Mohan SV, Rao NC, Srinivas S, Prasad KK, Karthikeyan J (2002) Treatment of simulated Reactive Yellow 22 (azo) dye effluents using Spirogyra species. Waste Manag 22: 575-582.
- Chung KT, Stevens SEJ (1993) Degradation of azo dyes by environmental microorganisms and helminthes. Environ ToxicolChem 12:2121-2132.
- Weisburger JH (2002) Comments on the history and importance of aromatic and heterocyclic amines in public health. Mutat Res 506-507: 9-20.
- Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30: 953-971.
- Slokar YM, Le Marechal AM (1998) Methods of decolorization of textile wastewaters. Dyes Pigments 37:335-356.
- O'Neill C, Hawkes FR, Hawkes DL, Lourenco ND, Pinheiro HM, et al. (1999) Colour in textile effluents: sources, measurement, discharge consents and simulation: a review. J ChemTechnolBiotechnol 74:1009-1018.
- Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. BioresourTechnol 77: 247-255.
- Fu Y, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. BioresourTechnol 79: 251-262.
- Tan NCG, Lettinga GH, Field JA (1999) Reduction of the azo dye mordant orange 1 by methanogenic granular sludge exposed to oxygen. BioresourTechnol 67: 35-42.
- Gupta VK, Rastogi A, Saini VK, Jain N (2006) Biosorption of copper(II) from aqueous solutions by Spirogyra species. J Colloid Interface Sci 296: 59-63.
- Aksu Z, Dönmez G (2003) A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 50: 1075-1083.
- Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. BiotechnolAdv 22: 161-187.
- Barr DP, Aust SD (1994) Mechanisms white rot fungi use to degrade pollutants. Environ Sci Technol 28: 78A-87A.
- Pointing SB (2001) Feasibility of bioremediation by white-rot fungi. ApplMicrobiolBiotechnol 57: 20-33.
- Nazia K, Muhammad A, Haq NB, Munir AS (2010) Ligninase synthesis and decolorization of Drimarene Blue K2RL by Pleurotusostreatus IBL-02 in carbon and nitrogen sufficient shake flask medium. Fresenius Environ Bull 19:63-68.
- Claus H, Faber G, König H (2002) Redox-mediated decolorization of synthetic dyes by fungal laccases. ApplMicrobiolBiotechnol 59: 672-678.
- Kudanga T, Nyanhongo GS, Guebitz GM, Burton S (2011) Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzyme MicrobTechnol 48: 195-208.
- Rodríguez Couto S, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. BiotechnolAdv 24: 500-513.
- Ambrósio ST, Campos-Takaki GM (2004) Decolorization of reactive azo dyes by Cunninghamellaelegans UCP 542 under co-metabolic conditions. BioresourTechnol 91: 69-75.
- Zhang X, Liu Y, Yan K, Wu H (2007) Decolorization of anthraquinone-type dye by bilirubin oxidase-producing nonligninolytic fungus Myrothecium sp. IMER1. J BiosciBioeng 104: 104-110.
- Liu YX, Zhang XY, Yan KL, Wu HJ (2007) Decolorization of indigo carmine by crude bilirubin oxidase from Myrothecium sp. Fresenius Environ Bull 11: 1389-1394.
- Liu Y, Huang J, Zhang X (2009) Decolorization and biodegradation of remazol brilliant blue R by bilirubin oxidase. J BiosciBioeng 108: 496-500.
- Tsujimura S, Tatsumi H, Ogawa J, Shimizu S, Kano K, et al. (2001) Bioelectrocatalytic reduction of dioxygen to water at neutral pH using bilirubin oxidase as an enzyme and 2,2'-azinobis(3-ethylbenzothiazolin-6-silfonate) as an electron transfer mediator. J ElectroanalChem 496:69-75.
- Durand F, Gounel S, Kjaergaard CH, Solomon EI, Mano N (2012) Bilirubin oxidase from Magnaportheoryzae: an attractive new enzyme for biotechnological applications. ApplMicrobiolBiotechnol 96: 1489-1498.
- Wong Y, Yu J (1999) Laccase-catalyseddecolorization of synthetic dyes. Water Res 33:3512-3520.
- Spadaro JT, Gold MH, Renganathan V (1992) Degradation of azo dyes by the lignin-degrading fungus Phanerochaetechrysosporium. Appl Environ Microbiol 58: 2397-2401.
- Knapp JS, Newby PS, Reece LP (1995) Decolorization of dyes by wood-rotting basidiomycete fungi. Enzyme Microbial Technol 17: 664-668.
- Soares GM, Costa-Ferreira M, Pessoa de Amorim MT (2001) Decolorization of an anthraquinone-type dye using a laccase formulation. BioresourTechnol 79: 171-177.
- Kapdan IK, Kargia F, McMullan G, Marchant R (2000) Effect of environmental conditions on biological decolorization of textile dyestuff by C. versicolor. Enzyme MicrobTechnol 26: 381-387.
- Asgher M, Kausara S, Bhattia HN, Shah SAH, Ali M (2008) Optimization of medium for decolourization of Solar golden yellow R direct textile dye by Schizophyllum commune IBL-06. IntBiodeteriorBiodegrad 61:189-193.
- Kaushik P, Malik A (2009) Fungal dye decolourization: recent advances and future potential. Environ Int 35: 127-141.
- Knapp JS, Newbym PS (1995) The microbial decolorization of an industrial effluent containing a diazo linked chromophore. Water Res 29: 1807-1809
- Rodríguez E, Pickard MA, Vazquez-Duhalt R (1999) Industrial dye decolorization by laccases from ligninolytic fungi. CurrMicrobiol 38: 27-32.
- Miranda M, Benito GG, Cristobal NS, Nieto CH (1996) Color elimination from molasses wastewater by Aspergillusniger. BioresourTechnol 57:229-235.
- Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH.BioresourTechnol 97: 512-521.
- Sulistyaningdyah WT, Ogawa J, Tanaka H, Maeda C, Shimizu S (2004) Characterization of alkaliphiliclaccase activity in the culture supernatant of Myrotheciumverrucaria 24G-4 in comparison with bilirubin oxidase. FEMS MicrobiolLett 230: 209-214.
- Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. BioresourTechnol 97: 1061-1085.
- Coulibaly L, Gourene G, Agathos NS (2003) Utilization of fungi for biotreatment of raw wastewaters. Afr J Biotechnol 2:620-630.
- Mou DG, Lim KK, Shen HP (1991) Microbial agents for decolorization of dye wastewater. BiotechnolAdv 9: 613-622.
- Hao OJ, Kim H, Chiang PC (2000) Decolourization of wastewater. Crit Rev Environ Sci Technol 30:449-505.
Figures at a glance
 |
 |
 |
 |
 |
| Figure S1 | Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14642
- [From(publication date):
June-2014 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 9949
- PDF downloads : 4693
